One of the reasons of implants failure are the stress forces appearing in the material–tissue interface due to the differences between their mechanical properties. For this reason, similar mechanical properties to the surrounding tissue are desirable. The synthesis of hydroxyapatite by solution combustion method and its processing have been studied in order to obtain fully dense ceramic bodies with improved mechanical strength. Combustion synthesis provides nanostructured powders characterized by a high surface area to facilitate the following sintering. Moreover, synthesis was conducted in aqueous and oxidizing media. Oxidizing media improve homogenization and increase the energy released during combustion. It gives rise to particles whose morphology and size suggest lower surface energies compared with aqueous media. The obtained powders were sintered by using a controlled sintering rate schedule. Lower surfaces energies minimize the shrinkage during sintering and relative densities measurements and diametral compression test confirm improved densification and consequently mechanical properties.
Una de las razones principales de fracaso de los implantes son las fuerzas de estrés que aparecen en la interface material-tejido debido a las diferencias existentes entre sus propiedades mecánicas. Por esta razón, es necesario que el implante posea propiedades mecánicas similares a las del tejido circundante. La síntesis de hidroxiapatita por el método de combustión y su procesamiento se han estudiado con el objetivo de obtener cuerpos cerámicos completamente densificados y, consecuentemente, con propiedades mecánicas mejoradas. El método de combustión provee de polvos nanoestructurados que se caracterizan por una superficie específica elevada que facilita el siguiente proceso de sinterización. Además, este proceso de síntesis se ha realizado en medio acuoso y oxidante. El medio oxidante homogeniza e incrementa la energía liberada durante la combustión. Esto da lugar a partículas cuya morfología y tamaño indican energías superficiales menores en comparación con las obtenidas en medio acuoso. Los polvos obtenidos se sinterizaron siguiendo un esquema de velocidad de calentamiento controlada. Partículas con bajas energías superficiales dan lugar a menores contracciones durante el proceso de sinterización y las medidas de densidad y los test de compresión diametral confirmaron la mejora de la densificación, así como de las propiedades mecánicas.
Most of the calcium in the human body is in solid state and stored in bone tissue as nanometric crystals of a calcium phosphate compound with structure and composition similar to hydroxyapatite (HA). Stoichiometric HA is represented by the chemical formula Ca10(PO4)6(OH)2, where the molar ratio Ca/P is 1.67 [1]. However, far from the above formula, the calcium phosphate found in the mineral constituent of bones is better described as a non-stoichiometric, calcium-deficient and carbonated apatite [2].
Synthetic HA ceramics are considered as bioactive, i.e. they are able to bond tightly to bone tissue without any intermediate connective tissue. However, HA is less soluble than other calcium phosphates [3,4] and consequently, it slightly biodegrades when is implanted. This is a problem to complete the regeneration process. Only the surface of the material interacts with bone tissue while the bulk remains unchanged. At the material–tissue interface stress forces are developed due to mismatch of the Young modulus between material and bone [5,6]. This may cause the long term failure of the implant, which usually takes place at the bulk of the ceramic. Thus, HA ceramics with improved fracture toughness [1,5] would be more useful for manufacturing structural load-bearing bone implants.
One way to improve the mechanical behavior is by controlling the sintering process in order to obtain tailored microstructures. Thermal treatments affect the final microstructure and this microstructure affects the mechanical properties. During the thermal treatment, grains connect and close the spaces between them resulting in larger grains. Moreover, mechanical properties such as resistance become worse with the increase of porosity. If the thermal treatment is realized in harmony with material shrinkage, densification improves by decreasing the porosity. To attempt this challenge, rate controlled sintering (RCS) was suggested. During sintering, porosity can be reduced by controlling the heating rate and shrinkage occurs in synchrony with densification rate.
Particle size of sintered powders plays an important role in the densification process during the thermal treatment. Nanoparticles have higher reactivity and, despite they compact worse, interparticle porosity is smaller and both characteristics should improve densification. Moreover, Nano-HA has great importance in medical applications due to the fact that this small grain size is similar to that of natural HA and showed improved properties with respect to micrometer grain size HA [6].
