Chitosan-coated Fe3O4 nanoparticles were prepared by coprecipitation followed by reaction with chitosan and different volumes of glutaraldehyde. Coating was modified by varying the volume of glutaraldehyde and reaction time. XRD pattern shows maxima compatible Fe3O4 structure. FTIR spectroscopía confirms the presence of chitosan, more evident in samples with higher chitosan content, as denoted by TGA. TEM images of samples with low glutaraldehyde content reveal particles coated with a homogeneous chitosan shell. Meanwhile, those prepared with high glutaraldehyde volume show nanoparticles dispersed in an organic matrix. Samples present almost superparamagnetic behavior with magnetization saturation values that are reduced as the content of organic matter increases. Regarding the stability of these samples in solution, the presence of a homogeneous coating improves the initial suspension, although it does not prevent its subsequent aggregation over time. However, this aggregation process is reduced for sample synthesized with 1mL of glutaraldehyde after 6h of reaction.
Se han preparado nanopartículas de Fe3O4 recubiertas con quitosano mediante coprecipitación seguida de reacción con quitosano y diferentes volúmenes de glutaraldehído. El recubrimiento fue modificado a través de la variación del volumen de glutaraldehído y el tiempo de reacción. Los patrones de DRX muestran máximos compatibles con la estructura del Fe3O4. La espectroscopía IRTF confirma la presencia de quitosano, que se hace más evidente para muestras con altos contenidos de quitosano, como se observa mediante ATG. Las imágenes de MET con volumenes pequeños de glutaraldehído revelan partículas recubiertas homogéneamente con una coraza de quitosano. Por su parte, aquellas preparadas con grandes cantidades exhiben nanopartículas dispersas en la matriz orgánica. Las muestras presentan un comportamiento prácticamente superparamagnéticos con valores de magnetización a la saturación que se reducen cuanto mayor es le contenido de materia orgánica. Atendiendo a la estabilidad de las muestras en disolución, la presencia de un recubrimiento homogéneo mejora la suspensión inicial del material, pero no previene la agregación con el tiempo. Sin embargo, este proceso de agregación se ve reducido para la muestra sintetizada con 1ml de glutaraldehído con un tiempo de reacción de 6 horas.
Over the years, the preparation of magnetic nanoparticles of two of the most common forms of iron oxides, magnetite (Fe3O4) and maghemite (γ-Fe2O3) has been the subject of great interest by scientists on the basis to its numerous technological applications. Among them, we can mention magnetic resonance imaging [1,2], hyperthermia [3,4], targeted drug delivery [5,6], biosensing [7,8] and adsorption of heavy metal ions [9–11] or organic pollutants [12].
Nanoparticles of Fe3O4 or γ-Fe2O3 oxides smaller than 15nm offer unique advantages over other materials. Among them, they can be synthesized by low-cost methods, are physically and chemically stable, biocompatible, non-toxic [13], present strong magnetic saturation and superparamagnetism at room temperature, which means that these nanoparticles can be temporarily magnetized in the presence of an external magnetic field with disappearance of the magnetization upon field removal [14,15]. Attending to its higher magnetization saturation value, based on its usefulness as a magnetic material capable of responding to an external magnetic field [16], Fe3O4 phase has been selected as the base of the research in this paper.
A variety of preparation methods based on aqueous and non-aqueous routes have been reported in the literature for the synthesis of Fe3O4 or γ-Fe2O3 nanoparticles as chemical coprecipitation [17,18], thermal decomposition [19,20], solvothermal synthesis [21,22] or sol–gel [23]. Depending on the method of synthesis used, the morphology of the powder and the size of the constituent particles will be affected and, ultimately, this will also influence the magnetic behavior of the prepared sample. At this point, it should be noted that coprecipitation method has advantages such as experimental simplicity, mild reaction conditions, relatively short reaction time and the use of water as a solvent [24].
On the other hand, it should be considered that once the nanoparticles of Fe3O4 or γ-Fe2O3 are obtained, these nanoparticles have hydrophobic surfaces with a large surface area to volume ratio in the absence of any surface coating material [25–27]. These particles agglomerate to form large clusters due to hydrophobic interactions between the particles, resulting in increased relative particle size of the cluster. To prevent the aggregation, a stabilizer of the nanoparticles, as a surfactant or a polymer, is usually added during the preparation. Dispersing agents such as oleic acid or oleylamine can perform a double function: control the growth of particles and prevent their aggregation [25–27]. In addition, a high-density coating is often implemented as an alternative strategy to stabilize iron oxide nanoparticles.
Magnetic nanoparticles can be coated using different inorganic and polymeric shells [24,28,29]. When preparing a compound suitable for application in biomedicine or removal of heavy metal ions, various types of materials such as silica [28,30], biomolecules [31,32], Au or Ag pure [33,34] and polymer [35–37] have been investigated as surface modifiers of iron oxide nanoparticles. Among them, natural biopolymers are favored due to their high biocompatibility and biodegradability [38,39]. Chitosan is a polysaccharide obtained by deacetylation of chitin, a polymer that is part of the external skeletons of many crustaceans and, therefore, one of the most abundant in nature [40]. Its cationic character is the differential point of other natural polymers, which in most cases are neutral or anionic macromolecules. The presence of free amino groups gives it the possibility of a subsequent functionalization. In this sense, chitosan can be incorporated either as a nanoparticle shell in a core–shell structure or act as a polymer matrix into which the magnetic material can be embedded. Focusing on the first process, which is the object of the research described here, it should be noted that multiple parameters could affect the stability of the chitosan cover around the magnetic nanoparticle. For this reason, the improvement of core–chitosan shell structures with chitosan has been reported in the literature using various chemical cross-linking processes [41,42]. Aldehydes such as glyoxal or glutaraldehyde have been commonly used to cross-link the chitosan chains by their reaction with the amino groups to form imines. These bonds modify the final properties of the materials prepared, determining their mechanical strength or their behavior in aqueous media. In fact, once the iron oxide coated nanoparticles are obtained, their environmental and public health impact will depend largely on how stable these particles suspended in the natural environment.
Based on the above considerations, results describing the preparation of chitosan-coated Fe3O4 samples are presented here. Some similar studies have been carried out, however, from our knowledge, in none of them is a study so broad and systematic that it also addresses the stability of this type of samples in aqueous solutions. A two-stage process, chitosan-coated Fe3O4 nanoparticles were prepared by varying the volume of glutaraldehyde and by increasing chitosan reaction time for the same glutaraldehyde content. The effects of the different chitosan thicknesses on the structural features, powder morphology, magnetic properties and stability of samples in solutions were evaluated.
