Through experience it has been accepted that bile in normal conditions remains sterile. Bactibilia is a common finding in individuals at high risk or with complicated cholecystolithiasis, however few data prevails about the prevalence of bactibilia in patients operated on for uncomplicated laparoscopic cholecystectomy. There is s common usage of preoperative and postoperative antibiotics in the different patients without the existence of any actual bacteriologic and epidemiologic evidence.
Material and methods183 patients with diagnosis of cholecystolithiasis postoperated of laparoscopic cholecystectomy had their bile sent to bacteriology.
ResultsBactibilia was identified in 31.95% of the cultures of mild cholecystitis and in 35.71% for moderate (p<0.0001). A total of 125 negative cultures were obtained (68.3) and 58 positive (31.69%) with a prevalence of enterobacteria group (43.10%) and Enterococcus (27.58).
ConclusionsComparing the groups according to severity there is a significant difference with regard to the presence of bactibilia, in addition to the bacterial groups cultivated. Fluoroquinolones and metronidazole is an option for the treatment of patients with the suspicion of bactibilia. The use of antibiotics is not justified in patients at low risk.
Se ha aceptado a través del tiempo que la bilis en condiciones normales es estéril. La bactobilia es un hallazgo común en individuos de alto riesgo o con cuadros de colecistolitiasis complicados, sin embargo, hay pocos datos con respecto a la prevalencia de bactibilia en pacientes sometidos a colecistectomía por colecistolitiasis no complicada. Es común el uso de agentes antibióticos preoperatorios y postoperatorios en los diferentes pacientes que son sometidos a colecistectomía laparoscópica, sin que exista una base bacteriológica y epidemiológica demostrada sobre el predominio bacteriano determinado, su resistencia y sensibilidad en nuestro medio.
Material y métodosPacientes con diagnóstico de colecistitis litiásica, a quienes se realizó CL con una muestra calculada por proporciones de 183 unidades (IC 95%).
ResultadosSe identificó bactibilia en el 31.95% de los cultivos de colecistitis leve y en el 35.71% de los cultivos de pacientes con colecistitis moderada (p<0.0001). Se recolectaron un total de 125 cultivos negativos (68.3%) y 58 positivos (31.69%) con un claro predominio del grupo de enterobacterias (43.10%) y Enterococcus (27.58%).
ConclusionesComparando los grupos de acuerdo al grado de severidad, hay una diferencia significativa en relación a la presencia de bactibilia, así como en el tipo de agentes aislados. Las fluoroquinolonas asociadas a metronidazol son una opción de tratamiento en pacientes en los que se sospecha bactobilia. Actualmente no está justificado el uso de antibioticoterapia en pacientes de bajo riesgo.
Because it is one of the most common causes of admission to hospital in our environment, the interest in gallbladder and biliary tract disease is constant. The prevalence of gallstone disease is very high; in the United States 20.5 million people have the condition, i.e., 6.3 million men and 14.2 million women. Twenty percent of people over the age of 65 have gallstones, and one million new cases are diagnosed every year. Several studies carried out in our country have demonstrated that the prevalence of this entity is approximately 14.3%.1
Experience tells us that bile under normal conditions is sterile. Similarly, it is well known that bactibilia is a common finding in individuals at high risk or with complicated cholecystolithiasis, including obstruction of the biliary tract, choledocolithiasis, those aged >70 years, acute lithiasic cholecystitis, afunctional gallbladders, and biliary prostheses. However, there is little data regarding the prevalence of bactibilia in patients who have undergone cholecystectomy due to uncomplicated cholecystolithiasis.2 Gutiérrez Banda et al., report in a Mexican case series (72 patients), an incidence of positive cultures of 13.9% (9.7% chronic cholecystitis and 4.2% acute), with predominance of the enteric coliform group.
This nosological entity can present with symptoms suggestive of inflammatory disease with or without superimposed infection. However, it is remarkable that many patients with no history of biliary surgery and no apparent infectious symptoms at time of surgery, present histological changes of the gallbladder walls compatible with infectious processes.
