Rectouretral fistulas are a rare disease, but represent an important problem for the patient that suffers them and a challenge for the urologist and colorectal surgeon who has to manage them.
A wide review has been performed focusing on etiopathogenic factors, diagnostic and therapeutic options including the analysis of different surgical techniques. PubMed, MEDLINE and EMBASE medical database were searched up to September 2014.
Las fístulas recto-uretrales constituyen una rara entidad, pero representan un problema trascendental para el sujeto que la padece y un reto para el urólogo y/o coloproctólogo que debe resolverlo.
Se realiza en este trabajo una amplia revisión sobre los factores etiopatogénicos, procedimientos diagnósticos y actitud terapéutica, analizando las diferentes opciones quirúrgicas descritas en la literatura, mediante búsqueda bibliográfica en PubMed, MEDLINE y EMBASE hasta septiembre de 2014.
Rectourethral fistulas (RUFs) are a rare disease, but represent an important problem for the patient who suffers them and a challenge for the urologist and/or coloproctologist who has to manage them.
The published series are short; the largest ones comprise two or three dozen cases seen over long periods of time. This circumstance has limited the availability of a large number of therapeutic options, the choice of which is based on the preference or greater experience of the respective surgeon. In recent years, however, there has been a greater trend towards streamlining treatment based on the aetiopathogenesis, type of fistula and degree of injury.
Now, despite its rarity and the technical difficulties for treatment approach, highlighted by all the authors (most papers describe it as a devastating pathology), healing figures are close to 100% with various therapeutic procedures, either with only one intervention or after several attempts.
This paper discusses the clinical aspects of this exceptional disease, as well as the main therapeutic options.
MethodA bibliographic search was conducted in PubMed, MEDLINE and EMBASE up to September 2014, in Spanish and English, using the key word “rectourethral fistulas”. Every article deemed important was evaluated with a special focus on the aetiopathogenesis, diagnostic methodology and therapeutic options. The highlighted articles mentioned in the previous search were also reviewed.
AetiopathogenesisAlthough congenital fistulas do exist, mainly associated with anorectal malformations, in this analysis we consider acquired rectourethral fistulas exclusively.
The causes are highly variable, but the vast majority are related to prostate cancer, either due to tumour invasion or, more frequently, as a consequence of its treatment; rectourethral fistulas are estimated to occur in around 1%–2% of all patients treated for prostate cancer.1–4
It is certainly interesting to analyse disease progression with respect to its exact cause and in relation to the incorporation of modern technologies, not only from a surgical perspective with the application of laparoscopic and robotic5–12 surgery, but also due to the arrival of new therapeutic options, such as external radiotherapy, brachytherapy, cryotherapy, high-intensity ultrasound and radiofrequency, mainly.13–26
Also of utmost importance is the spectacular increment of RUFs in relation to external radiotherapy and brachytherapy in recent years. In this sense, Lane27 underscores that, out of 315 cases of RUF collected until 1997, only 12 (3.8%) had received pelvic radiotherapy, whereas, since 1998, 113 of the 228 published cases (49.6%) had received that treatment. Still, as explained by this author, not all fistulas can be exclusively attributed to radiotherapy, since many of these patients had undergone some kind of surgery, instrumental manipulation and biopsies, and all these factors could have contributed to the fistulas being triggered, a situation stressed by other authors.28–30 Other circumstances, such as nutritional status, immunodepression, smoking and older age have also been implied, although definitive conclusions could not be drawn.30,31
The frequency of RUF occurrence in association with these factors varies in different series: it develops in 0.53%–9% after radical prostatectomy1,32; 0.4%–8.8% after brachytherapy28,29,33,34; 0%–6% after external radiotherapy30; 0.4%–3% after cryotherapy23,25,35 and 2.2% after high-intensity ultrasound.16
The time of onset of the fistula ranges between four and seven days following a radical prostatectomy36; between four and seven weeks after cryotherapy or ultrasound and up to three years following the administration of brachytherapy.28–30,34
Genitourinary traumas are less frequent, but undoubtedly important; for example, pelvic fractures, war wounds, transrectal biopsies and some very anecdotal traumas, such as those resulting from enema cannulas or haemorrhoid sclerosing injections,13,36–38 as well as pelvic inflammatory or infectious processes, such as Crohn's disease, recurrent anal abscesses and tuberculosis. The occurrence of a RUF due to invasive rectal cancer, although possible, is exceptional.
DiagnosisThe diagnosis is made based on symptoms and appropriate diagnostic tests.
The primary symptoms are the presence of pneumaturia and/or faecaluria and the passage of urine through the anus. The possibility of urinary infections is permanent.
In variable proportions, haematuria and perianal or perirectal pain may be added; occasionally, a rectal examination may allow the location or suspicion of the existence of the fistulous orifice.28,30,39,40
Diagnostic tests are intended to confirm the presence of the rectourethral communication and its location and to rule out the presence of a superimposed disease.
The most often proposed studies are rectoscopy, cystoscopy, urethroscopy and cystourethrography, with different authors recommending them.20,22,24,41
Performing a contrast enema, CT, MRI and endorectal ultrasound may provide data in selected cases, especially to rule out abscesses or tumour infiltration. There are some anecdotal cases on the finding of a RUF after performing a PET-CT scan.42
All these diagnostic tests provide the most complete evaluation of the fistula and allow choosing the most adequate treatment.
