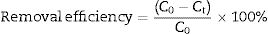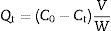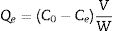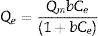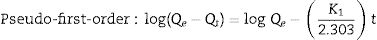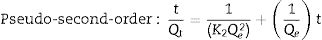Removal of heavy metal Mn2+ ions in water is of great importance for human health and it is urgently needed to develop efficient adsorption materials. Here, a green and effective strategy to prepare mesoporous micro/nanostructured lithium disilicates (LDs) by employing the cation surfactant hexadecyltrimethyl-ammonium bromide (CTAB) as morphology control agent in hydrothermal environment, and investigated its adsorption behavior toward Mn2+ ions. The LDs possessed branched structures that were consisted of scattering pyramidal rods bestrewn with secondary nucleated and aggregated nanoparticles. Due to the mesoporous structures and negatively charged surfaces, LDs exhibited a high adsorption capacity up to 346.84mgg−1 with corresponding removal efficiency up to 99.82% when initial Mn2+ concentration was 82mgL−1, and their maximum adsorption capacity reached up to 785.25mgg−1 toward Mn2+ of 250mgL−1. Results indicated that the isotherm adsorption behavior of LDs was well described by mono-layer Langmuir model and kinetic adsorption fitted well with pseudo-second-order model, implying them the excellent chemical adsorbent to remove Mn2+ from wastewater. We believe this CTAB-modified approach could be extended to prepare other lithium silicates with mesoporous structures, rendering them wider applications in environmental protection.
La eliminación de los iones Mn2+ de metales pesados en el agua es de gran importancia para la salud humana, y se necesita urgentemente para desarrollar materiales de adsorción eficientes. Aquí, una estrategia verde y eficaz para preparar disilicatos de litio mesoporosos micro/nanoestructurados (LD) mediante el empleo del tensioactivo catiónico bromuro de hexadeciltrimetilamonio (CTAB), como agente de control de morfología en el entorno hidrotérmico, e investigó su comportamiento de adsorción hacia los iones Mn2+. Los LD poseían estructuras ramificadas que consistían en varillas piramidales dispersas mejor cortadas con nanopartículas secundarias nucleadas y agregadas. Debido a las estructuras mesoporosas y a las superficies cargadas negativamente, los LD exhibieron una alta capacidad de adsorción de hasta 346,84 mg g-1 con la eficiencia de eliminación correspondiente de hasta el 99,82% cuando la concentración inicial de Mn2+ era de 82 mg L-1, y su capacidad máxima de adsorción alcanzó hasta 785,25 mg g-1 hacia Mn2+ de 250 mg L-1. Los resultados indicaron que el comportamiento de adsorción isotérmica de los LD fue bien descrito por el modelo de Langmuir de capa única y la adsorción cinética se ajustó bien con el modelo de seudo-segundo orden, lo que implica el excelente adsorbente químico para eliminar Mn2+ de las aguas residuales. Creemos que este enfoque modificado por el CTAB podría ampliarse para preparar otros silicatos de litio con estructuras mesoporosas, haciéndolos más amplios en aplicaciones de protección ambiental.
Water pollution by heavy metal ions, major produced from human activities and the substantial industry discharge such as mining, painting, car radiator manufacturing, batteries, and so on, is a serious environmental problem for their toxic effects on human health and living organisms [1–5]. To date, various techniques have been investigated to decontaminate heavy metal ions from wastewater including chemical coagulation [6], adsorption [7,8], ion exchange [9,10], membrane filtration [11,12], and electrochemical methods [13]. Among these methods, adsorption has become the most attractive treatment to remove heavy metal ions from wastewater owing to the high efficiency, easy operation, cost effectiveness, environmental friendliness and so on. Up to now, a variety of adsorbents have been tested and investigated on heavy metal ions removal. Among them carbon materials, such as activated carbon [14,15], biochars [16], carbon nanotubes [17], graphene [18,19] and so on, are regarded as effective adsorbents, but their expensive costs of manufacturing, surface/structure-modifying and regeneration obstruct their further application. Therefore, the development of new adsorbents is still required to uptake heavy metal ions from contaminated water or waste.
