Clear cell carcinoma originating in the abdominal wall is a rare event. It is generally associated with endometrial tissue implants left behind after a caesarean section or other gynaecological operations. Its pathophysiology is complex and controversial.
Clinical caseThe case is presented of a 45 year-old female with history of three caesarean sections, who was seen due to having a tumour mass of 6 months onset in the anterior abdominal wall. Imaging studies confirmed its location, and due to measuring 9cm×7cm it was suspected to be an urachal tumour. A resection with wide margins was performed. The histopathology report was of a clear cell adenocarcinoma originated in ectopic endometrial tissue, with negative margins.
ConclusionThis is a very rare case, with few cases reported in the literature. This diagnosis should be included in tumours of the abdominal wall.
El carcinoma de células claras originado en la pared abdominal es una entidad rara y generalmente está asociado a implantes iatrogénicos de tejido endometrial, por una incisión quirúrgica durante la operación cesárea u otros procedimientos ginecológicos, su fisiopatología es compleja y controversial.
Caso clínicoPaciente del sexo femenino de 45 años con antecedente de 3 cesáreas, que acude con masa tumoral en la pared anterior del abdomen de 6 meses de evolución. Los estudios de imagen confirmaron su localización, con dimensiones de 9 por 7cm que hicieron sospechar en un tumour del uraco; con ese diagnóstico se decidió llevar a resección amplia. El reporte final de patología fue de adenocarcinoma de células claras originado en foco endometriósico, con márgenes quirúrgicos negativos.
ConclusiónEsta entidad es excepcionalmente rara, hay pocos casos publicados, su conocimiento es esencial para incluirla en el diagnóstico diferencial de tumores de pared abdominal.
The presence and growth of endometrial tissues outside the uterus is called endometriosis and if it forms a cystic mass it is known endometrioma1; its prevalence is variable,2 during reproductive age, ranging from 10% to 30–50% when it is associated with pelvic pain and infertility. Average age of presentation is 30 (range between 25 and 35). The pelvic cavity is the main site (75% in the ovary) but any part of the body may be affected1 (lung, eye, brain, soft tissues). The abdominal wall is the most frequent extra-pelvic site.3,4 Its physiopathology is complex and controversial, and several theories exist to explain its origin, including1: retrograde menstruation, epithelium cell metaplasia, vascular and lymphatic dissemination, activation of embryonic remains and iatrogenic implants during an open surgery or laparoscopic procedure of gynaecological surgery such as caesarean section (57% cases) and hysterectomies.
Incidence is between 0.03% and 3.5%.3,5,6 Malignant transformation is extremely rare,7,8 but has been described since 1925.9
It appears10–13 as a subcutaneous progressive growth tumour mass associated with cyclical pain during menstruation.
Diagnostic approach includes: ultrasound of the abdominal wall, biopsy for reporting malignancy, which is complemented by computed axial tomography for assessing spread and resectability.
Differential diagnostics include benign causes, such as incisional hernia, haematoma of the abdominal rectus muscle lining, and primary or metastatic neoplasias of the abdominal wall.
Treatment is extensive resection with reconstruction of the abdominal wall which generally leads to good oncological outcome. However, experience is limited due to the rare occurrence of this entity.
ObjectiveWe present a clinical case which mimicked an urachal tumour.
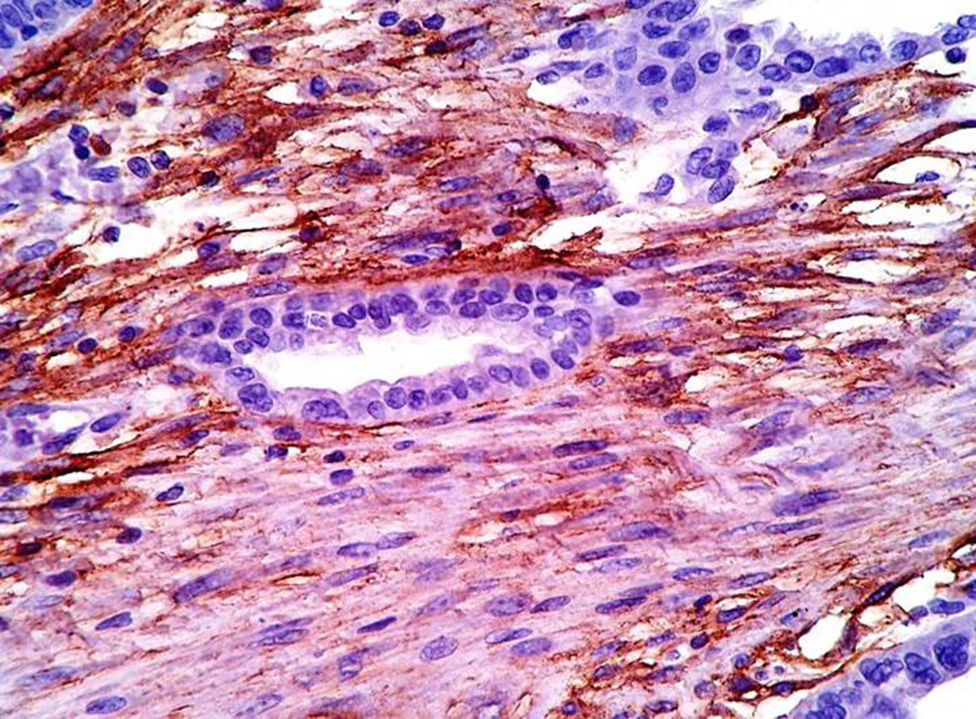
Clinical caseA female patient aged 45, with a history of dysmenorrhoea and 3 previous caesarean sections who presented with a tumoral mass in the anterior abdominal region at infra-umbilical level on the left side, of 6 months onset (Fig. 1). Axial computed tomography confirmed its location and dimension of 9cm×7cm, with infiltration of abdomen rectus muscles and fatty tissue, and also compression of the bladder (Fig. 2). She was diagnosed with a urachal tumour and an exploratory laparotomy was performed with resection en bloc of the abdominal wall with a 2cm margin and reconstruction of the abdominal wall (Fig. 3). Histopathological diagnosis was: carcinoma of primary clear cells of the abdominal wall caused by endometriosic deposit with negative surgical margins (Figs. 4–6).