TreatmentTaking into account its low incidence, its wide pathological variability and its aetiopathogenic characteristics, as well as each patient's different circumstances, it is easy to understand the lack of a methodical guide for a generalised therapeutic plan. Consequently, most of the time the course of action provided is based on the habits and experience of the medical team in charge; there are no available data comparing progression and outcomes according to the procedure used. In general, the choice is based on the time when the fistula is detected (during surgery or post-surgery), clinical symptoms, aetiology, associated pathological abnormalities, urinary and faecal functions, age, life expectancy and overall condition of the patient.4,20–22,25,27,31,32,43,44
The use of a faecal or urinary diversion will be decided on a case-by-case basis.
Thus, we will try to reflect the current situation of this issue, ordering our discussion in the following sections:
- 1.
Possibilities of conservative treatment.
- 2.
Surgical options.
The spontaneous closure of the fistula with transurethral bladder catheterisation or diversion by suprapubic puncture is exceptional; nevertheless, it may be attempted in patients without signs of sepsis or faecaluria.32,45,46 A “conservative treatment” includes the possibility of a urinary and/or faecal diversion which, although not being an excessively aggressive treatment, significantly affects the patient's quality of life.18
The number of resolutions is highly variable, ranging from 14% to 54% in the different series.32,47–49
As a “conservative” treatment, we can also consider the application of different types of fibrin sealants on the fistulous tract, although their use is limited to isolated cases.50–53
If effective, these sealants would evidently be the ideal treatment: non-invasive, repeatable and enabling any other intervention. However, the outcomes are not spectacularly good and the procedure is not free from complications.54
Lastly, in cases with good anal sphincter continence, therapeutic “abstention” could provide good quality of life, with minimum symptoms, comparable to the situation after an ureterosigmoidostomy, although with the permanent risk of urinary tract infections and secondary malignisation.
In summary, the possibility of a conservative treatment exists in the terms explained above. The decision should be made on a case-by-case basis considering the type of lesion and the patient's characteristics.
Surgical OptionsSeveral aspects are unanimously highlighted in relation to the surgical treatment of RUFs: the first attempt of repair is the most favourable one55; the optimal approach is the one the surgeon is most familiar with to carry out the necessary surgical actions required in each case43; deciding on the most adequate time for the repair is of paramount importance56; there is no unanimity with respect to the need of a faecal diversion as a step prior to the surgical correction,18,25,32,39,47,48,57–60 except for RUFs secondary to radiotherapy and, particularly, to brachytherapy, where a double diversion is recommended prior to any other treatment,4,21,22,27,31 with Linder24 even suggesting the option of a permanent diversion given the poor surgical outcomes in these patients.
After various publications over the last century, in 1958 Goodwin61 highlighted the most important aspects of the repair, which may be summarised, with small variations, in the following premises: good mobilisation and excision of the fistulous tract, adequate mobilisation of the surrounding tissues, healthy margins in urethra and rectum, urethral closure, rectal closure in two planes (both tension-free sutures), avoiding overlapping of both suture lines, and interposition of healthy tissue whenever it is considered adequate.
There are many surgical options described, from the route of approach to the exact details about how to handle the urethra, rectum and adjacent tissues, all aspects that have been included in broad revisions.22,39,41,55,62
The routes of approach used are the following: abdominal, transanal, transrectal, transsphincteric and perineal. Their main characteristics are:
Abdominal ApproachMinimally used because, since, although it enables the interposition of the omentum, it involves greater surgical difficulty and duration, a longer hospital stay, longer recovery time and more postoperative pain.58,63
The laparoscopic approach has been used in some cases, but only exceptionally.64,65 The lesions originated during a laparoscopic prostatectomy can also be repaired via this route.6
In extremely complex cases that would require implanting a permanent colostomy, Chirica66 proposes a coloanal anastomosis with Soave's technique.
Transanal ApproachIts advantages are its simplicity and limited morbidity. On the contrary, its disadvantages are poor visibility, limited capacity to manoeuvre the instruments and the impossibility of tissue interposition.