In recent years, silicate materials have attracted much attention in wastewater treatment as adsorbents due to their low cost, excellent thermal and chemical stability, and environmentally benign nature [20–23]. Some works have shown that their surfaces are negatively charged, which makes them good candidates to remove cation heavy metal ions [22,24]. Particularly, silicates with desired functional 3D structures organized by 1D or 2D building blocks have gained increasing attention compared with their counterpart solid particles due to their amazing versatility in new applications with enhanced [22–30]. Among these micro/nanostructured silicates, lithium disilicates (Li2Si2O5, LDs) become more attractive due to their unique sandwich structure composed of [SiO4] tetrahedron frameworks with mobile Li+ ions residing between layers, which endows them with ideal candidates as adsorbents in water purity [24,31,32]. Many methods, like [29,30], solid state [33], combustion [34] and sol–gel [35], have been used to prepare micro/nanostructured Li2Si2O5 particles with controllable morphologies. However, owing to the distinct feature of fast reaction related to characteristic nature of Li+ ions, irregular blocks with heterogeneous phases were often obtained. In addition, less attention has been paid to the synthesis and investigation of micro/nanostructured Li2Si2O5 particles for wastewater treatment. Our previous work [36] reported that a single-step hydrothermal process could synthesize monophase 3D Li2Si2O5 hollow flower-like microstructures, which exhibited good adsorption performance toward cation organic dye of methylene blue. Despite the recent success in the preparation of high-purity Li2Si2O5 particles, it is still a significant challenge to develop tunable structures for the removal of heavy metal ions in wastewater treatment.
In this study, we developed a facile hydrothermal strategy to prepare micro/nanostructured LDs by employing the cation surfactant CTAB as morphology control agent. The mesoporous LDs particles possessed branching structures that were consisted of scattering pyramidal rods bestrewn with secondary nucleated and aggregated nanoparticles, which allowed them the excellent adsorption performance of Mn2+ from wastewater. The plausible growth mechanism, isothermal and kinetic adsorption models were discussed in detail. The results suggested that the mesoporous LDs represented enormous potential in removing heavy metal ions for the wastewater remedy. Moreover, the CTAB-mediated method could be extended to prepare other silicates by virtue of its advantages such as large scale production, mild reaction conditions, environmentally benign, easy to operate, and so on.
ExperimentalMaterialsAll the chemicals were used as received and were of analytic grade without further purification. Tetraethyl orthosilicate (TEOS, ≥99.8%), hexadecyltrimethyl-ammonium bromide (CTAB, ≥99.8%), lithium hydroxide monohydrate (LiOH·H2O, ≥99.0%), manganese sulfate monohydrate (MnSO4·H2O, ≥99.0%), absolute ethanol and deionized water were purchased from Sinopharm Chemical Reagent Co, Ltd. (China).
Synthesis of LDsLDs were prepared through a simple surfactant-mediated hydrothermal process. Typically, m g (where m=0.25 and 0.5) CTAB were dispersed and dissolved in a mixture solution of 10mL ethanol and 60mL deionized water under stirring for 20min at a rate of 500rmin−1, respectively. Next, 0.672g LiOH H2O was added into the above solution under vigorous stirring for 5min. Afterwards, 3.334g TEOS was dropped in under mechanical stirring and the mixtures were further stirred for another 2h. Finally, the above solutions were then transferred into 100mL Teflon-lined stainless steel autoclaves and heated at 180°C for 48h. After the autoclaves cooled to room temperature, the white LDs powders were collected by filtered and washed with deionized water and absolute ethanol 3 times, respectively, and were subsequently air-dried in an oven at 80°C for 24h.