Solution combustion synthesis (SCS) gives the possibility to produce a big variety of materials, morphologies and sizes [7–11]. Especially in the case for what concerns the size, combustion synthesis is an excellent way to obtain submicronic structured materials. For this reason, SCS method has been chosen to synthesize HA [12–17] in order to obtain nanoparticles and improve densification [18]. During combustion a quick exothermic reaction takes place, delivering high amount of energy [13]. It promotes wide thermal gaps which allow to obtain particles in the nanometer size [19].
The objective of this work is the obtaining of dense HA materials with improved mechanical resistance. With this aim a RCS has been carried out on HA nanostructured particles obtained by combustion. Furthermore, it will be demonstrated how mechanical properties can be improved by controlling the synthesis conditions.
Materials and methodsRaw materials employed for solution combustion synthesis were, Ca(NO3)2·4H2O (99.5% PA-ACS, Panreac Química S.A.), (NH4)2HPO4 (98% PA-ACS, Panreac Química S.A.) and Urea (NH2)2CO (99.5% ACS, Panreac Quimica S.A.) as fuel [13]. The quantities employed were calculated to obtain 5g of final product considering the reaction stoichiometry [13]. A second synthesis was carried out adding 5cm3 of HNO3 (65% Pro Analysis Merck) as internal oxidant or oxidant excess [13] to ensure total fuel combustion and pH control [20].
In both cases, raw materials were separately dissolved in 100cm3 of deionized water and put in a porcelain capsules. The capsules were placed in a furnace where the mixtures are heated at 500°C during 30min to assure the drying and the corresponding combustions.
In order to break down the obtained agglomerates, the synthesis products were passed in an agate mortar and milled by attrition during 8h. Then, particle size distributions were measured by laser scattering using Mastersizer S (Malvern, UK) after Agatha mortar and after attrition milling. Specific surface area was measured before and after milling in a Monosorb. Mod MS 13 (Quantachrom, EE.UU). Dilatometry was realized in order to design the thermal treatment using a dilatometer Setsys 16/18 (Setaram, France). For dilatometry analysis Rate Controlled Shrinkage (RCS) schedule was used.
The obtained HA powders were compacted by uniaxial pressing (200MPa) to ensure a proper compaction overcoming the high particle friction between nanostructured particles. Then, sintering was carried out using a rate controlled thermal cycle to decrease porosity formation. Thermal treatments were performed using a heating rate of 5°C/min until 900°C, where shrinkage starts according to the dilatometries of both products, and 1°C/min until 1230°C for 2h. Because shrinkage starts at 900°C, heating rate is decreased in order to minimize porosity formation due to a quick contraction of material. 1230°C was selected as sintering temperature in order to get the maximum densification avoiding HA decomposition. Finally, cooling rate selected was 5°C/min. The final phase composition was analyzed by X-ray diffraction (XRD) and the relative density was evaluated by Archimedes method as well as geometrically.
Phase compositions were studied by XRD with an X-ray diffractometer Siemens, D 5000 model from Germany. Mechanical properties of sintered materials were studied using diametral compression test [21], also known as Brazilian test (Instron, UK). Cylinders with 5mm in diameter and 10mm in height were used for the mechanical test (n=16). Resistance results were analyzed using Weibull statistics and discussed considering the microstructure images obtained by Field Emission Scanning Electron Microscopy (FESEM, Hitachi Mod. S-4700, Japan). The polished section and the fracture of both materials were observed and their microstructures were analyzed. To prepare the polished sections, materials must be previously embedded in Epoxy resin. Once Epoxy resin is hardened samples are cut and the section is polished.