The patient is currently disease free 16 months after treatment.
DiscussionEndometriosis is a chronic benign gynaecological type condition, defined by the ectopic localisation of glands and endometrial stroma, frequently during reproductive age. The natural course of the disease is unpredictable, infiltrating and invasive and there is a high probability of general spread which is generally resolved during menopause.7 Implants in the abdominal wall during a caesarean or a hysterotomy have an incidence below 1%,3 although it is estimated that at least 1% of endometriosic implants present malignant transformation7; the atypical progression mechanism to carcinoma is unknown,14 as it appears to be related to the hormonal exposure to chronic inflammation which involves genetic damage. There are 3 theories to try to explain carcinogenesis and the first suggests that the endometriosic epithelium serves as a precursor of its cancer, in such a way that the normal ovarian surface epithelium leads to ovarian tumours, which may be epithelium, stromal or borderline. The second refers to the similarity in the cancer process of the endometrial neoplasy, which is governed by constant oestrogens and leads to an endrometrial tumour. The third which is considered the most precise, suggests that the carcinogenesis is due to the influence of a micro ambiance on the inside of the endometriosis which is exposed to oxidative stress and chronic inflammation. This leads to slow-growing atrophied, phenotype tumours, such as clear cell carcinoma.15
The most common histologic type from extraovarian endometriosisis clear cell adenocarcinoma (62%).16 Only 15 cases of this have been reported since 1980.17,18
Suspected diagnosis of an endometrioma or its malignancy is based on the presence of a subcutaneous tumour of the abdominal wall, with progressive growth which is adjacent to a surgical scar. It is also associated with cyclical pain during menstruation (dysmenorrhoea),19,20 and sustained in the histopathological criteria of Sampson–Scott, which are: the coexistence of the benign and malignant endometrial tissue in the tumour, histologic in appearance and consistent with endometrial origin, showing the dysplasic phase in the endometrial tumoral tissue and excluding other primary tumour sites. The atypical dysplasic or endometriosis phase in accordance with Liu et al. in 1988,18 which is characterised by endometrial glands with architectural and cytological atypia (hyperchromatic nuclei with moderate to marked peomophism, with increased nucleus-cytoplasm relations, cellular overposition and stratification).21 In our case the criteria of Sampson–Scott were fulfilled, but they are not always able to be valued due to the fact that an extensive sample is required on multiple levels and extensive malignant transformation prevents the observation of benign endometriosic foci.17,18,22
The demonstration of endometrial stromal cells is vital. On occasions, it may mimic stromal reaction or chronic inflammatory infiltration. An important immunomarker is the CD10 expression of the stromal cells, since this is sensitive and specific of endometriosis in extragonad sites.23,24 Nuclear expression of P53 is also reported because it is raised in the malignant transformation region and absent in benign endometriosis and has an 87% sensitivity and 92% specificity in atypical endometriosis and cancer associated with endometriosis.23 In our case both CD10 and P53 were positive.
The differential diagnostics include: metastasis of kidney cells carcinoma to abdominal wall, but it is less probable if pleomorfism and studded cells are identified. The mesothelioma may present with a cystic or solid pattern with marked pleomorfism, although the studded cells and clear cytoplasm are rare,25 and immunohistochemistry markers are useful for distinguishing them. The mesothelioma tested positive for CQ 5/6, calretinin, D240 whilst the clear cell carcinoma was negative. Another clinically differential diagnosis in this case was the urachal carcinoma, owing to its proximity with the bladder dome. However, it was not histologically compatible nor immunophenotypically compatible in any of its subtypes, among the most frequent of which were the mucinous, enteric and signet ring cells, since in our case they tested negative for CDX2 and CQ 20. A third diagnosis which was considered was cystic peritoneal lymphangioma, due to the patient's previous caesarean sections and the cystic growth pattern.
Histologically, it is characterised for presenting lymphoid, smooth muscle nodules in its walls and the lining layer of cells express markers including: D240, CD 31 and CD 34; however, our case was negative for these markers and morphologically it presented areas of clear cells and studded cells, which are rare in cystic lymphangioma.
Despite the low evidence in the reference consulted, due to the rarity of this entity, the recommendable treatment is resection with wide margins,26 which produces a good outcome and survival rate of over 80% at 5 years.
ConclusionMalignant transformation of endometriosic foci deposited during gynaecological surgery has been well reported. Clinical suspicion should be alerted by any large, painful nodule of rapid growth in caesarean scars; pathology plays an essential role in its diagnosis.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Sosa-Durán EE, Aboharp-Hasan Z, Mendoza-Morales RC, García-Rodríguez FM, Jiménez-Villanueva X, Peñavera-Hernández JR. Adenocarcinoma de células claras originado de endometriosis en pared abdominal. Cirugía y Cirujanos. 2016;84:245–249.