The simplest procedure is Latzko's technique, with mucosal denudation and closure of the fistulous orifice without excision of the tract; it results in minimum postoperative pain, does not require a diverting stoma and may be repeated as many times as necessary. It is most often used in rectovesical and vesicovaginal fistulae; it may be useful for small and distal RUFs.49,57
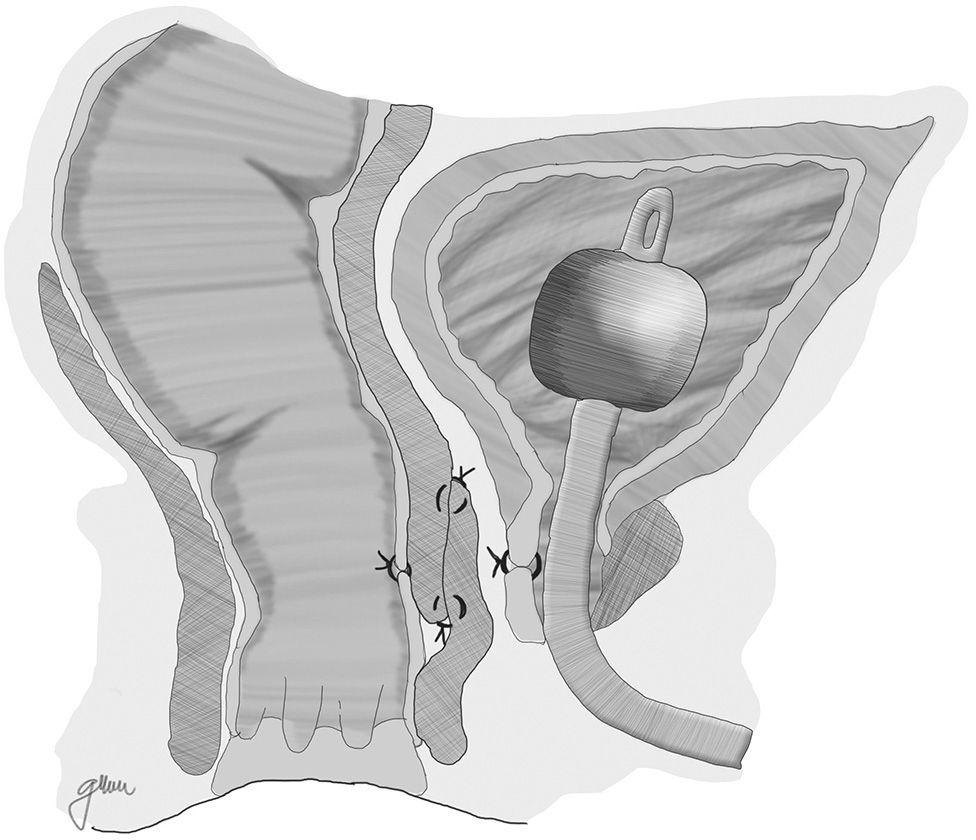
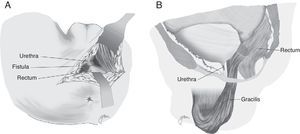
Another option is a total rectal wall advancement flap with closure of the urethral orifice, avoiding an overlap of the suture lines, which has yielded favourable results (Fig. 1).67 In order to separate both structures, an interposition with Alloderm grafts, biocompatible material made from human cadaveric dermis that provides an acellular matrix and enables the vascularisation and growth of native tissue, has been used in a few cases.14