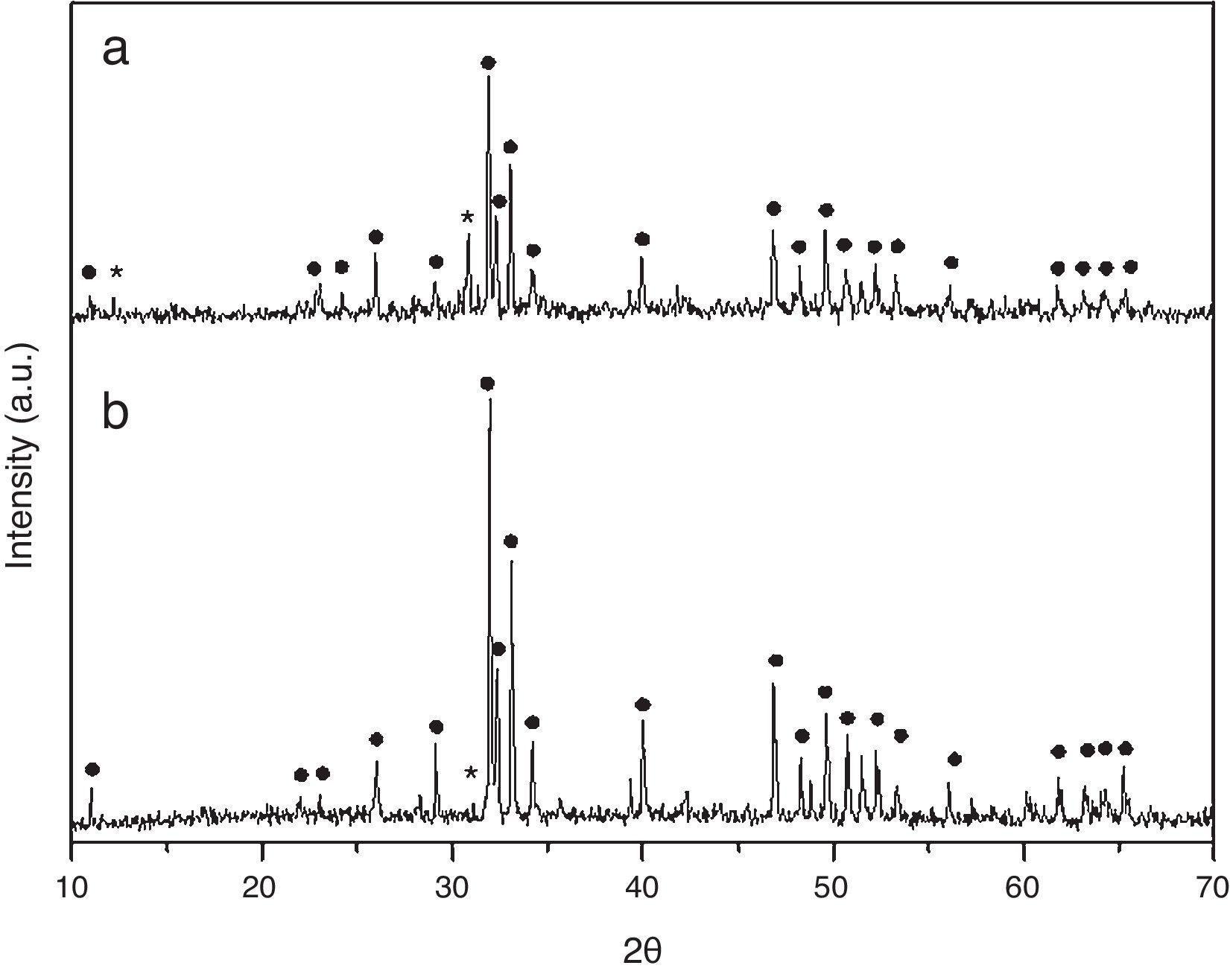
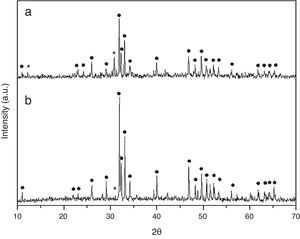
Results and discussionCrystall phase characterization of HA obtained by combustion in two different media, aqueous and oxidizing media (by HNO3 addition), shows in both cases two crystalline phases. The major crystal phase is HA and the minor phase is β-tricalcium phosphate (β-TCP) [13].