There are different guidelines in the literature on the correct use of antimicrobial prophylaxis in surgery and although most of their recommendations coincide, there remain some inconsistencies. None of the guidelines suggest the use of antibiotic prophylaxis before laparoscopic cholecystectomy (LC) for patients at low risk.3
Preoperative and postoperative antibiotics are commonly used in the different types of patients undergoing LC, with no demonstrated bacteriological or epidemiological basis regarding the specific bacterial predominance, its resistance or sensitivity in our environment.
Microbial resistance is a growing public health problem associated with increased morbidity and mortality and which has repercussions for both patients and institutions. The inappropriate use of antibiotics is the principal cause of microbial resistance. This is why the frequency of biliary infection in patients undergoing LC needs to be established. Expenditure on antibiotics can be reduced by knowing the specific bacterial sensitivity and the type/s of bacteria identified that are most common in bile and their spectrum of sensitivity to antibiotics. This information will be useful to design guidelines for antibiotic prophylaxis for LC.
ObjectivesGeneral objective: to identify the bacterial flora present in bile and gallbladder wall cultures of patients undergoing LC due to simple or complicated cholecystolithiasis in the University Hospital Dr. José Eleuterio González.
Specific objectives: to determine the genus and species of the bacteria most commonly isolated in samples of bile, gallbladder wall and of both from LC patients. To evaluate the role of prophylactic antibiotics in LC. To determine the profile of sensitivity and resistance to antimicrobials of the species isolated.
Material and methodsAn observational, methodological, cross-sectional, descriptive and prospective study including patients diagnosed with mild, moderate or severe lithiasic cholecystitis, classified according to the Tokyo guidelines that underwent LC in the University Hospital Dr. José Eleuterio González, of both sexes and aged between 13 and 85 years. A sample of 183 units was obtained using a formula for calculating proportions with a zα value of 1.96 with a two-tailed significance level of 95% and expected prevalence of 14%.
The clinical findings and data relating to diagnoses of acute cholecystitis were collected using the following variables: gender, age, comorbidities, clinical symptoms, ultrasound findings of the gallbladder and biliary tract, results of laboratory tests and classification of grade of severity.
The samples for culture were taken using a sterile microbiological technique during the operation by laparoscopic puncture or immediately during extraction of the gallbladder and sent for analysis.
Antibiotics were chosen for the antibiogram by selective reporting in accordance with the hospital's infectology department, classified into blocks A, B and C. For the Enterobacteriaceae family, block A: penicillin, ampicillin and gentamicin; block B: amikacin, amoxicillin/clavulanic acid, cefuroxime, cefotaxime, ceftazidime, ciprofloxacin, levofloxacin, meropenem and trimethoprim/sulfamethoxazole. For Acinetobacter spp., block A: ampicillin/sulbactam, ceftazidime and ciprofloxacine and block B: amikacin, cefepime, doxycycline, tigecycline, colistin, fosfomycin and meropenem. For Pseudomonas spp. block A: ceftazidime and gentamicin and block B: amikacin, cefepime, ciprofloxacin, meropenem piperacillin/tazobactam. For Staphylococcus spp./Streptococcus spp. block A: penicillin, erythromycin, clindamycin, cefoxitin and trimethoprim/sulfamethoxazole; block B: doxycycline, vancomycin and levofloxacin and block C: daptomycin, linezolid and rifampicin. For Enterococcus spp., block A: ampicillin and penicillin; block B: vancomycin and gentamicin, and block C: linezolid and tetracycline.
The statistical analysis was performed using measures of frequency, Pearson's chi-square and Fischer's exact test for categorical variables and measures of central tendency or dispersion, and the student's t-test for quantitative variables with IBM SPSS version 20 (SPSS, Inc., Armon, NY). Statistical significance was defined as p<0.05.
ResultsThe study included 183 patients, 151 females (82.5%) and 32 males (17.5%), with a median age of 35 years (IQR 27–45 years) who underwent randomised bacteriological bile and gallbladder analysis. One hundred and twelve gallbladder wall cultures (61.2%) were included and 71 bile cultures (38.8%).