A correction by endoscopic microsurgery (TEM-TAMIS) has also been proposed, with different treatments of the fistulous tract, urethral orifice and rectal opening.15,51,68–72
Posterior Transrectal Approach (Kraske Procedure)Described by Kraske in 1885, its value is essentially historical, given the multiple (both urinary and faecal) complications it involves.73,74
Posterior Transsphincteric Approach (York-Mason Procedure)It accesses the RUF via a posterior transsphincteric approach and opening of the posterior rectal wall.
Described by Kilpatrik [sic: Kilpatrick] and Mason in 1969,74 with parasacral-coccygeal transsphincteric opening, and modified in 1970 by York-Mason.75
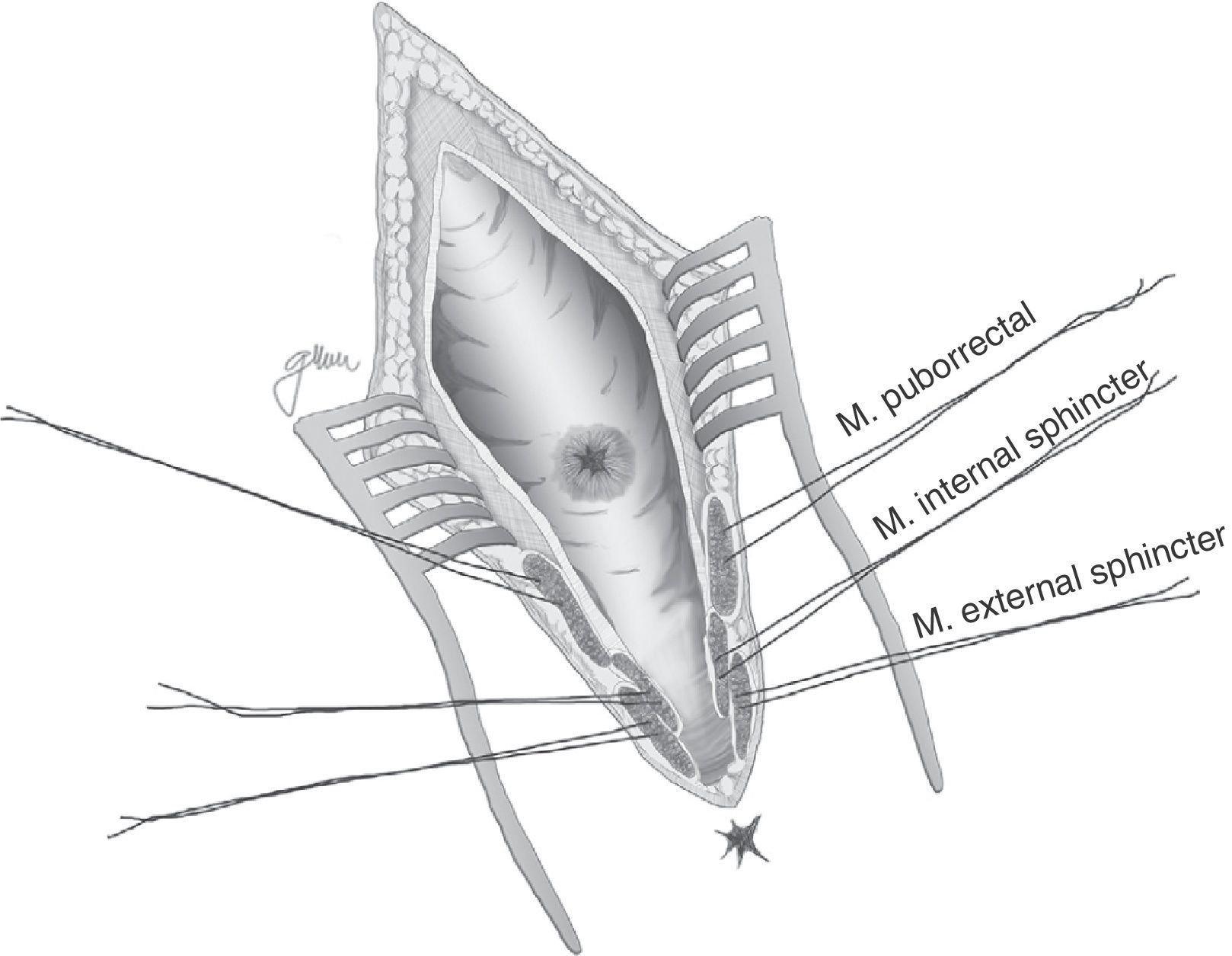
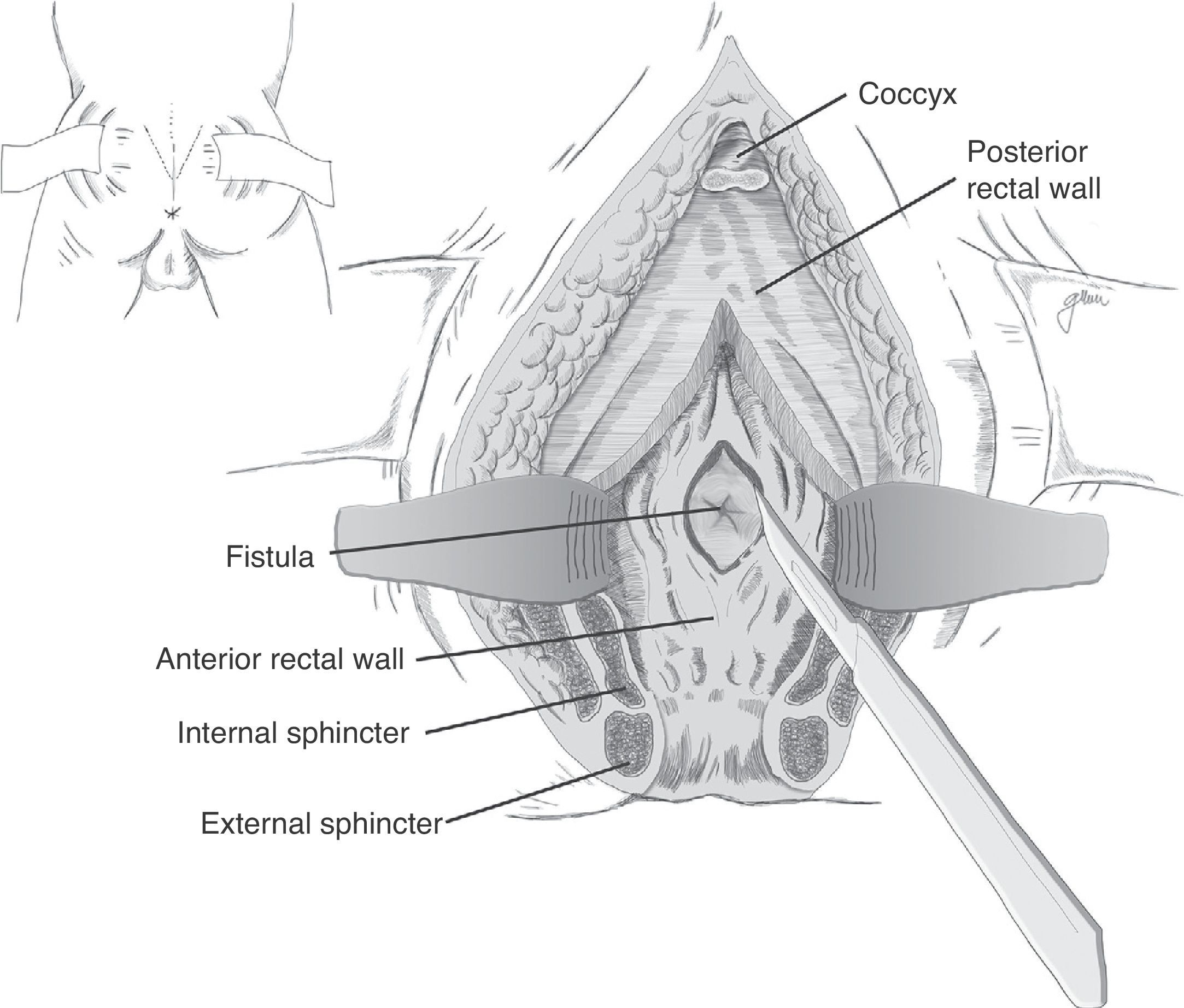
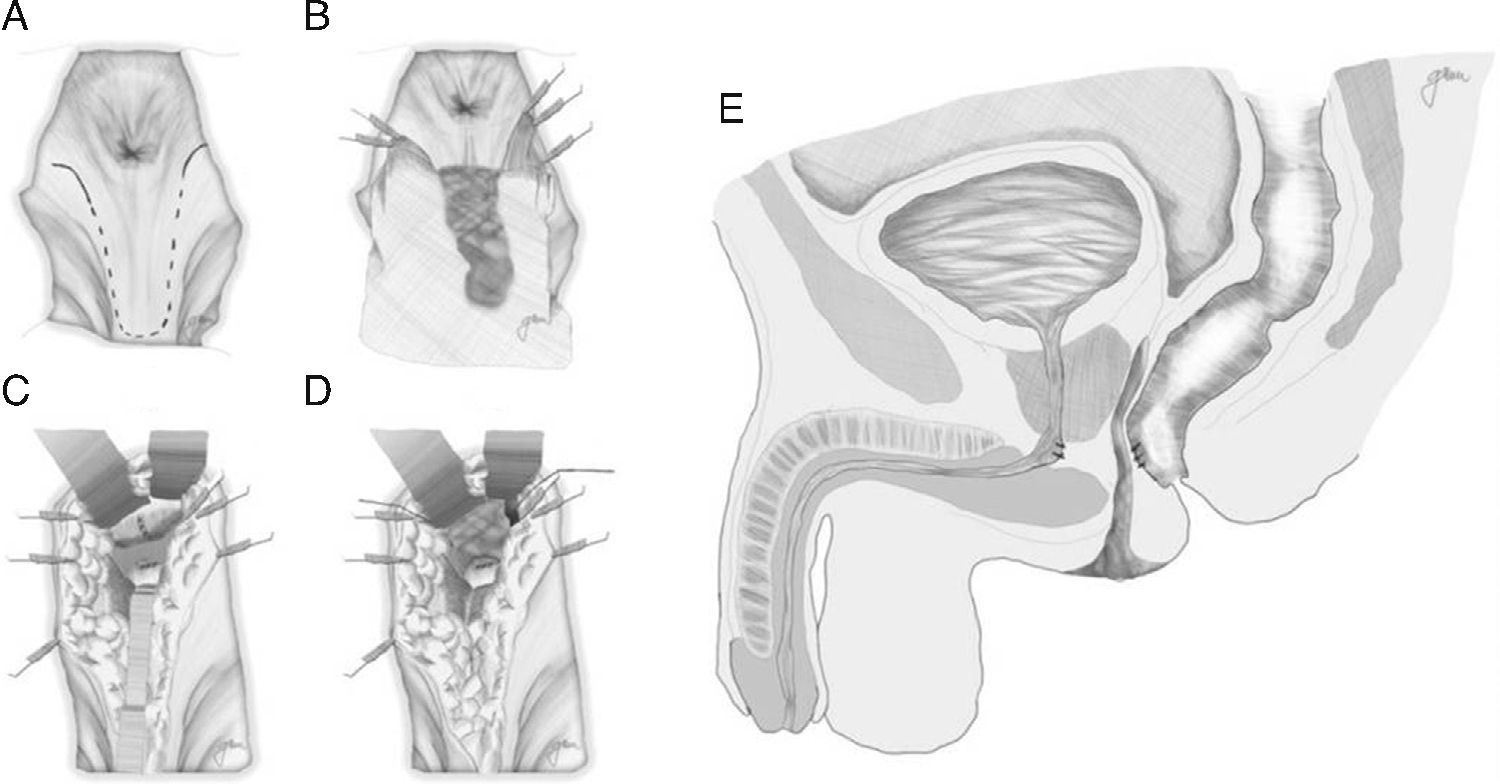
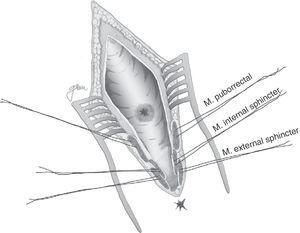
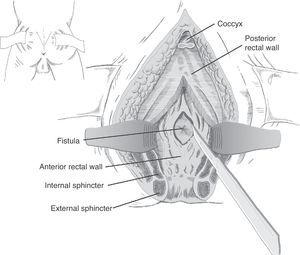
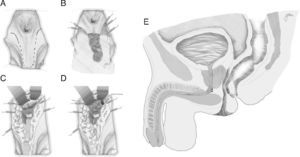
Following mechanical bowel preparation and antibiotic prophylaxis, it essentially consists in a preoperative 18Fr bladder catheterisation, prone jack-knife position and separation of both buttocks, oblique incision from the left side of the sacrum and coccyx up to the posterior anal margin, sectioning the entire sphincter complex (external sphincter, internal sphincter and puborectalis/levator), and leaving them marked with dots for an easier later repair. Then, an opening is made on the posterior rectal wall, which enables a perfect view of the anterior rectal aspect with its fistulous orifice (Fig. 2). Discrete modifications, such as the opening in the middle line18 (Fig. 3) or on the right side,58 have been made without changing the basics of the procedure.