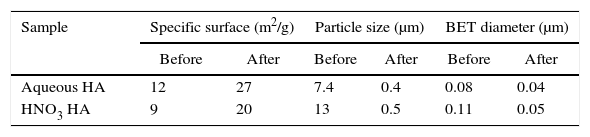
Specific surface is bigger for HA obtained in aqueous media (Table 1), 12m2/g compared to 9m2/g measured for the products synthesized in presence of HNO3. It is probably due to the higher energy involved in the reaction when internal oxidant (HNO3) is added. Energy released is firstly invested in the maintaining of the reaction but also for grain growth after that. The bigger are the grains the smaller is the specific surface area. After attrition milling particle size decreases due to fracture of nanostructured particles. Particle sizes of HA powders after attrition are similar but specific surface is lower for powders obtained by combustion in acid media as compared to the product in aqueous media. It can be also explained considering the higher energy involved when HNO3 is added.
It can be also observed a big difference between the particle size and the much smaller BET diameter that the obtained by laser scattering. It means presence of agglomerates. There are also morphological differences between the products obtained by combustion in two different media. These differences can be explained observing the samples by SEM.
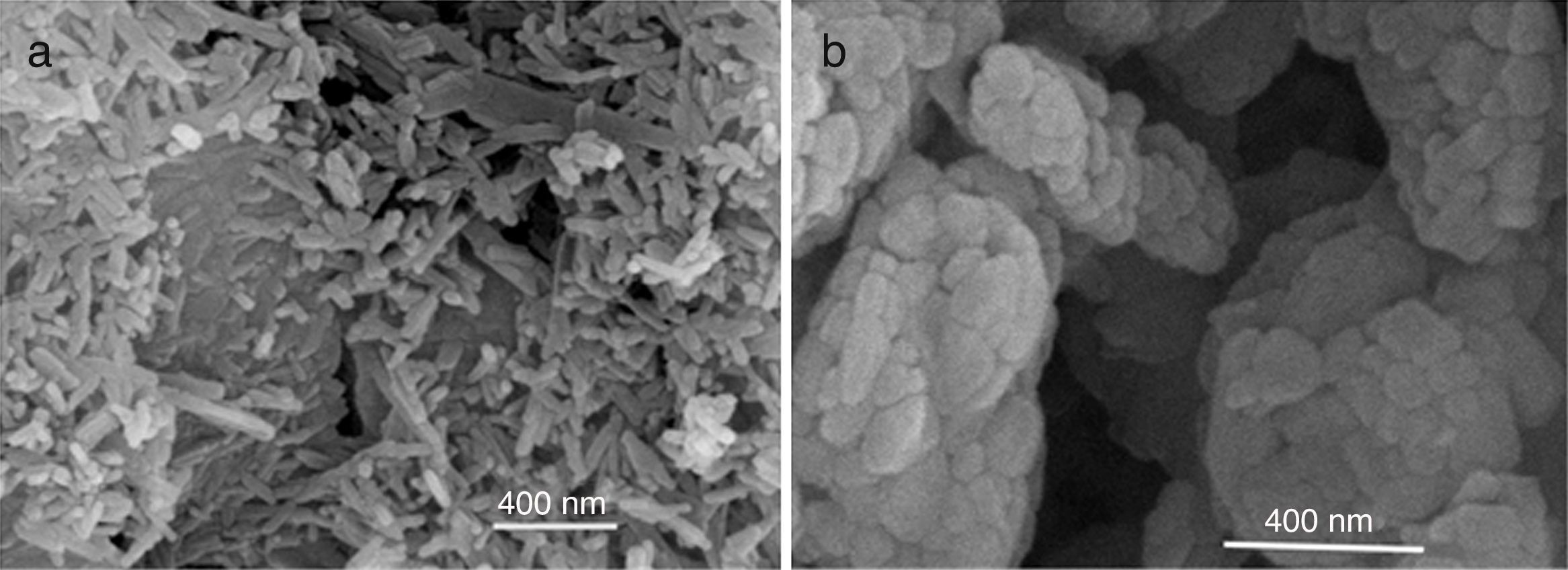
SEM micrographs of the products show important differences (Fig. 1). In both cases a submicronic structure can be observed. Powders obtained in aqueous media shows agglomerates formed by parallelepiped grains while powders obtained in oxidizing media show rounded shaped agglomerates formed by smaller globular grains in the submicronic range. This globular shape also supports both: higher energy released during the reaction and smaller specific surface area of powders obtained in the case of oxidizing media.
