The aim of the present study was to study the diagnostic efficacy of the percutaneous puncture of pancreatic tissue.
Material and methodsA retrospective study was conducted on patients with suspicion of pancreatic neoplasm, and with a percutaneous biopsy of pancreatic tissue, from 2000 to 2011. For the statistical comparative analysis, the sample was stratified by tumor size: ≤3cm and >3cm.
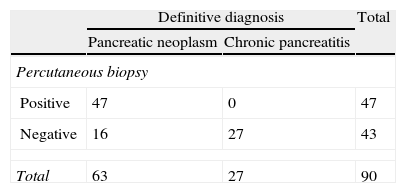
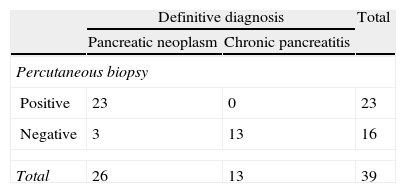
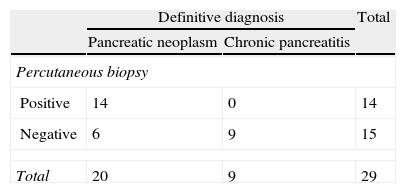
ResultsA total of 90 biopsies were performed. Pancreatic neoplasm diagnosis was made in 47 cases (52%), with 16 false negatives (18%), no false positives, and chronic pancreatitis in 24 cases (27%). The efficacies of the test results were: an overall sensitivity of 75% (95% CI: 62%–85%), a specificity of 100% (95% CI: 87%–100%), a positive predictive value of 100% (95% CI: 92%–100%), and a negative predictive value of 63% (95% CI: 46%–77%). For tumor sizes ≤3cm the sensitivity was 70% (95% CI: 45%–88%), with a specificity of 100% (95% CI 66%–100%), a positive predictive value of 100% (95% CI: 76%–100%), and a negative predictive value 60% (95% CI: 32%–83%). For tumors greater than 3cm, the sensitivity was 88% (95% CI: 70%–98%), the specificity was 100% (95% CI: 75%–100%), with a positive predictive value of 100% (95% CI: 85%–100%) and a negative predictive value of 81% (95% CI: 54%–96%).
ConclusionsPancreatic percutaneous biopsy efficacy was strongly determined by lesion size. For tumor sizes less than 3cm, the sensitivity and negative predictive value are unacceptably low, as negative results would not be reliable.
El objetivo del presente estudio fue analizar la eficacia diagnóstica de la punción percutánea de tejido pancreático.
Material y métodosEstudio retrospectivo de pacientes con sospecha de neoplasia de origen pancreático, con biopsia percutánea de tejido pancreático, desde el 2000 hasta el 2011. Para el análisis estadístico comparativo se estratificó la muestra por tamaño, en menores o iguales a 3cm frente a mayores.
ResultadosSe realizaron un total de 90 biopsias. Se llegó al diagnóstico de neoplasia pancreática en 47 casos (52%), 16 falsos negativos (18%), 0 falsos positivos y al de pancreatitis crónica en 24 casos (27%). Los resultados de rendimiento de la prueba fueron: sensibilidad (S) global del 75% (intervalo de confianza [IC] 95%: 62–85%), especificidad (E) del 100% (IC 95%: 87–100%), valor predictivo positivo (VPP) del 100% (IC 95%: 92–100%) y valor predictivo negativo (VPN) del 63% (IC 95%: 46–77%). En masas ≤3cm la S fue del 70% (IC 95%: 45–88%), la E del 100% (IC 95%: 66–100%), el VPP del 100% (IC 95%: 76–100%) y el VPN 60% (IC 95%: 32–83%). Frente a masas mayores de 3cm que presentaron una S del 88% (IC 95%: 70–98%), una E del 100% (IC 95%: 75–100%), un VPP del 100% (IC 95%: 85–100%) y un VPN del 81% (IC 95%: 54–96%).
ConclusionesLa rentabilidad de la biopsia percutánea pancreática está fuertemente condicionada por el tamaño de la lesión. Para tamaños tumorales menores de 3cm la sensibilidad y el valor predictivo negativo son inaceptablemente bajos, por que lo que resultados negativos no serían fiables.