Following that, the entire fistulous tract is resected, including the rectal wall, the urethral wall and the surrounding tissues that enable the correct suture of healthy tissues; the urethral and rectal sutures should not overlap. Occasionally, performing a rectal advancement flap is recommended, as shown in Fig. 1.48
With small variations, and without different transcendental surgical actions, this is the technique recommended by numerous authors because of its ease, accessibility, lack of complications and satisfactory outcomes, particularly in the case of small-sized RUFs,5,18,43,55,56,58,76,77 although it may be applied in other more complex techniques by combining procedures.18,55,76,78 Not all the authors close the urethral orifice, unless such closure is achieved in a tension-free manner and on healthy tissue37,48,79–81; whereas the recommendation of closing the rectal orifice in two planes is unanimous: submucosa and muscle in the first plane and everted mucosa in the second plane, both with reabsorbable material.
The final step is the closure of the posterior rectal wall and sphincter, subcutaneous and skin reconstruction, leaving a suction drainage at subcutaneous level for 24–48h.
The bladder catheter should remain placed for six to eight weeks. With respect to the need of a faecal diversion, even though most authors recommend it, the possibility of omitting it is also accepted in cases of small fistulae, without extensive fibrosis and in the absence of uncontrolled systemic infection, sepsis or abscess.58
If a colostomy is made, the closure will take place between two and three months after the procedure. Prior to suppressing the diversion, the complete closure of the fistula should be confirmed by cystoscopy, retrograde cystourethrogram, rectoscopy and even opaque enema, to confirm there is no communication.
Healing percentages of this procedure range from 85% to 100%; in some cases several surgical procedures are required to achieve healing, which provides evidence that this technique may be performed repeatedly, without an increase in morbidity.48,58
Basically all the series report that anal continence is not affected.58