Of the 183 patients studied, 100% presented with initial symptoms of colic-type pain in the right hypochondrium and epigastrium, 74 patients had associated nausea and vomiting (40.4%) and only 3 patients (1.63%) reported episodes of jaundice. The most common comorbidities were: systemic arterial hypertension (13.1%), diabetes mellitus (9.2%), resolved biliary pancreatitis (4.9%), and medium-high risk of choledocolithiasis (7.1%). One hundred and seventeen patients (63%) had no associated comorbidities at the time of the study.
One hundred and sixty-nine patients (92.34%) were classified with a diagnosis of mild cholecystitis and 14 patients (7.65%) with moderate cholecystitis, no patients were recruited with severe cholecystitis. Of the mild cholecystitis cases, 85.7% were female and 85.7% (p=0.999) of the moderate cases were female.
The median gallbladder wall thickness was 0.30cm (IQR 0.2–0.38cm, SD±0.35), and the bile duct measured 0.40cm (IQR 0.2–.38cm, SD±0.24).
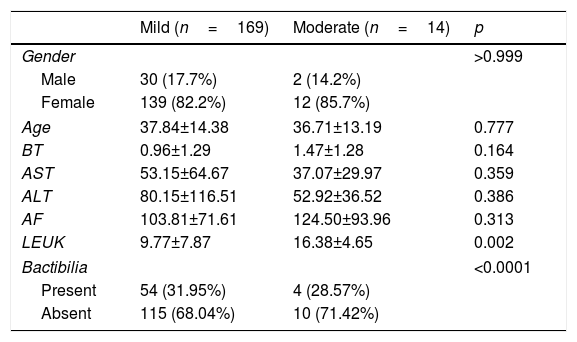
The characteristics of the patients recruited are given in Table 1, which shows a clear predominance of females in both groups according to severity of disease. The mean age was 37.84 SD±14.38 for patients with mild cholecystitis and 36.71 SD±13.19 for those with moderate cholecystitis (p=0.777). Statistically representative difference (p=0.002) in relation to leukocytosis in the moderate cholecystitis group, with a mean of 16.38 SD±4.65. Bactibilia was identified in 31.95% of the mild cholecystitis cultures and in 35.71% of the moderate cholecystitis cultures (p<0.0001).
Patient characteristics according to grade of severity.
| Mild (n=169) | Moderate (n=14) | p | |
|---|---|---|---|
| Gender | >0.999 | ||
| Male | 30 (17.7%) | 2 (14.2%) | |
| Female | 139 (82.2%) | 12 (85.7%) | |
| Age | 37.84±14.38 | 36.71±13.19 | 0.777 |
| BT | 0.96±1.29 | 1.47±1.28 | 0.164 |
| AST | 53.15±64.67 | 37.07±29.97 | 0.359 |
| ALT | 80.15±116.51 | 52.92±36.52 | 0.386 |
| AF | 103.81±71.61 | 124.50±93.96 | 0.313 |
| LEUK | 9.77±7.87 | 16.38±4.65 | 0.002 |
| Bactibilia | <0.0001 | ||
| Present | 54 (31.95%) | 4 (28.57%) | |
| Absent | 115 (68.04%) | 10 (71.42%) | |
ALT: alanine aminotransferase; AST: aspartate alaninotransferase; BT: total bilirubin; AF: alkaline phosphatase; LEUK: leukocytes.
A total of 125 (68.3%) negative cultures were collected (68 gall bladder wall and 57 bile cultures) and 58 positive (31.69%) (44 gallbladder wall and 14 bile). Of the total positive cultures (58 cultures), 67.24% reported a single microbiological agent, while 32.75% showed polymicrobial activity. Of the 151 female patients, 31.7% had positive cultures. Of the 32 male patients, 31.25% had positive cultures (p=0.999).