Pancreatic cancer is the fourth leading cause of cancer death in developed countries.1–4 Despite new diagnostic and therapeutic strategies, the prognosis of this neoplasm is still dire: overall 5-year survival is less than 5%.3,5–7 In patients with resectable neoplasms, surgery is the therapy that obtains the best results, and percutaneous biopsy is unnecessary. However, less than 20% of patients with pancreatic neoplasm are candidates for surgical treatment, and therefore percutaneous biopsy plays an important role.3,8–10
In several international publications,1,2,11–13 percutaneous needle biopsy (guided by either ultrasound or computed tomography) reached sensitivities (S) and negative predictive values (NPV) of around 70%, and specificities (E) and positive predictive values (PPV) close to 100%. It is a simple and inexpensive technique with a low rate of complications.
The aim of this study was to determine the diagnostic efficacy of percutaneous needle biopsy of pancreatic tissue, analyzing the S, E, and predictive values of the test in our hospital.
Material and MethodsThis was a retrospective study that reviewed the medical records of patients with clinical and radiological suspicion of pancreatic neoplasm who underwent pancreatic tissue biopsy between January 1, 2000 and December 31, 2011.
The inclusion criterion was: suspected pancreatic neoplasm (based on symptoms and imaging tests) that was initially not treatable with surgery. Exclusion criteria were: resectable pancreatic masses with indication for surgery, or pancreatic neoplasms with biopsy (percutaneous or surgical) in other locations without biopsy of pancreatic tissue.
The different variables used for the present study were: age, sex, size and location of the pancreatic mass, pancreatic biopsy (percutaneous, excisional or incisional surgical), pathology study and definitive diagnosis.
The definitive diagnosis of pancreatic neoplasm was considered as those patients with biopsy that was positive for cancer, both percutaneous (pancreatic tissue or possible metastases) as well as incisional surgical biopsy, or from the definitive surgical specimen. Likewise, also included in the study were those patients with clinical evolution compatible with a neoplastic process.
The remaining patients who did not meet the above criteria were diagnosed with chronic pancreatitis.
In all cases, we carried out both cytology and fine-needle biopsy of pancreatic tissue under local anesthesia, taking 2 different samples, one with an 18G needle and another with a 20G needle, respectively, guided by either real-time ultrasound or CT. The result was considered positive if both tests were positive or if only the biopsy was positive, uncertain if the cytology was positive and the biopsy negative, and negative if both were negative.
For the statistical analysis, only the total number of biopsies was considered. Thus, 2 biopsies in one same patient were analyzed as 2 different procedures. An analysis was done by intention to treat. For the different calculations, the patients were stratified into 2 groups: one according to size (diameter less than or equal to 3cm versus larger diameters) and the other according to the location of the pancreatic mass (head and uncinate process versus body and tail).
As measurements of the efficacy of this diagnostic test, we used the S, Sp, PPV, NPV and ROC of the different subgroups with their corresponding 95% confidence intervals.
The different analyses were done using the STATA v11 statistical package.
ResultsDuring the 11 years of the study, 81 patients underwent pancreatic tissue biopsy: 49 men (60%) and 32 women (40%), with a mean age of 64 (range 32–89). The most frequent location was the head of the pancreas with 34 cases (42%), followed by the uncinate process with 16 cases (20%), the body of the pancreas with 12 patients and the tail of the pancreas with 13 patients. In 6 cases, the location was not determined. Mean tumor size was 3.5cm (range 1.3–10).
Due to the radiological and analytical suspicion, it was necessary to perform another percutaneous biopsy in 9 cases. Therefore, 90 percutaneous biopsies were performed (88 of which were guided by ultrasound and 2 by computed tomography).
The definitive diagnosis of pancreatic carcinoma was established in 63 cases: 47 using pancreatic percutaneous needle biopsy (82%), 6 cases with percutaneous biopsy of possible metastases, 5 cases with incisional surgical biopsy and no cases with the surgical specimen. In 5 cases there was no other choice than to be guided by the evolution of the symptoms (progression of the disease compatible with malignant tumor): local progression, appearance of distant metastases and finally death of the patient. The most frequent histological type was pancreatic adenocarcinoma in 56 patients (98% of the total), with one case of renal carcinoma metastasis (2%), and 24 cases (30%) of chronic pancreatitis.