Perineal ApproachIn 1958, based on previous descriptions by Weyrauch (1951) and Wilhelm (1955), Goodwin61 recommended the perineal approach for the treatment of complex RUFs and established the basic criteria and surgical actions for such intervention, which basically are: patient in lithotomy position and catheterised, wide perineal exposure, transection of the fistula, release of the urethral and rectal orifices, suture in two planes forming a right angle between them, adequate separation of such sutures by the interposition of the levator, urinary drainage via urethral or suprapubic catheter for at least two weeks, colostomy in selected cases and perineal drainage. With these essential principles, he was able to obtain a 100% healing rate.
Apart from all those surgical actions, in the case of complex or large RUFs or RUFs with fibrosed, infected and/or abscessed tissue, it is critical to interpose healthy tissue between both suture lines to provide a higher healing rate. Although the omentum is useful, it requires an abdominal approach; on the other hand, in cases of previous abdominal surgery, it is not always possible to use it and, therefore, other structures have been proposed in recent years, mainly the gracilis, dartos, bulbocavernosus and gluteal muscles, apart from the peritoneal and levator muscles previously used.
In 1979, Ryan80 used the gracilis muscle in three cases and obtained healing in all of them. Since then, this technique has been widely recommended by many authors, with minimum complications and high healing rates, and it can be widely used regardless of age, sex or body configuration,4,17,18,20,47,50,59,82–87 although the published series are very limited.
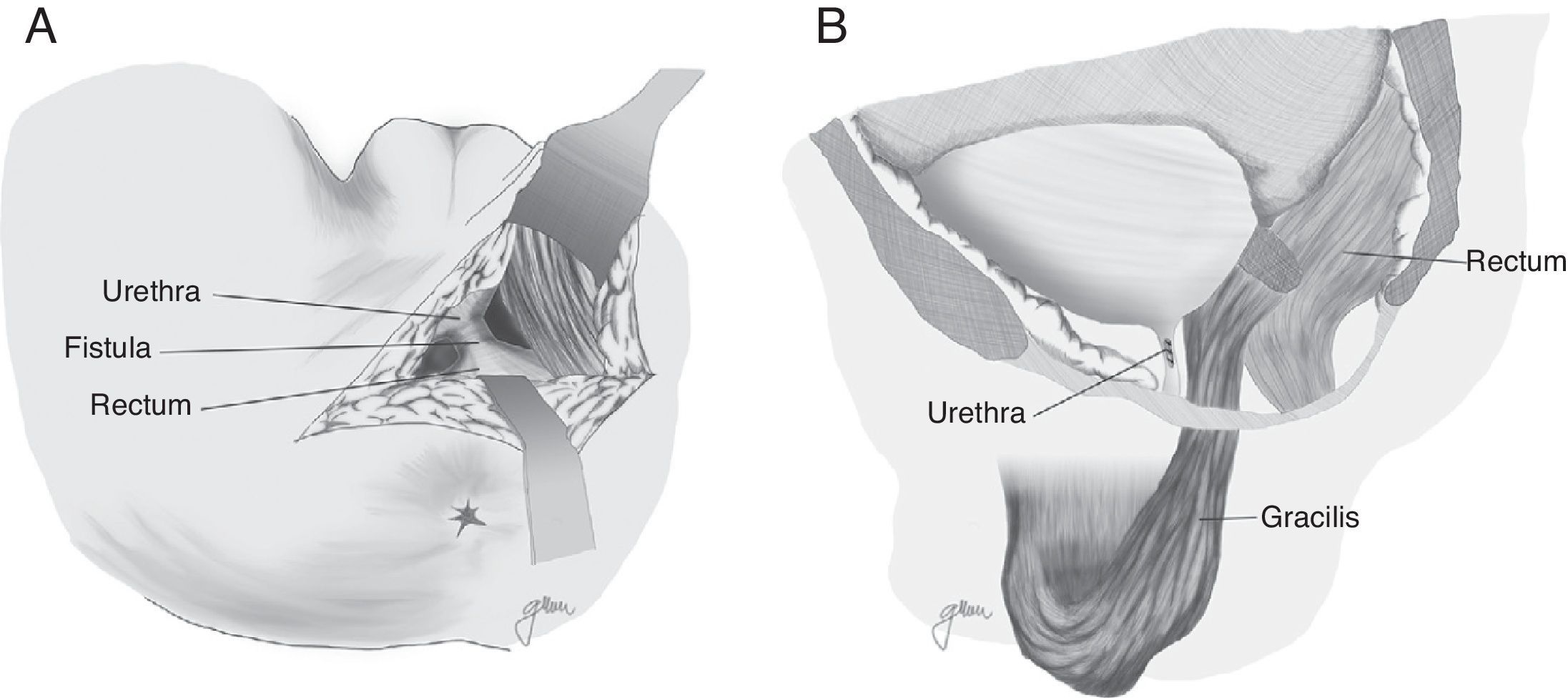
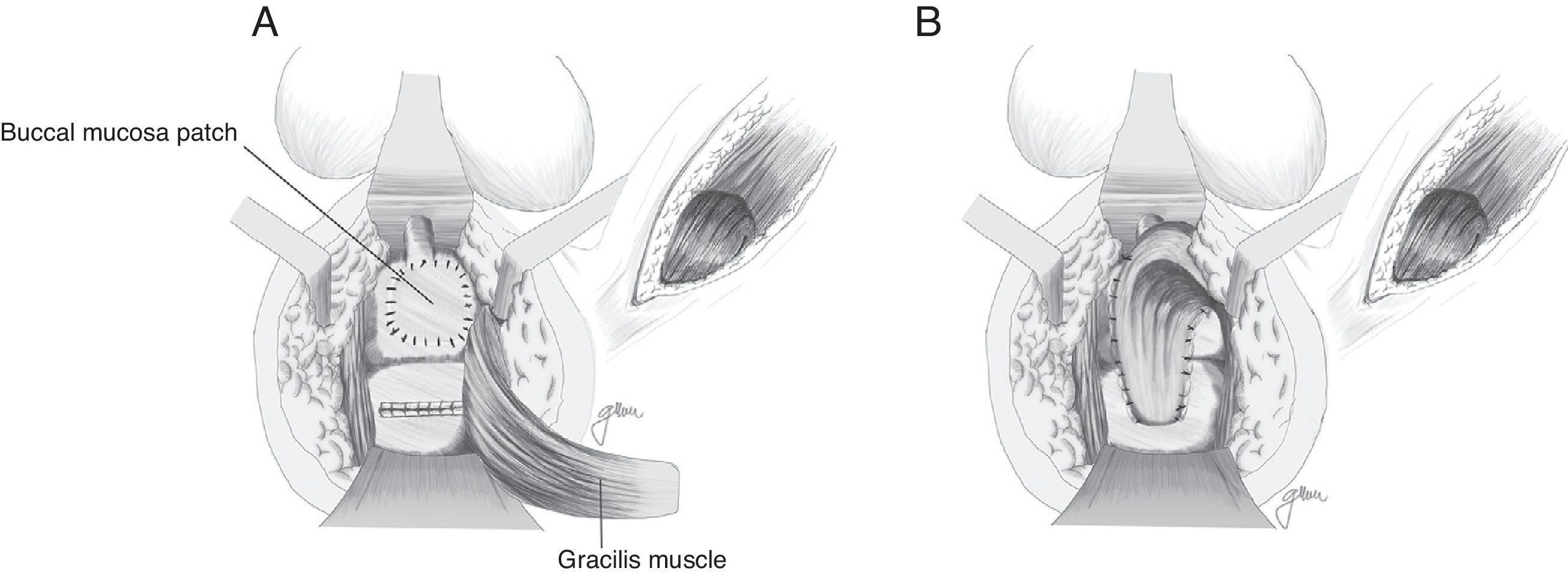
Apart from the surgical actions established by Goodwin,61 the gracilis muscle is released along its entire length respecting the neuromuscular structures in its proximal part, preparation of a subcutaneous tunnel in the perineum, and rotation and implantation between rectum and urethra (Fig. 4). The shifting of the gracilis muscle may trigger a flap necrosis, so the surgical team should be prepared for a bilateral disection82 or a second transposition.50 The endoscopic release of the gracilis muscle has been described, which would avoid some incisions.88
Bladder catheterisation is recommended for six weeks, but there is no unanimity about the need of a faecal diversion.84 However, it is certain that it should always be used in cases of previous surgeries, not sufficiently solid repairs, active infection and large fistulous orifices, avoiding rectal distension and increased pressure during the healing period, minimising the risk of suture dehiscence. Ileostomy has also been proposed as a faecal diversion, which may be performed through a laparoscopic approach.