Of the 183 study subjects, 144 (78%) had a gallbladder wall measuring less than 0.4cm, from which 94 (65.3%) had a negative culture and 50 (34.7%) a positive culture. Thirty-nine (21.31%) had a gallbladder wall measuring more than 0.4cm, from whom 31 (16.93%) cultures were negative and 8 (4.3%) were positive (p=0.120). Of the 58 positive cultures, 75.9% were gallbladder wall, while 24.1% were bile (p=0.006).
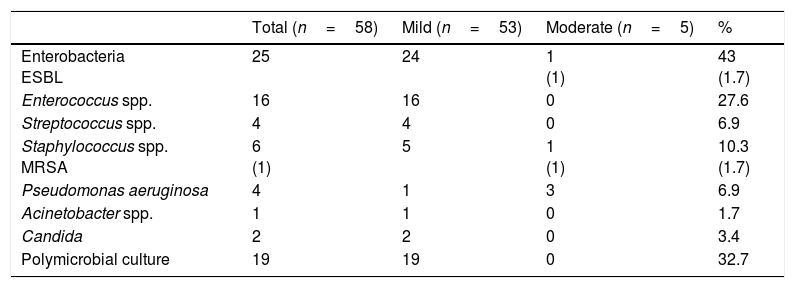
The bacteriological analysis findings are shown in Table 2. The agents isolated in the positive cultures were entrobacteria (25 cultures, 43%) (Enterobacter cloacae (E. cloacae), Enterobacter aerogenes, Enterobacter faecium, Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, Citrobacter diversus, Morganela morgani), the most prevalent being E. cloacae with 11 cultures (19%) and 2 cultures (12%) of Klebsiella had isolated extended-spectrum beta lactamase, Enterococcus spp. (16 cultures, 27.6%) (Enterococcus faecalis), Streptococcus spp. (4 cultures, 6.9%), Staphylococcus coagulase negative (5 cultures, 8.6%) Staphylococcus aureus MRSA (1 culture, 1.7%), Pseudomonas spp. (4 cultures, 6.9%) (Pseudomonas aeruginosa [P. aeruginosa]), Acinetobacter spp. (1 culture, 1.7%) (Acinetobacter baumanii), Candida spp. (2 cultures, 3.4%). Nineteen cultures with poly microbial activity showed diverse bacterial associations: enterobacteria with Enterococcus (12 cultures, 63%), enterobacteria of different genera (2 cultures, 10.52%), Pseudomonas with Staphylococcus (2 cultures, 10.52%), Enterococcus with Staphylococcus (2 cultures, 10.52%). Acinetobacter with Staphylococcus (one culture, 5.2%).
Bacteriological profile according to grade of severity.
| Total (n=58) | Mild (n=53) | Moderate (n=5) | % | |
|---|---|---|---|---|
| Enterobacteria ESBL | 25 | 24 | 1 (1) | 43 (1.7) |
| Enterococcus spp. | 16 | 16 | 0 | 27.6 |
| Streptococcus spp. | 4 | 4 | 0 | 6.9 |
| Staphylococcus spp. MRSA | 6 (1) | 5 | 1 (1) | 10.3 (1.7) |
| Pseudomonas aeruginosa | 4 | 1 | 3 | 6.9 |
| Acinetobacter spp. | 1 | 1 | 0 | 1.7 |
| Candida | 2 | 2 | 0 | 3.4 |
| Polymicrobial culture | 19 | 19 | 0 | 32.7 |
ESBL: extended-spectrum beta lactamase; MRSA: methicillin-resistant Staphylococcus aureus.