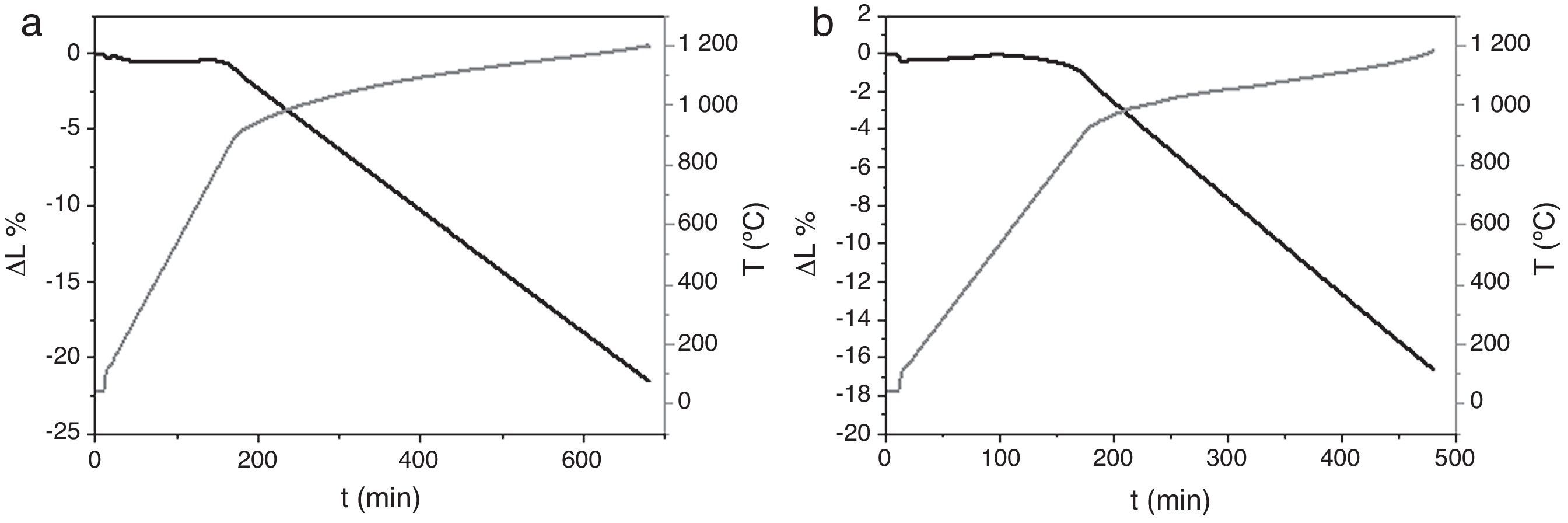
Dilatometries of powders, using a RCS schedule, are shown in Fig. 2. In both cases, they show that shrinkage starts approximately at 900°C. Also, the heating rate during these experiments was similar and close to 1°C/min. For this reason, the thermal treatment choice was: Initial heat with a heating rate of 5°C/min until 900°C. It is here where contraction begins. From this temperature the heating rate was reduced to 1°C until 1230°C, maintaining this temperature for 2h. Heating rate is reduced in order to maintain the shrinkage rate in values around 0.05%/min. to prevent remaining porosity. 1230°C is selected as sintering temperature in order to get maximum densification avoiding HA decomposition. XRD shows (Fig. 3) that phase composition of powders, based on HA as majority phase and β-TCP as secondary and minor phase, is still maintained after the sintering process.