ExperimentalPreparation of Fe3O4 sample by coprecipitationFor the preparation of samples of the same composition, precursor agents (FeCl3·6H2O and FeCl2·4H2O) in a 2:1 ratio were dissolved in 150mL of deionized water in argon atmosphere. The resulting solution was taken into a glass reactor under both mechanical stirring and argon flow. This flow provides an inert atmosphere that avoids the presence of oxygen in the reaction that would allow the formation of maghemite (γ-Fe2O3) or hematite (α-Fe2O3) as secondary phases. In addition, to achieve an alkaline reaction medium, 20mL of 25% NH3 was added, detecting a color change of the solution from blood red to black by formation of iron hydroxides. The reactor was connected to a thermal water bath warming up to 75°C, being stirred for 30min. Then, 1mL of oleic acid (OA) is added to favor the dispersion of nanoparticles [43,44] and the reaction system was maintained under mechanical stirring for 1h. A solid black powder was obtained, which was separated from the liquid by magnetic decantation and was washed several times with a mixture of ethanol/water, drying finally in a stove at 50°C.
Preparation of chitosan-coated Fe3O4 samples0.125g of previously synthesized Fe3O4 sample, dispersed with 0.5mL of oleic acid (OA) and mixed with 0.25g of commercial chitosan (Sigma-Aldrich, Medium molecular weight, 75–85% deacetylated) in 50mL of 2.0wt% acetic acid solution was poured into a beaker and stirred using an ultrasonic bath for 20min. As is known, the acid medium favors the solubility of chitosan [45] and its interaction with the Fe3O4 powder by the protonation of the amino groups present in the chitosan [46]. The resulting mixtures were poured in a glass reactor, being stirred for 20min and heated to 40°C. In order to induct a crosslinking reaction, different volumes of glutaraldehyde (GA) (25% by weight) were added (0, 0.5, 0.75, 1, 1.5 and 2mL) on prepared solutions as described above. Aldehyde groups from GA react with amine moieties of chitosan, leading to crosslinked polymer chains. All reactions with GA were carried out for 3h.
In a parallel study, different chitosan-coated Fe3O4 samples were prepared by adding 1mL of GA, but now varying the reaction time of Fe3O4 sample with chitosan at 6 and 12h.
In all cases, gray color powders were isolated from the liquid by magnetic decantation and they were washed several times with a mixture of deionized water and ethanol. Subsequently, they were dried in an oven at 50°C.
CharacterizationThe different prepared samples of Fe3O4 composition were characterized by X-ray diffraction (XRD) employing an X́Pert-MPD Philips diffractometer with Cu Kα radiation. A step scan of 0.04° (2θ) in the range 10–70° and a counting time of 1s for each step were employed for data collection. Chitosan and Fe3O4 associations were evaluated by using Fourier-transform infrared spectroscopy (FTIR). Spectra were recorded on a Prestige-21 Fourier-transform spectrophotometer using the KBr pellet technique within the (4000–400)cm−1 range. To prove the encapsulation efficiency and the thickness of the coatings, transmission electron microscopy (TEM) images were obtained on a JEOL 2100F transmission electron microscope, operating at 200kV and equipped with a field emission electron gun providing a point resolution of 0.19nm. For TEM observations, the powders were dispersed