Adsorption experimentsFor the adsorption of toxic metal ions, Mn2+ from the source of MnSO4 H2O was used as the model. In the kinetic studies, a series of suspensions (23.6mg LDs powders and 100mL of a 82mgvL−1 Mn2+ solution) were stirred (600rmin−1) for different time at the initial pH value of 6.3 without adjustment. In a typical procedure to obtain the adsorption isotherm, 23.6mg LDs powders were mixed with 100mL solutions of different Mn2+ concentrations (50, 80, 100, 150, 200 and 250mgL−1) and stirred for 6h (sufficient for ensuring adsorption equilibrium). After adsorption, the adsorbed LDs were separated by centrifugation at a rate of 5000rmin−1 and dried at 80°C for 24h, and the supernatants were analyzed using an inductively coupled plasma atomic emission spectroscopy (ICP-AES, Shimadzu, ICPE-9000) to measure the residual Mn2+ concentrations. The removal efficiency and adsorption capacity toward Mn2+ were calculated according to Eqs. (1)–(3).
where Qt and Qe are the adsorption capacities (mg/g) at time t and at equilibrium, respectively. C0, Ct and Ce (mgL−1) are the concentrations of Mn2+ at initial, time t and equilibrium, respectively. V is the solution volume (L) and W is the weight of the absorbents LDs used (g). After being dried, the surface bonding state of the LDs was measured on a Thermo Fisher Scientific X-ray photoelectron spectrometer (XPS) equipped with a monochromatic Al Kα X-ray source (1486.6eV) and hemispherical electron energy analyzer. All binding energies were calibrated by using the containment carbon C1s=284.6eV.CharacterizationThe LDs samples were analyzed by a DX-2700 X-ray diffractometer (XRD; Empyrean, PANalytical, the Netherlands) using Cu Kα radiation (λ=0.15406nm) at a scanning rate of 0.02s−1 in a 2θ range from 10° to 80°. The crystallinity of each phase were quantitatively analyzed and obtained