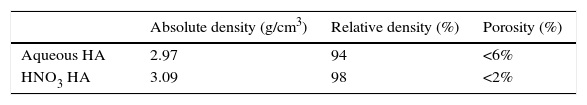
Value of density for aqueous HA, after sintering, was 2.97g/cm3 (94% of relative density). For HA obtained in acid media with HNO3 is 3.09g/cm3 (98% of relative density). All these data are summarized in Table 2.
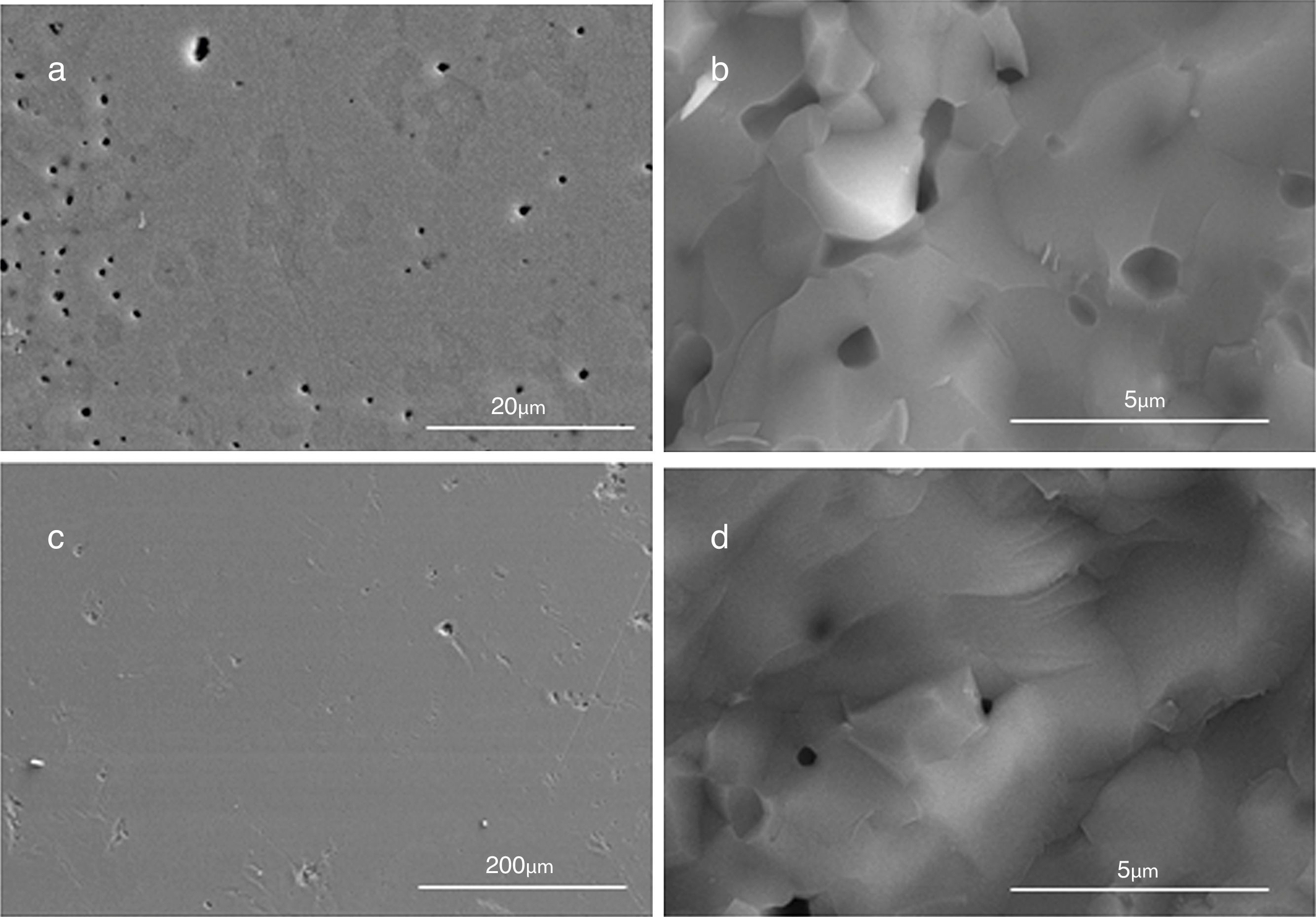
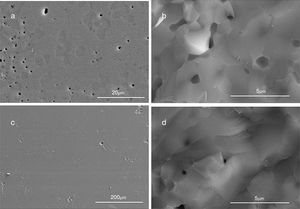
SEM micrographs of the section and fractures of bulk materials show important differences depending on the combustion media in which HA was obtained (Fig. 4). It is clear that samples obtained from powders synthesized in oxidizing media show a lower porosity than those obtained in aqueous media (Fig. 4a and c). Moreover, samples obtained from powders synthesized in aqueous media show a heterogeneous pore distribution (Fig. 4a). It looks like there was a problem of differential sintering, with porosity surrounding hard agglomerates. Furthermore, regarding to the pore size, smaller pores have been observed in the fracture of samples obtained from powders synthesized in oxidizing media compared to those in aqueous media (Fig. 4b and d). It could be explained by the different specific surface area of powders obtained in different media. The particle morphology could play also an important role during sintering. The geometry of particles can be approached to a parallelepiped, in case of aqueous media, and to a sphere (equiaxial), in case of HNO3 media. Surface energy for parallelepiped particles is higher than for spheres. In case of HA obtained in aqueous media a higher specific surface area as well as higher surface energy can give rise to a quick shrinkage during the thermal treatment and as a consequence the remaining porosity is higher.