Its healing rate ranges from 78% to 100%, with one or several interventions.80,82,83,87
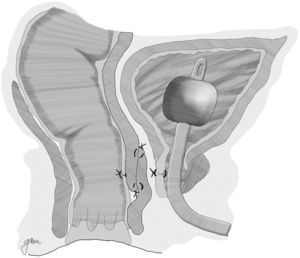
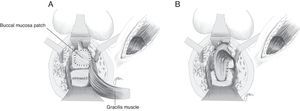
In 1999, Youssef89 proposed for the first time the interposition of a dartos pedicled flap between the rectum and the urethra (Fig. 5). It is an easy-to-obtain, well-vascularised flap that forms a full septum between rectum and urethra, separating the suture lines and filling the space present between them, thus facilitating healing and preventing recurrences. He achieved healing without complications or recurrences in his 12 patients, so he proposes it as a potential ideal solution. Other authors underscore the good outcomes obtained when this technique is used.44,81,90–93
Recently, Iwamoto94 proposed the interposition of the lateral vastus muscle; Krand13 and Abdalla95 suggested the gluteus maximus muscle, and Solomon96 proposed the bilateral interposition of the puborectal muscle. Easiness of dissection, rich vascularisation, anatomical proximity and absence of side effects are highlighted in all cases. Its use has been merely nominal until the present day.
Based on the use of the buccal mucosa to carry out urethroplasties,97–99 Lane27 proposed implanting a buccal mucosal patch for the closure of broad ureteral orifices, ensuring healthy and epithelised tissue; in most cases, it is accompanied by a coloanal pull-through anastomosis.
Technical variations have been conducted following the implantation of buccal mucosa. Thus, Spahn100 uses it alone, without any other tissue interposition; Zinman,33 after its implantation and rectal closure, interposes the gracilis muscle. This step is also adopted by Vanni,17 who highlights its application particularly in patients exposed to previous radiotherapy and with difficulty for the primary closure (Fig. 6). The use of a porcine submucosa patch has also been proposed, although we have only found one described case.101
By rule of thumb, the bladder catheter or the suprapubic diversion is maintained for two to four weeks if the RUF is secondary to prostatectomy, and for six to eight weeks after radiotherapy; as for faecal diversion, the mean period accepted is three months. The closure of the fistula should be confirmed in all cases, as stated above.
The complications described for the perineal approach include sexual impotence due to neuro-vascular lesion, urethral stenosis, urinary incontinence and complications derived from the muscle pedicle.
Further Routes and OptionsIn addition to the approaches described above, which are the most common ones, other approaches have been used more or less anecdotally. Thus, in 1973, Gecelter102 recommended the anterior transanorectal route. It consists of a deep incision in the anterior middle line, from the scrotum to the anus, including the superficial perineal fascia, the central tendinous portion of the perineum, internal sphincter and external sphincter up to the prostatic capsule, the level at which the fistula is removed and the urethral and rectal orifices are sutured. Castillo7 points out that this approach provides good exposure, satisfactory access to well-vascularised tissue and is a relatively easy-to-perform procedure.
Finally, in certain extremely complex cases, a cystoprostatectomy, proctectomy, permanent urinary and faecal diversion,17,27,30,47,57 and even pelvic exenteration, may be needed.19,22
In short, many procedures are recommended but there is no definite guideline that will advise the surgeon on the best problem-solving approach with the least complications. In this sense, Rivera76 makes a classification of the type of fistula based on its size, location and on whether it is secondary to irradiation, together with the therapeutic recommendation considered most appropriate. Nevertheless, this classification is based only on 14 cases and even the author concludes on the need of greater experience and longer follow-up periods to confirm the validity of the 100% healings obtained.
Recently, Hanna4,22 proposed an algorithm based on the fistula aetiology: post-radiotherapy or not.
ConclusionsAlthough rare, rectourethral fistulas are an important disease whose main aetiology is related to prostate cancer, either after surgical treatment or secondary to radiotherapy or other therapeutic options.
Clinically, they are easy to identify by the presence of pneumaturia and/or faecaluria and by the passage of urine through the anus. However, diagnostic tests should be performed for confirmation, notably rectoscopy, cystoscopy, urethroscopy and cystourethrography. Performing contrast enemas, CT, MRI and endorectal ultrasound may provide data in selected cases.
Treatment should be selected on a case-by-case basis. Although certain favourable cases may be solved with conservative treatment, surgical treatment is usually required, with or without urinary and/or faecal diversion.
Options are abundant and the choice should be adapted primarily to the surgical team's experience and to the fistula type, location and aetiology. With that approach, healing rates range from 80% to 100% after one or several interventions.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: Cerdán Santacruz C, Cerdán Miguel J. Fístulas recto-uretrales adquiridas: etiopatogenia, diagnóstico y opciones terapéuticas. Cir Esp. 2015;93:137–146.