in n-butanol and drops of the corresponding suspensions were deposited on carbon-coated copper grids. The stability of the synthesized covered samples with respect to heating in the presence of air was evaluated from thermogravimetric analysis, recorded on TGA Q50 (TA Instruments). The run was carried out in air at a heating rate of 10°C/min. M–H curves were registered in a coercivity spectrometer developed by the University of Kazan (Coercivity Spectrometer J-meter) [47]. Finally, the suspension stability of the chitosan-coated Fe3O4 samples and their aggregation capacity over time were analyzed by dynamic light scattering (DLS) experiments using a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestshire, UK). All the DLS measurements were performed at 25°C using as radiation the red line (wavelength, λ=632nm) of a He–Ne laser.
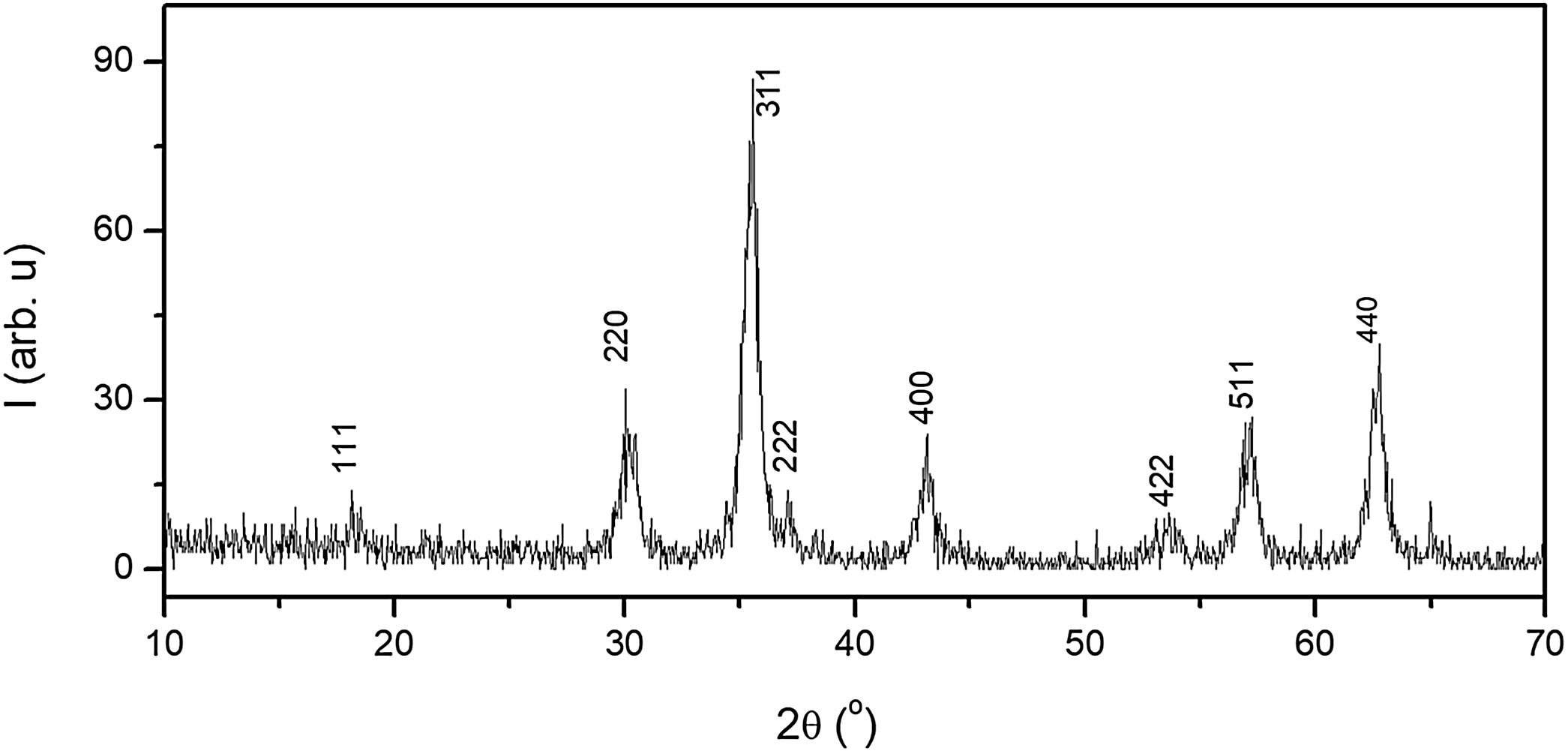
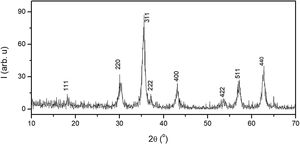
Results and discussionStructural characterizationFig. 1 shows the XRD patterns of the Fe3O4 synthesized sample. All observed reflections can be indexed to a cubic symmetry of space group Fd3¯m with Z=8, compatible with an inverse spinel-type structure, characteristic of Fe3O4 [ICDD 82-1533]. XRD data were analyzed by the Rietveld method using the FULLPROF program and, the results, which we published in previous work [48], finding a cell parameter a=8.3781(5)Å. The result highlights the existence of 95% of the magnetite phase together with 4.7% of the maghemite phase and, therefore, partial oxidation of the sample thus synthesized.
An estimation of the average crystalline size of the as-synthesized sample using the Scherrer formula [49]. The diffraction maxima used for the calculation were (220), (311), (511 and (440). It was found a value of 11.5nm in size for prepared the nanoparticles. This particle size is of the same order as determined in samples of the same composition prepared by the same synthesis method [50–52].
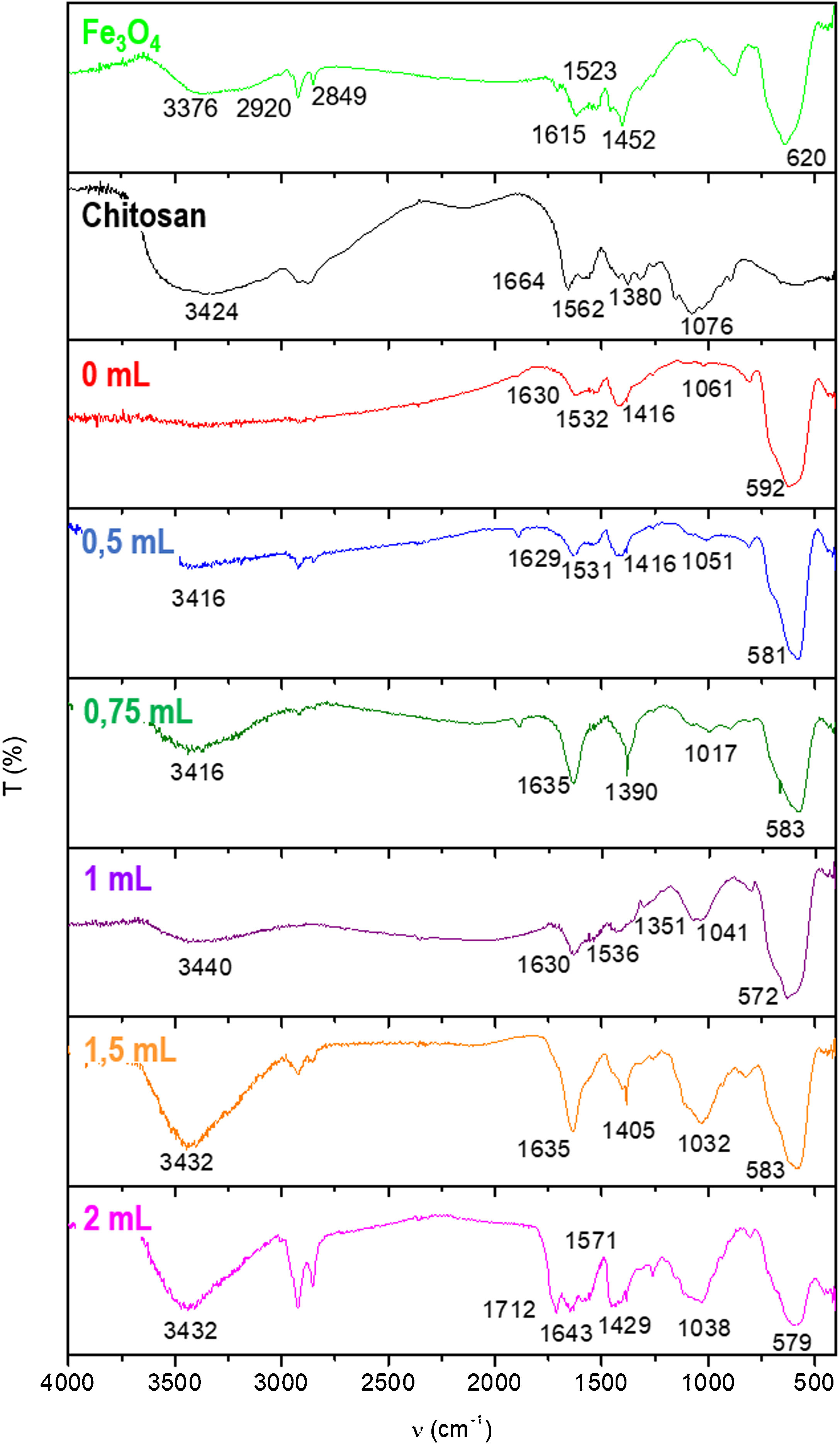
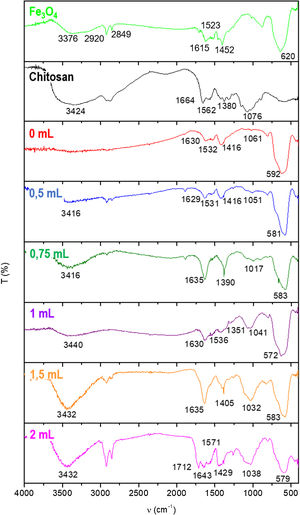
Fig. 2 shows the FTIR spectra of Fe3O4 sample, pure chitosan and chitosan-coated Fe3O4 samples for x=0, 0.5, 0.75, 1, 1.5 and 2mL of glutaraldehyde volume. In the spectrum of Fe3O4 sample, a high-intensity band can be observed at about 600cm−1 assignable to intrinsic stretching vibration of FeO bonds located at tetrahedral site of the inverse spinel structure type of this oxide [53]. This band is also present in the spectra of the coated samples. In the rest of the spectrum, a broad band with a maximum close to 3400cm−1, probably attributable to the OH stretching of water adsorbed on the surface of these materials, can be observed and another band at 1630cm−1 also assignable to the H2O bending vibrations of water adsorbed. The band at 3376cm−1 are related with the presence of OH groups from water or rests of free. The bands 2920–2850cm−1 were attributed to asymmetric and symmetric CH2 stretching of the oleic acid, which remains in nanoparticles surface. It is also reflected by the presence of the band at 1615cm−1, associated with CC double bonds, with a wide shape due to the appearance in this area of the signal of OH bending band. The doublet appearing at 1452 and 1523cm−1 correspond to the asymmetric and symmetric stretching vibrations of the carboxyl group when is coordinated to the surface as carboxylate (COO−) [54,55].