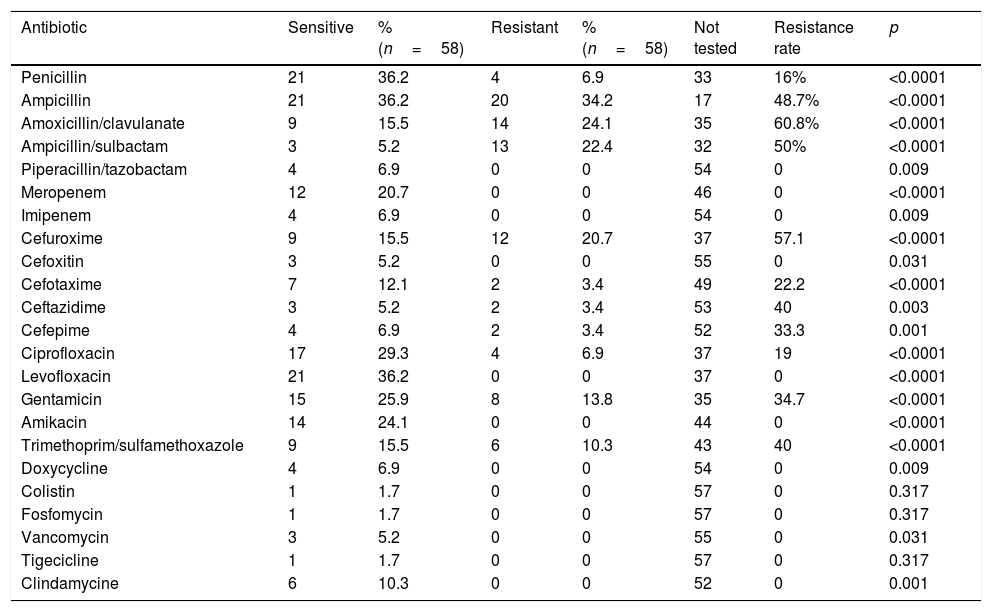
Of the 25 cultures that were positive for enterobacteria, penicillin was tested in 8%, which were resistant. Of the 16 cultures of Enterococcus, 87.5% were sensitive to penicillin. One hundred percent of the Streptococcus and 50% of the Staphylococcus were also sensitive to penicillin. An antibiogram with selective reporting was obtained from the 58 positive cultures, which showed a resistance profile according to Table 3. In the beta-lactam group, the combination of piperacillin with tazobactam showed the lowest resistance rate, followed by penicillin at 16%, tested in up to 43.1% of the positive cultures. Ampicillin, amoxicillin/clavulanate and ampicillin/sulbactam, presented the highest resistance rates, 48.7%, 60.8% and 50% respectively. The carbapenems, represented by imipenem and meropenem on the local antibiogram, showed no resistance in the positive cultures where they were tested (27.6%). Of the cephalosporin family, the second generation showed a resistance rate of 57.1%; for the third generation, represented by cephotaxime and ceftazidime, the incidence rates were 22.2% and 40% respectively, as a group they were tested in up to 20% of the positive cultures. Cefepime was tested in 10.3% of the positive cultures and a resistance rate of up to 33.3% was found. The quinolones (ciprofoxacine and levofloxacine) were tested in 72.4% of the positive cultures, with a resistance rate of 19%. The aminoglycosides (gentamicin and amikacin) were tested as a group in 63.8% of the cultures, their resistance rates were 34.7% and 0% respectively. Trimethoprim/sulfamethoxazole showed a resistance of up to 40% of the cultures where tested. The remaining antibiotics (doxycycline, colistin, fosfomycin, erythromycin, vancomycin, tigecycline, clindamycin) of the local antibiogram showed no resistance, as a group they were tested in 30% of the positive cultures.
Resistance rate per antibiotic.