The S of percutaneous needle biopsy was 74% (95% CI: 62%–84%), Sp was 100% (95% CI: 87%–100%), PPV 100% (95% CI: 92%–100%), NPV 62% (95% CI: 46%–77%) and ROC area 87% (95% CI: 81%–92%).
As can be observed in Table 1, percutaneous biopsy obtained 16 false negatives (20%), one of which was due to insufficient sample, with a later diagnosis of liver metastasis confirmed by biopsy.
The division into 2 groups according to tumor size gave the following results: in pancreatic masses ≤3cm, the S was 70% (95% CI: 45%–88%), Sp was 100% (95% CI: 66%–100%), PPV 100% (95% CI: 76%–100%) and NPV 60% (95% CI: 32%–84%) and the ROC area 85% (95% CI: 78%–91%). Tumors larger than 3cm presented an S of 88.5% (95% CI: 70%–98%), Sp 100% (95% CI: 75%–100%), PPV 100% (95% CI: 85%–100%) and NPV 81.3% (95% CI: 54%–96%) and an ROC area of 94% (95% CI: 88%–100%). The results are detailed in Tables 2 and 3.
As for location, the lesions located in the head and body of the pancreas presented an S of 72% (95% CI: 56%–85%), Sp of 100% (95% CI: 75%–100%), PPV 100% (95% CI: 89%–100%), NPV 52% (95% CI: 31%–72%) and an ROC area of 86% (95% CI: 79%–92%). Tumors of the head and tail obtained an S of 75% (95% CI: 48%–93%), Sp of 100% (95% CI: 73%–100%), PPV 100% (95% CI: 73%–100%), NPV 75% (95% CI: 47%–93%) and an ROC area of 87% (95% CI: 76%–98%).
DiscussionThe S and NPV of percutaneous needle biopsies of the pancreas, as has been reported extensively in the international literature1,2,11–13 and as can be seen with the results of our series, are low. In our study, however, they did present high Sp and PPV as all the cases with positive biopsy were later confirmed as malignant. In this way, a positive result would be very reliable since the Sp is very high. In contrast, a negative result would not be reliable, as the NPV and S are in general unacceptably low (too many false negatives).
The CI in these indices of diagnostic performance is 50%, which indicates that this index would be useless for diagnosis since its diagnostic value is like a coin-toss. Statistically, it would be equivalent to statistical insignificance. Nevertheless, with a relatively limited sample such as that of our study, this situation is enhanced in appearance.
To correctly interpret a CI in this situation, and from the standpoint of the possible clinical significance or importance of the result, we should focus on the upper limit of the interval, which shows where it is possible for the diagnostic yield of the index in question to reach (in the best-case scenario).
On the other hand, in biopsies such as these, the main items to consider are the S and the NPV, since what matters in a tumor whose image is suggestive of malignancy is to rule out that malignancy and thus avoid surgical interventions, which are sometimes very aggressive, and absolutely unnecessary oncologic treatments. To do so, a high S is needed (few false negatives on biopsy) as well as a high NPV to confirm, in the end, a probably certain negative result. This occurs when both items clearly exceed 80% in their estimation or at least in the upper limit of their CI. If this occurs, despite the statistical non-significance, the result has a value that should not be disregarded from a statistical standpoint.