In the chitosan pure spectrum, characteristic bands of its functional groups are also observed: bands at 1664 and 1562cm−1 are assignable to the OH flexion, the band at 1380cm−1 to the flutter of CH2 while the bands at 1076cm−1 were attributed to CO and COC bonds [56]. All these bands appear somewhat displaced in the spectra of chitosan-coated Fe3O4 samples, which may indicate the possible association of chitosan and Fe3O4 sample. This effect has been previously observed and described by Long et al. [57].
Spectra of samples synthesized with increasing GA volumes show bands that are visualized in spectra of pure chitosan and Fe3O4 sample. In the spectrum of the sample prepared by the addition of 2mL of GA an additional band at 1712cm−1 also appears, which can be assigned to the strain CO bond and related to the high percentage of glutaraldehyde present [58].
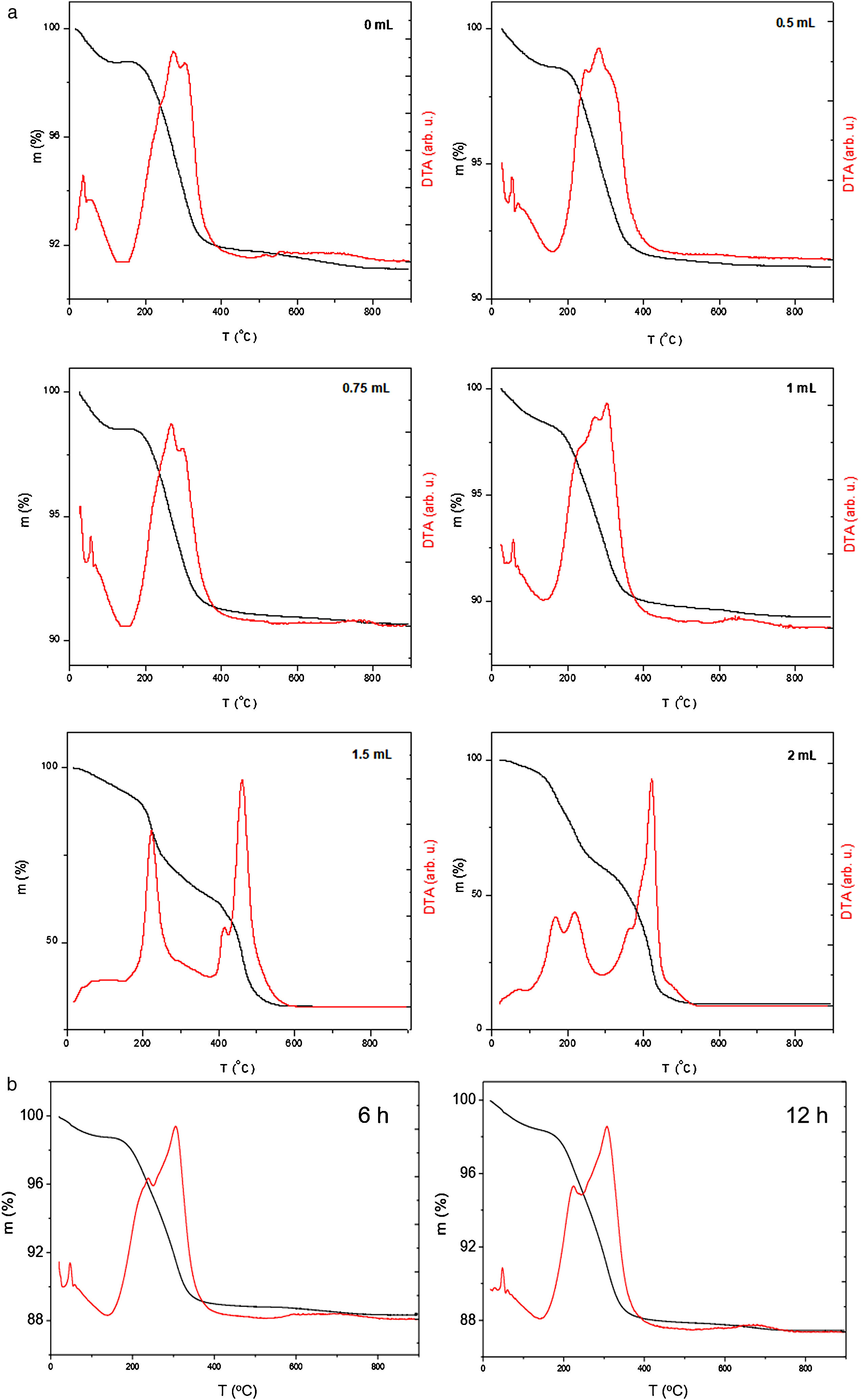
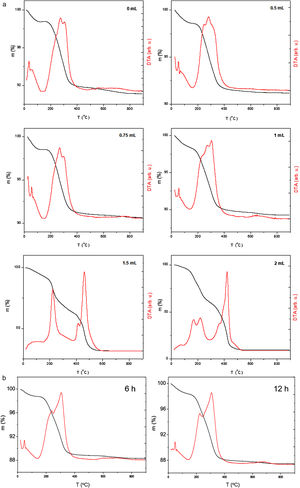
Thermogravimetric analysis (TGA)The thermal stability on air of all synthesized samples was evaluated from the thermogravimetric analysis. TGA/DTA curves of chitosan-coated Fe3O4 samples prepared with GA volume variation and increasing the reaction time are shown in Fig. 3a and b, respectively, where a weight loss below 150°C is found due to the adsorbed water on the surface. In addition, a significant weight loss between 150 and 400°C can be also observed. In fact, while in TGA curve of sample prepared without glutaraldehyde (0mL) a loss of mass by decomposition at lower temperatures is seen, in TGA curve of sample that contain the greatest amount of crosslinking agent (2mL) these losses are caused by combustion of the organic layer and takes place gradually until it reaches 600°C. These results are in accordance with those described elsewhere [59,60]. Chitosan begins to decompose approximately at 200°C, although weight losses also observed at temperatures above 500°C when the chitosan is crosslinked with glutaraldehyde. In TGA/DTA curves of samples prepared with low content of GA, the weight loses determined is close to 10%, increasing progressively from 8.88% (for 0mL of GA) to 8.90 (0.5mL of GA), 9.34 (0.75mL of GA) and 10.70% (1mL of GA). For chitosan-coated Fe3O4 samples prepared with a large amount of glutaraldehyde, exist an excessive weight loss (68.35% for 1.5mL of GA and 90.88% for 2mL of GA). These losses increase gradually in TGA of chitosan-coated Fe3O4 samples prepared with 1mL of GA and increasing the chitosan reaction time (see Fig. 3b) (10% for 3h, 11.6% for 6h and 12.6% for 12h).