Diametral compression tests indicate that the samples obtained from powders synthesized in oxidizing media have higher resistance (55MPa) than samples obtained from aqueous media (33MPa). These results are in agreement with porosity and microstructure shown before. These values are also similar (in the case of aqueous media) and 60% higher (in the case of oxidizing media) to the reported in the literature for zirconia reinforced hydroxyapatite [22].
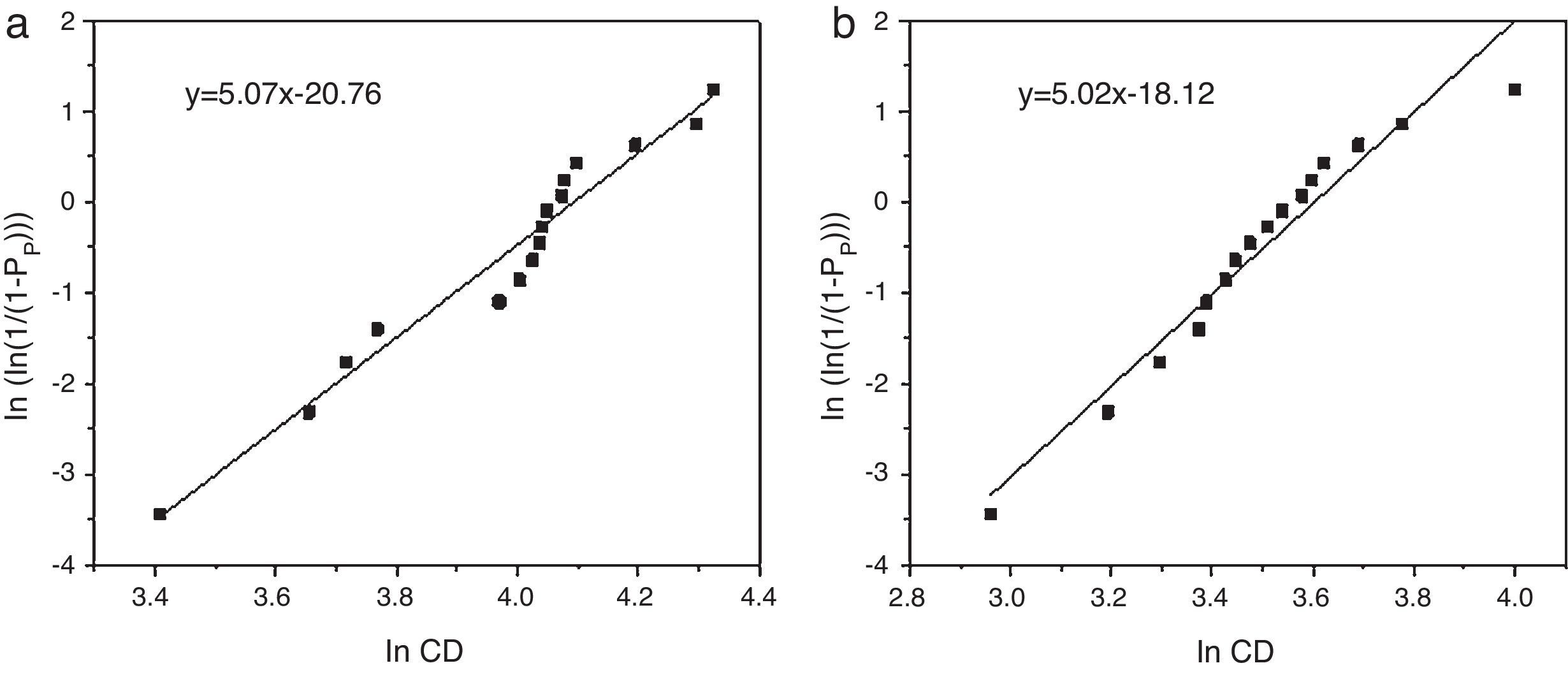
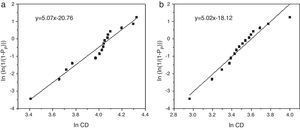
Weibull modulus was calculated for both materials (Fig. 5). Values calculated were 5.07 for samples obtained from powders synthesized in aqueous media (Fig. 5a) and 5.02 in oxidizing media (Fig. 5b). Usually, the higher is the porosity the lower is the Weibull modulus. Nevertheless both samples show similar values, around m=5.
These results suggest that morphology and reactivity of powders have a big influence over the densification process. In the worst case, using a well-designed sintering cycle the same mechanical property as zirconia reinforced materials is obtained and exceeding it by 60% with the best features powder, it means spherical morphology [22].