| Antibiotic | Sensitive | % (n=58) | Resistant | % (n=58) | Not tested | Resistance rate | p |
|---|---|---|---|---|---|---|---|
| Penicillin | 21 | 36.2 | 4 | 6.9 | 33 | 16% | <0.0001 |
| Ampicillin | 21 | 36.2 | 20 | 34.2 | 17 | 48.7% | <0.0001 |
| Amoxicillin/clavulanate | 9 | 15.5 | 14 | 24.1 | 35 | 60.8% | <0.0001 |
| Ampicillin/sulbactam | 3 | 5.2 | 13 | 22.4 | 32 | 50% | <0.0001 |
| Piperacillin/tazobactam | 4 | 6.9 | 0 | 0 | 54 | 0 | 0.009 |
| Meropenem | 12 | 20.7 | 0 | 0 | 46 | 0 | <0.0001 |
| Imipenem | 4 | 6.9 | 0 | 0 | 54 | 0 | 0.009 |
| Cefuroxime | 9 | 15.5 | 12 | 20.7 | 37 | 57.1 | <0.0001 |
| Cefoxitin | 3 | 5.2 | 0 | 0 | 55 | 0 | 0.031 |
| Cefotaxime | 7 | 12.1 | 2 | 3.4 | 49 | 22.2 | <0.0001 |
| Ceftazidime | 3 | 5.2 | 2 | 3.4 | 53 | 40 | 0.003 |
| Cefepime | 4 | 6.9 | 2 | 3.4 | 52 | 33.3 | 0.001 |
| Ciprofloxacin | 17 | 29.3 | 4 | 6.9 | 37 | 19 | <0.0001 |
| Levofloxacin | 21 | 36.2 | 0 | 0 | 37 | 0 | <0.0001 |
| Gentamicin | 15 | 25.9 | 8 | 13.8 | 35 | 34.7 | <0.0001 |
| Amikacin | 14 | 24.1 | 0 | 0 | 44 | 0 | <0.0001 |
| Trimethoprim/sulfamethoxazole | 9 | 15.5 | 6 | 10.3 | 43 | 40 | <0.0001 |
| Doxycycline | 4 | 6.9 | 0 | 0 | 54 | 0 | 0.009 |
| Colistin | 1 | 1.7 | 0 | 0 | 57 | 0 | 0.317 |
| Fosfomycin | 1 | 1.7 | 0 | 0 | 57 | 0 | 0.317 |
| Vancomycin | 3 | 5.2 | 0 | 0 | 55 | 0 | 0.031 |
| Tigecicline | 1 | 1.7 | 0 | 0 | 57 | 0 | 0.317 |
| Clindamycine | 6 | 10.3 | 0 | 0 | 52 | 0 | 0.001 |
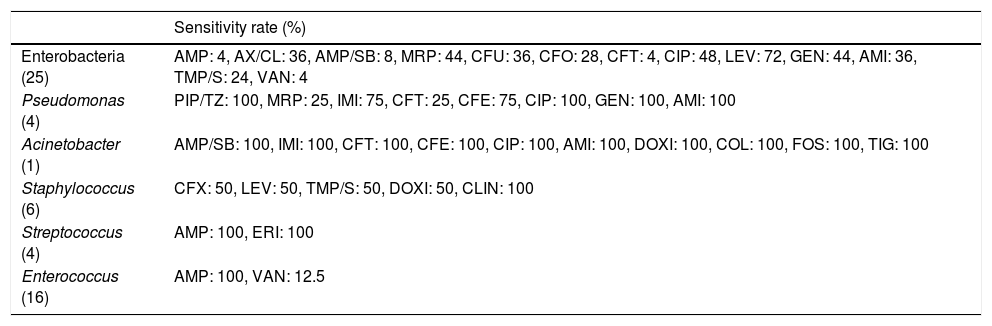
Table 4 shows the sensitivity rate per bacteria group. The group of enterobacteria showed 100% sensitivity to levofloxacin, aminoglycosides, carbapenems and vancomycin. The agents tested in the Pseudomonas culture showed excellent sensitivity. Ampicillin showed full sensitivity for the gram positives of the Streptococcus and Enterococcus groups when it was used. The culture reported with Acinetobacter showed no resistance to any of the antibiotics tested.
Sensitivity rate per genus.