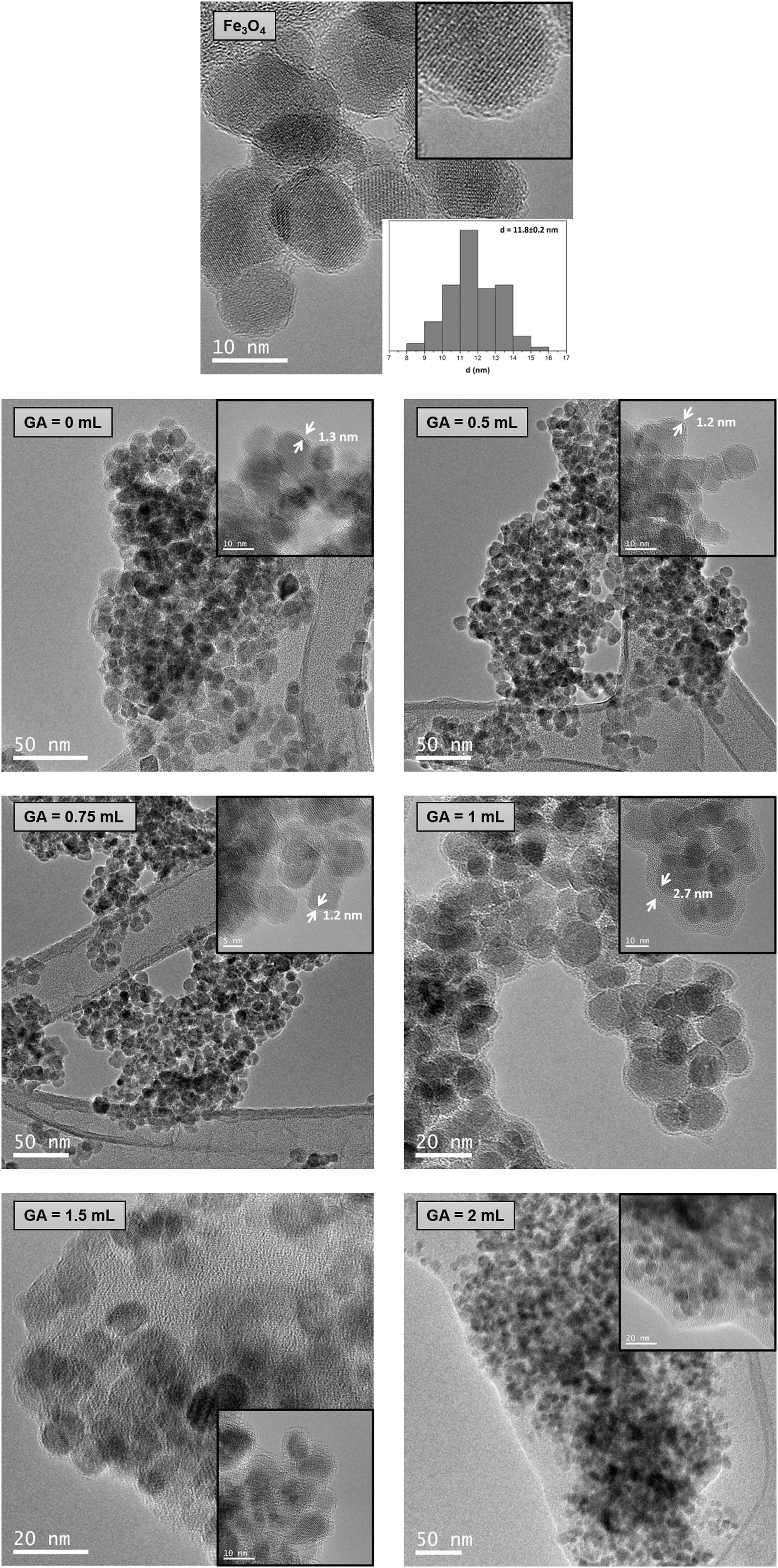
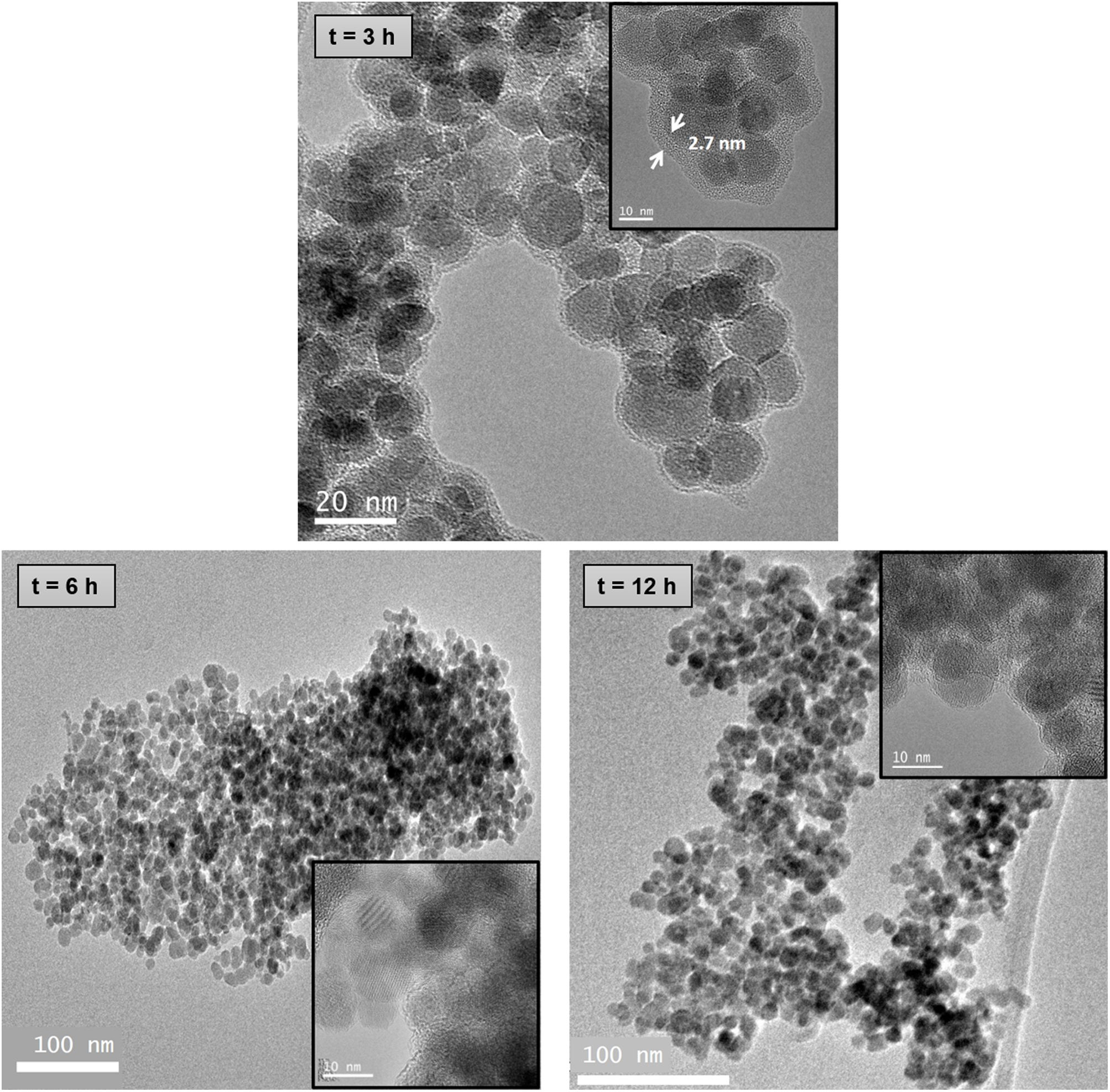
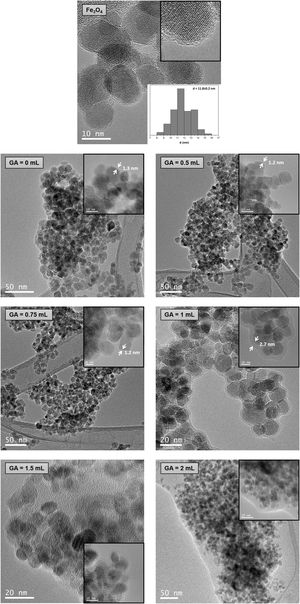
Transmission electron microscopy (TEM)TEM images of Fe3O4 and chitosan-coated Fe3O4 samples are shown in Fig. 4. Size calculations were made by using the ImageTool software, measuring at least 80 particles for every sample. TEM image of Fe3O4 sample (Fig. 4a) reveals spherical particles with an average diameter of 11.8nm. This result is in good agreement with the average size calculated by XRD. A similar arrangement of nanoparticles can be seen in TEM images of chitosan-coated Fe3O4 samples prepared with low content of glutaraldehyde (0, 0.5 and 0.75mL) with a lower powder aggregation, although these nanoparticles are now coated with a homogeneous and thin chitosan shell of 1.2–1.3nm. This thin shell would justify the similar losses of organic matter determined from TGA curves. TEM image of the chitosan-coated Fe3O4 sample prepared with 1mL of GA shows nanoparticles surrounded by a chitosan shell of 2.7nm. The thickness has increased and this result is in good agreement with the high organic content determined by TGA analysis (10.70%) in this sample. By contrast, several amorphous zones are seen in TEM images of the chitosan-cover Fe3O4 samples prepared with 1.5 and 2mL of GA, which cannot be considered as coating of the Fe3O4 nanoparticles surface and, therefore, the excess polymer seems to act as an organic matrix where the Fe3O4 nanoparticles are dispersed. These results are in good agreement with the high organic content determined by TGA (68.35% and 90.88% for samples prepared with 1.5 and 2mL of GA, respectively).
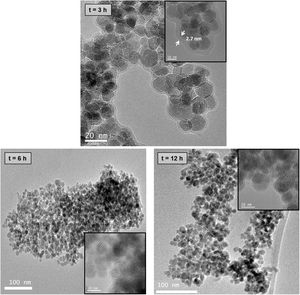
TEM images of chitosan-coated Fe3O4 samples prepared with 1mL of GA and increasing the chitosan reaction time (3, 6 and 12h) are shown in Fig. 5. As the reaction time increases from 6 to 12h, also Fe3O4 nanoparticles dispersed in an organic matrix can be seen here. These results indicate that an increase in the reaction time with chitosan does not favor the process of coating the nanoparticles and if the segregation of excess organic.