ConclusionsHA powders obtained by combustion have shown phase composition based on HA as main phase and β-TCP as secondary phase. Combustion gives rise to homogeneous phase composition grains with homogeneous shapes and sizes on the submicronic level. Moreover, different particles in terms of morphology can be obtained by controlling the media. Spherical (equiaxial) particles obtained in oxidizing media suggest low superficial energies.
In addition, these powders consisting of submicronic structured agglomerates of particles can be processed to obtain dense materials. After the compaction and sintering by RCS of the two different HA powders, they show excellent densification but important differences in terms of porosity. Powders obtained in HNO3 media give rise to materials with a lower porosity volume as well as pore size than powders obtained in aqueous media. It is related to a lower shrinkage during the sintering as a result of the low superficial energy of particles. Consequently, it is related with the differences observed in terms of mechanical properties. Materials obtained by densification of powders synthesized in oxidizing media, show strength value 60% higher than those obtained in aqueous medium or zirconia reinforced hydroxyapatite described in the literature.
The most important is that powders synthesized by combustion can be processed by conventional methods, obtaining materials with improved mechanical properties compared to powders obtained by standard routes.
The authors wish to thank the financial support from Ministry of Economy and Competitiveness of Spain under the projects MAT2013-48426-C2-1R, CSIC-201460E066 and CSIC-201760E022.