by Rietveld refinement method using JADE 6 software. The morphologies and elemental composition of the LDs samples before and after adsorption were studied by field-emission scanning electron microscopy (FESEM, S-4800, Hitachi, Tokyo, Japan) equipped with an energy-dispersive X-ray analyzer (EDX, Oxford INCA). All samples were ultrasonically dispersed with absolute ethanol at 25°C for 2min and dried on carbon-coated copper grids. The microstructure information was obtained by high-resolution transmission electron microscope (HRTEM) and selected area electron diffraction (SAED) with an accelerating voltage of 200kV (JEM-2100, JEOL, Japan). Nitrogen (N2) adsorption–desorption isotherms, including the Brunauer–Emmett–Teller (BET) surface area, the Barrett–Joyner–Halenda (BJH) pore diameter and pore distribution were determined by a Micromeritics ASAP2000 V3.01 analyzer.
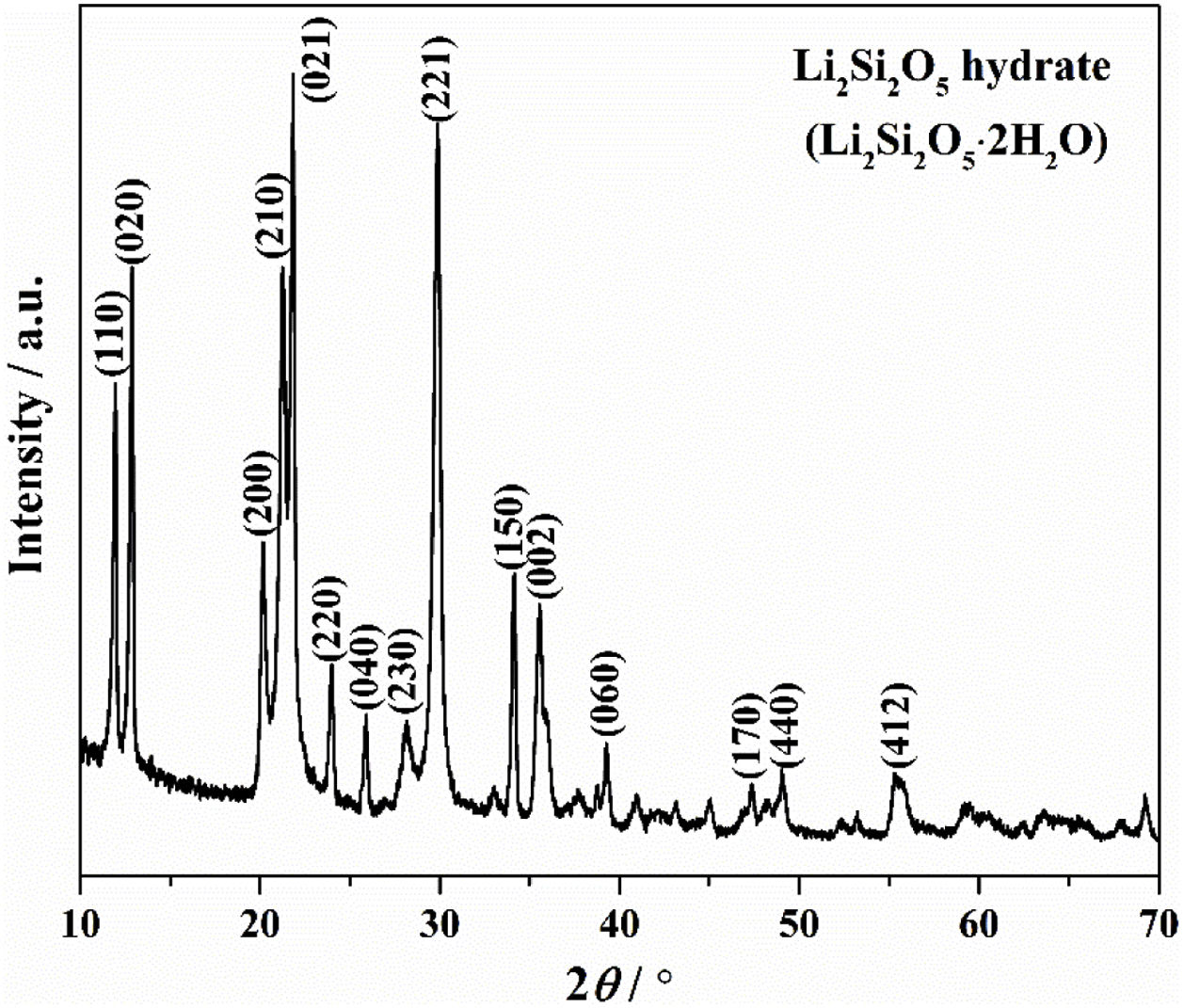
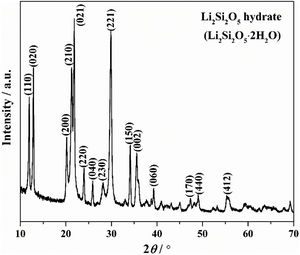
Results and discussionThe crystallinity and crystalline phase of the as-prepared LDs was characterized by XRD. The XRD pattern of the LDs sample prepared by using 0.25g CTAB is shown in Fig. 1. All peaks showed the characteristics of well-crystallized Li2Si2O5 hydrate (Li2Si2O5·2H2O) correspond to ICDD card no. 33-0816, and the main characteristic diffraction peaks at 2θ of 11.87°, 12.84°, 20.12°, 21.24°, 21.77°, 23.9°, 25.8°, 28.04°, 29.76°, 34.06° and 35.45° could be assigned to (110), (020), (200), (210), (021), (220), (040), (230), (221), (150) and (002) crystal planes, representing a high crystallinity of 98.12 (R=7.15)%. This indicated that orthorhombic Li2Si2O5 hydrate with an ideal chemical formula of Li2Si2O5·2H2O was the only crystalline phase existing in the obtained LDs, which could be concluded that CTAB mediating achieved the crystallization and purity of lithium silicates.