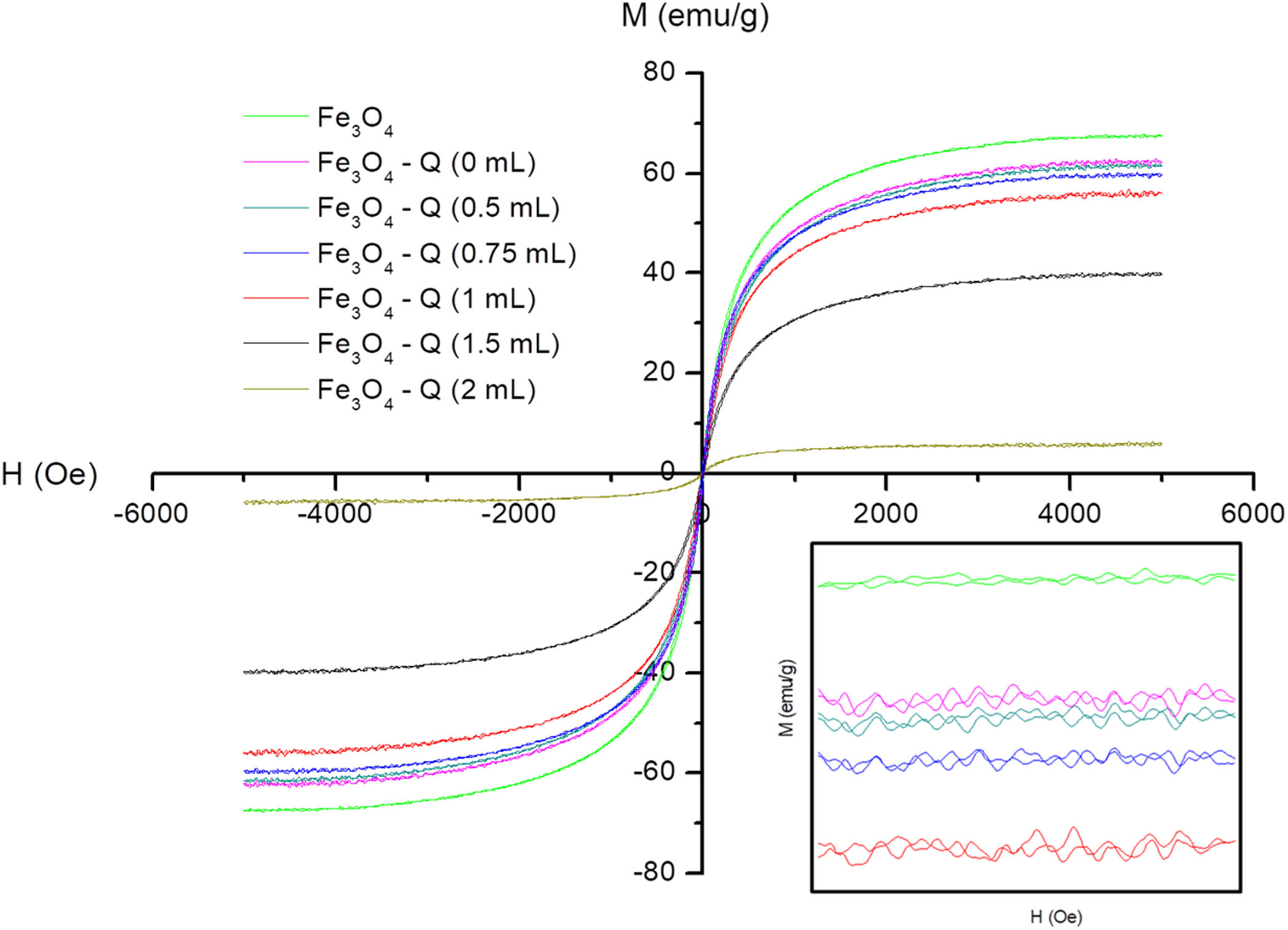
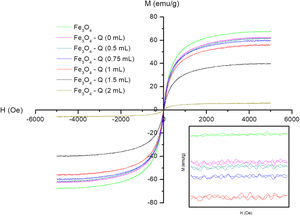
Magnetic behaviorThe magnetic field dependence of magnetization at room temperature of all synthesized sampled is presented in Fig. 6. Table 1 summarizes the magnetic parameters determinates from these representations.
Fe3O4 sample and chitosan-coated Fe3O4 samples show a practically superparamagnetic behavior with very low coercive fields (between 8 and 14Oe) and Mr values (between 1.99 and 0.19emu/g). On the other hand, it can be observed that Fe3O4 sample presents a reduction of the saturation magnetization values (Ms=67.48) with respect to the theoretical value of bulk magnetite (92emu/g) [61]. This result can be explained by both the partial oxidation, as it was found in XRD experiments, and the canted spin on the surface of this kind of nanoparticles, which results in a non-contribution of its magnetic moment to total magnetization [62]. A reduction of Ms is evidenced with the chitosan cover with respect to the value of the uncoated sample, Table 1. The non-magnetic character of chitosan and its content makes possible the noticeable reduction of Ms. Much more evident for the samples prepared with greater GA content (1.5 and 2mL) that have a higher chitosan content. These results are in good agreement with that reported by Freire et al. among others [63–65], with the percentages determined from TGA curves and with the powder morphology observed in the TEM images of Fig. 5.
For chitosan-coated Fe3O4 samples prepared with 1mL of GA with increasing the reaction time, can be also observed a slight reduction of Ms, in good agreement with the contents determined by TGA. In summary, the decrease of Ms is a multiple source phenomenon that depends on the GA concentration and reaction time, being the first the parameter that influences most of the experiment carried out.
Dynamic light scattering (DLS)Once the Fe3O4 and chitosan-coated Fe3O4 samples are obtained, their environmental and public health impact will depend largely on how stable these particles suspended in the natural environment. For this reason, we analyze in this work the effect of chitosan shell-thickness on the aggregation capacity of nanoparticles. It is noted that the absence of charge in the chitosan under the conditions of measurement (neutral pH) prevents the electrostatic repulsion of the nanoparticles with each other and the resulting colloidal stabilization for larger times [66].