| Sensitivity rate (%) | |
|---|---|
| Enterobacteria (25) | AMP: 4, AX/CL: 36, AMP/SB: 8, MRP: 44, CFU: 36, CFO: 28, CFT: 4, CIP: 48, LEV: 72, GEN: 44, AMI: 36, TMP/S: 24, VAN: 4 |
| Pseudomonas (4) | PIP/TZ: 100, MRP: 25, IMI: 75, CFT: 25, CFE: 75, CIP: 100, GEN: 100, AMI: 100 |
| Acinetobacter (1) | AMP/SB: 100, IMI: 100, CFT: 100, CFE: 100, CIP: 100, AMI: 100, DOXI: 100, COL: 100, FOS: 100, TIG: 100 |
| Staphylococcus (6) | CFX: 50, LEV: 50, TMP/S: 50, DOXI: 50, CLIN: 100 |
| Streptococcus (4) | AMP: 100, ERI: 100 |
| Enterococcus (16) | AMP: 100, VAN: 12.5 |
AMI: amikacin; AMP: ampicillin; AMP/SL: ampicillin sulbactam; AX/CL: amoxicillin/clavulanate; CFE: cefepime; CFO: cefoxitin; CFT: ceftazidime; CFU: cefuroxime; CFX: cefotaxime; CIP: ciprofloxacin; CLIN: clindamicine; COL: colistin; DOXI: doxycycline; ERI: erythromycin; FOS: fosfomycin; GEN: gentamicin; IMI: imipenem; LEV: levofloxacin; MRP: meropenem; PIP/TZ: piperacillin/tazobactam; TIG: tigecicline; TMP/S: trimethoprim/sulfamethoxazole; VAN: vancomycin.
Although the Tokyo guidelines for classifying acute cholecystitis recommend antimicrobial therapy according to grade of severity, they do not include a detailed description of the bacteriological profile of bile in relation to grade of severity.4 This is why we consider it extremely useful for treating physicians to know and understand the types of bacteria, resistance profiles and frequency of polymicrobial cultures according to grade of severity, to thus be able to select the most appropriate antimicrobial therapy.
It is well known that bactibilia is a common finding in patients at high risk, with biliary tract obstruction, biliary tract surgery, of advanced age and with cholangitis.5–7 The Tokyo guidelines recommend that bile cultures should be taken whenever there is the opportunity, especially in severe cases.8 In some series, it is reported that advanced age,9,10 the male sex and elevated total bilirrubin11 can also be predictors of positive cultures. A sample of 183 patients was recruited in this sample, of which 92% were classified as mild cholecystitis, and the remainder as moderate. A statistically significance difference was demonstrated in relation to the grade of severity and presence of positive cultures (31.9% vs 35.71%; p<0.0001); however, in this study, the differences between groups in terms of total bilirubin level, age and gender as factors associated with the presence of bactibilia, did not prove statistically significant. All of the patients that presented jaundice as a symptom reported positive cultures without previously having undergone invasive procedures. It has been reported in the literature that serum C reactive protein is an important marker of severity and even mortality,12,13 included in a definitive diagnosis of acute cholecystitis,14 but not in the classification of grade of severity in the Tokyo guidelines. This is not routinely requested as part of diagnosis in our environment. However, we consider that, since it is an important predictor of bactibilia and prognosis, it should be included as part of the integral approach to patients with cholecystis.
The Tokyo guidelines include the characteristics of local inflammation as mild or moderate inflammatory changes to differentiate between grades of severity, which does not enable a clear classification.15 In our study we included the ultrasound findings of the gallbladder wall and linking them with the laboratory test results we found no significant difference in relation to severity and findings of local inflammation or the presence of polymicrobial cultures, only leukocytosis was shown to be a statistically significant predictor of bactibilia (p=0.002). However, a difference was found in relation to the type of agent isolated, in moderate cholecystitis the most prevalent was P. aeruginosa, and the rest were an extended-spectrum beta lactamase Klebsiella and a Staphylococcus MRSA. Thirty-two percent of the positive cultures had polymicrobial activity, all of which belonged to the mild cholecystitis patient group. In addition, 34.7% of the patients with mild cholecystitis and no gallbladder wall thickening presented positive cultures, as against 4.3% positive cultures in patients with a gallbladder wall greater than 4 millimetres. There was no statistically different difference (p=0.120) between groups according to grade of severity or local alterations of the gallbladder wall to indicate a positive culture.