In contrast, when a result does not have a CI of 50% (which would mean being statistically significant), first we would have to look at the estimate, which should be equally high to accept the test as effective, but considering (this time) the lower limit of the interval because, as the result is significant, it is desirable to know whether in the worst case it could be clinically important or not.14
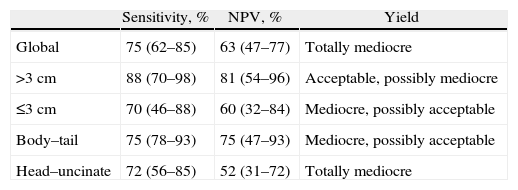
Given these considerations, as a whole the results obtained are mediocre, since the S and NPV are lower than 80%, in addition to the 50% CI of the NPV as well as the low lower limits of the CI for the S (62%) and higher ones for NPV (77%). Likewise, in tumors smaller than 3cm, neither of the values surpass 80% and, furthermore, both CI include 50%; however, the upper limit of both is high (88% and 84%), and therefore a mediocre result would be obtained with the possibility of being acceptable. On the other hand, in masses greater than 3cm, it would give an acceptable diagnostic yield with the possibility of being mediocre, with slightly higher S and NPV, with percentages higher than 80%, without including in the CI the 50%, but with lower CI limits (70% and 54%, respectively). As for the location, in proximal tumors a mediocre total performance is obtained, since the specific percentages are lower than 80% and despite the fact that the CI of the S does not include the 50%, the lower limit of its CI is low (56%), with an unacceptable NPV (point estimation of 52%, including the CI and with an upper limit of 72%). In distal masses, the diagnostic yield is mediocre and may be acceptable since, although both the S as well as the NPV include 50%, both obtain a point estimation close to 80% (75%) and a high upper limit of the CI (93%). Table 4 summarizes the diagnostic performance in the different subgroups.
Overall Performance as well as for the Different Needle Biopsy Subgroups.
| Sensitivity, % | NPV, % | Yield | |
| Global | 75 (62–85) | 63 (47–77) | Totally mediocre |
| >3cm | 88 (70–98) | 81 (54–96) | Acceptable, possibly mediocre |
| ≤3cm | 70 (46–88) | 60 (32–84) | Mediocre, possibly acceptable |
| Body–tail | 75 (78–93) | 75 (47–93) | Mediocre, possibly acceptable |
| Head–uncinate | 72 (56–85) | 52 (31–72) | Totally mediocre |
NPV, negative predictive value.
In spite of the increase in S and NPV in some subgroups, in general the preoperative biopsy is only indicated in potentially unresectable masses, in patients with high surgical risk or in those patients included in neoadjuvant protocols.15 Therefore, this is not a good technique for the screening of space-occupying lesions of the pancreas as they lack a high S, which would overlook a large number of neoplasms as false negatives.
Recently, the use of endoscopic ultrasound (EUS) has become more widespread since this technique is capable of detecting small tumors that are not visible with computed tomography, conditioning an increase in S (around 90%) as well as NPV of small-needle biopsies. Furthermore, it has great value for tumor staging based on location, invasion of neighboring organs such as the duodenal wall or vascular structures, existence of collateral circulation and paratumor or metastatic lymphadenopathies.6,8,11 Nonetheless, it is a complex procedure that requires a long learning curve, patient sedation, expensive instruments, and it is not available at all hospitals.
The basic use of percutaneous needle biopsy is in the field of oncology because it provides differentiation between histologic types, immunohistochemistry characteristics of the tumor and possible differential diagnoses. Although pancreatic cancer is relatively resistant to chemo- and radiotherapy, in recent years new lines of research have been initiated3,16–18 that increase survival and even, in some cases, reduce the tumor size up until the time when surgical treatment is indicated. Likewise, oncologists are reticent to begin neoadjuvant treatment in those patients who, despite having a high suspicion for treatable pancreatic neoplasm (compatible symptoms and imaging tests), present negative biopsies. This point of view should be reconsidered: although histologic confirmation presents many advantages, as previously commented, it should be kept in mind that up to 40% of these patients could present negative biopsies, meaning that there could be undertreatment in this subgroup.
The diagnostic yield of percutaneous pancreatic biopsy is strongly conditioned by the size of the lesion. Thus, it is a good diagnostic option in patients with unresectable pancreatic neoplasms larger than 3cm, fundamentally in those hospitals that do not have endoscopic ultrasound.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Fortea-Sanchis C, Gómez-Quiles L, Martínez-Ramos D, Escrig Sos J, Paiva-Coronel GA, Queralt-Martín R, et al. Rentabilidad diagnóstica de la punción percutánea pancreática en función del tamaño de la lesión. Cir Esp. 2013;91:361–365.