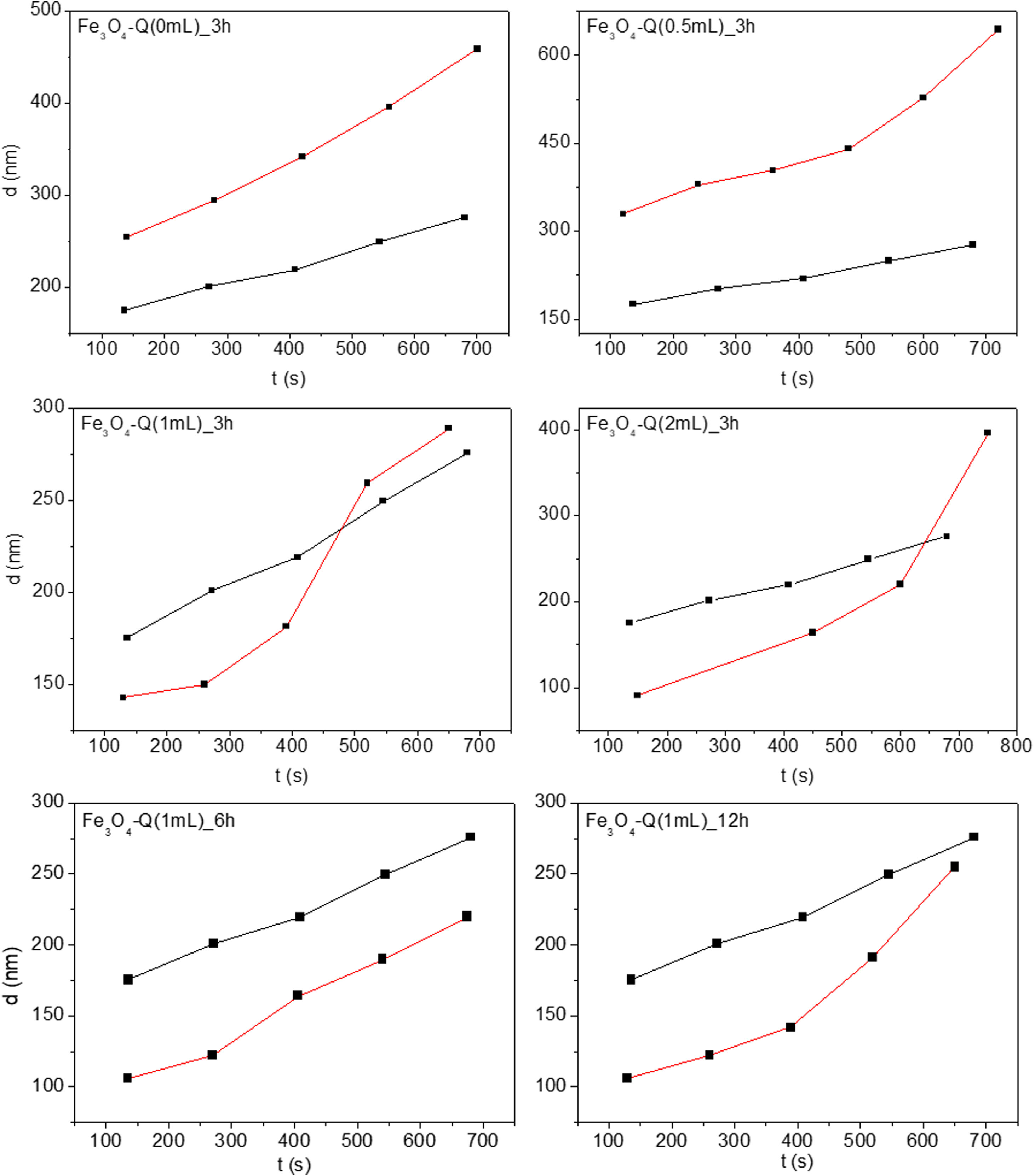
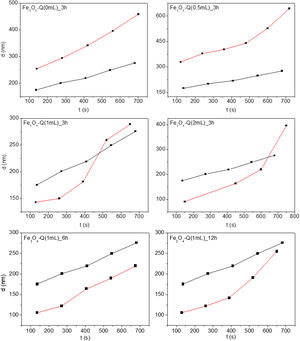
Fig. 7 shows the hydrodynamic diameter evolution overtime for 0.01g/L of distilled water for all synthetized chitosan-coated Fe3O4 samples. As a reference, the same variation for Fe3O4 sample suspended in water is also shown (black lines). An increase of the hydrodynamic size over time can be observed for the uncoated nanoparticles, which can be explained as a result of a process of aggregation in situ, generating aggregates with a minimum size of 175.4nm. These aggregates are much larger than those determined by TEM for the corresponding nanoparticles. This fact reflects the tendency of the Fe3O4 sample to form nanoparticles aggregates over time due to its high surface/volume ratio, in good agreement with what has been described in the literature for particles of such size [67].
Larger values of hydrodynamic diameter are visualized in the chitosan-coated Fe3O4 samples prepared with GA=0 and 0.5mL. For these samples, the amount of chitosan is very low, favoring the aggregation process. In the chitosan-covered Fe3O4 samples prepared with GA=1 and 2mL, smaller size of the aggregate is initially observed. This phenomenon can be explained considering a better dispersion in water of samples as a result of the presence of chitosan. However, the observed evolution of hydrodynamic diameter over time is far from the linearity found for the Fe3O4 sample, giving rise to a curve that shows an increase of the size of the aggregates of nanoparticles that accelerate over time. The results of these studies found that the presence of a homogeneous chitosan coating improves the initial suspension of these samples, although they do not prevent the aggregation of constituent particles over time.
The hydrodynamic diameter evolution over time for chitosan-coated Fe3O4 samples prepared with 1mL of GA at different reaction times also suffers the equivalent aggregation phenomenon. However, the formation of nanoparticle aggregates seems to be reduced and even disappear in the sample obtained after 6h of reaction with chitosan. In fact, TEM image of this sample (see Fig. 5) shows a homogeneous chitosan shell surrounding not a particle, but a group of nanoparticles of Fe3O4 of 12nm. This fact leads to a reduction in surface/volume ratio, minimizing the surface energy, reducing aggregation and contributing to the stabilization of chitosan covered Fe3O4 nanoparticles in suspension. For this reason, this sample seems to be the most suitable for use in the natural environment.
ConclusionsThe effects of the different chitosan thicknesses have been evaluated from the preparation of chitosan-coated Fe3O4 nanoparticles. X-ray patterns of Fe3O4 samples show reflections indexed to a cubic symmetry of space group Fd3¯m with Z=8, compatible with an inverse spinel structure. FTIR spectra of synthesized samples evidence the presence of chitosan which becomes more noticeable in samples with higher chitosan content and TGA curves show a significant weight loss which is the result of the combustion of the organic thickness. TEM images of chitosan-coated Fe3O4 samples synthesized with a lower GA volume show particles coated with a homogeneous chitosan shell of 1.2–1.3nm. TEM images of samples synthesized with higher GA volume show dispersed nanoparticles in an organic matrix. The content of organic matter reduces the magnetization saturation values of these superparamagnetic samples. The presence of a homogeneous coating improves the suspension of the samples in solution, although it does not prevent their aggregation over time. However, this aggregation process seems to be reduced and even disappear for the sample synthesized with 1mL of glutaraldehyde after 6h of reaction with chitosan.
FundingFundación Neurociencias y Envejecimiento has supported this work through project 359/2014 as well as MINECO through the project MAT2016-80182-R.