The prevalence of positive cultures in the literature is ambiguous, between 14% and 72%. In our study, the prevalence of cultures was 31.69% – double that expected; with a clear predominance of the enterobacteria group (43.10%) and Enterococcus spp. (27.58%). These findings are compatible with the literature16–19 and confirm the principal hypothesis of this study. The Enterococcus spp. group, at up to 36.7%, and enterobacteria, at 29.5%, predominate in some series. The Pseudomonas spp. (6.89%) and Acinetobacter spp. (1.72%), group presented a relatively high incidence compared to that commonly reported,20,21 and in line with the study by Asai et al. Seventy-five percent of cultures positive for Pseudomonas spp. were taken from patients with a diagnosis of moderate cholecystitis, representing 6.9% of the total positive cultures. In light of the above, empirical antipseudomas therapy is not justified in patients at low risk.
The information provided by the antibiogram has great clinical and epidemiological impact. It is a very important tool in organisational support strategies for the best use of antibiotics. The selection of the most appropriate antimicrobials to report is a decision that has to be taken by every clinical laboratory, after consulting the specialists most involved in the treatment of infectious diseases. Their clinical interest must prevail. Reported antibiotics must be of demonstrated clinical efficacy. The prevalence of resistance in the hospital and the out-of-hospital area, cost, approved clinical indications for use and the latest consensus recommendations on first choice and alternatives must also be evaluated. Selective reporting means that some antibiotics are only reported in certain circumstances (selectively), which each laboratory should protocolise to coincide with the conditions of their environment. According to the results obtained, amoxycillin/clavulanate has the greatest resistance rate (60.8%), followed by cefuroxime (57.1%), ampicillin/sulbactam (50%) and ampicillin without beta-lactamase inhibitor (48.7%). Likewise, the third generation cephalosporins have resistance rates of up to 40%. Penicillin was tested in 32.4% of the positive cultures showing a resistance rate of 16%; thus constituting a management option. The fluoroquinolones, marked in the selective reporting by ciprofloxacin and levofloxacin, with resistance rates of 19% and 0% respectively, appear to be an excellent option for initial management of patients at risk of bactibilia. Amikacin was tested in 24% of the positive cultures and showed no resistance. By contrast, gentamicin was tested in 25% of the positive cultures showing a resistance rate of up to 37.7%. The current most commonly used antibiotic therapy regimens are piperacillin/tazobactam, ceftriaxone plus metronidazole and levofloxacin with metronidazole.22 On defining the antibiotic spectrum, according to the bacteria group found in the cultures of our study, we found that the enterobacteria group, being the most frequently isolated agents, presented a high rate of resistance to 3rd generation cephalosporins; this is why we suggested restructuring first line treatment. Fluoroquinolones combined with metronidazole could be an excellent option for patients suspected to have bactibilia (leukocytosis, jaundice, moderate severity grade). However, antibiotic therapy is not appropriate for patients at low risk.
ConclusionIt is extremely useful for treating physicians to know and understand the type of bacteria, resistance profiles and frequency of polymicrobial cultures according to grade of severity, and to be able to select the most appropriate antimicrobial therapy. We found a higher than expected prevalence of positive cultures with a clear predominance of enterobacteria in the cultures analysed. Comparing the groups according to severity grade, there was a significant difference in relation to the presence of bactibilia, and in the type of agents isolated. There needs to be consensus as to the criteria for classifying severity. Serum C reactive protein, being an important predictor of bactibilia and prognosis, should be included as part of the integral approach to patients with cholecystitis.
The resistance and sensitivity profiles of the most frequently isolated bacteria must be adjusted in the guidelines for managing patients at risk of bactibilia and according to grade of severity. Fluoroquinolones combined with metronidazole are a treatment option for patients suspected to have bactibilia (leukocytosis, jaundice, moderate severity grade), but clinical judgement must always prevail. Currently, the use of antibiotic therapy is not appropriate for low risk patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Cueto-Ramos R, Hernández-Guedea M, Pérez-Rodríguez E, Reyna-Sepúlveda F, Muñoz-Maldonado G. Identificación de flora bacteriana en cultivos de bilis y pared de vesícula biliar de pacientes sometidos a colecistectomía laparoscópica en el Hospital Universitario «Dr. José Eleuterio González». Cir Cir. 2017;85:515–521.