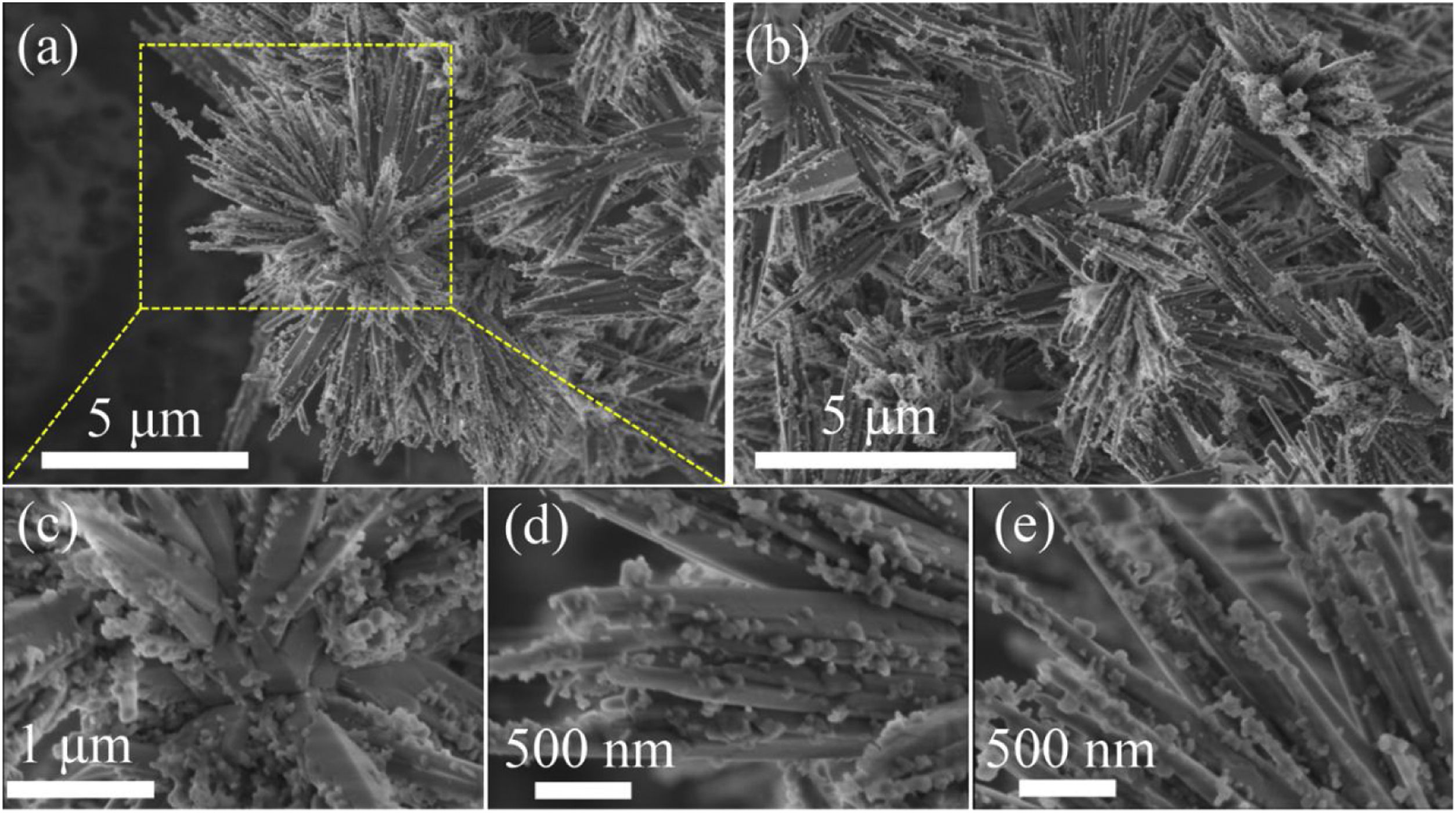
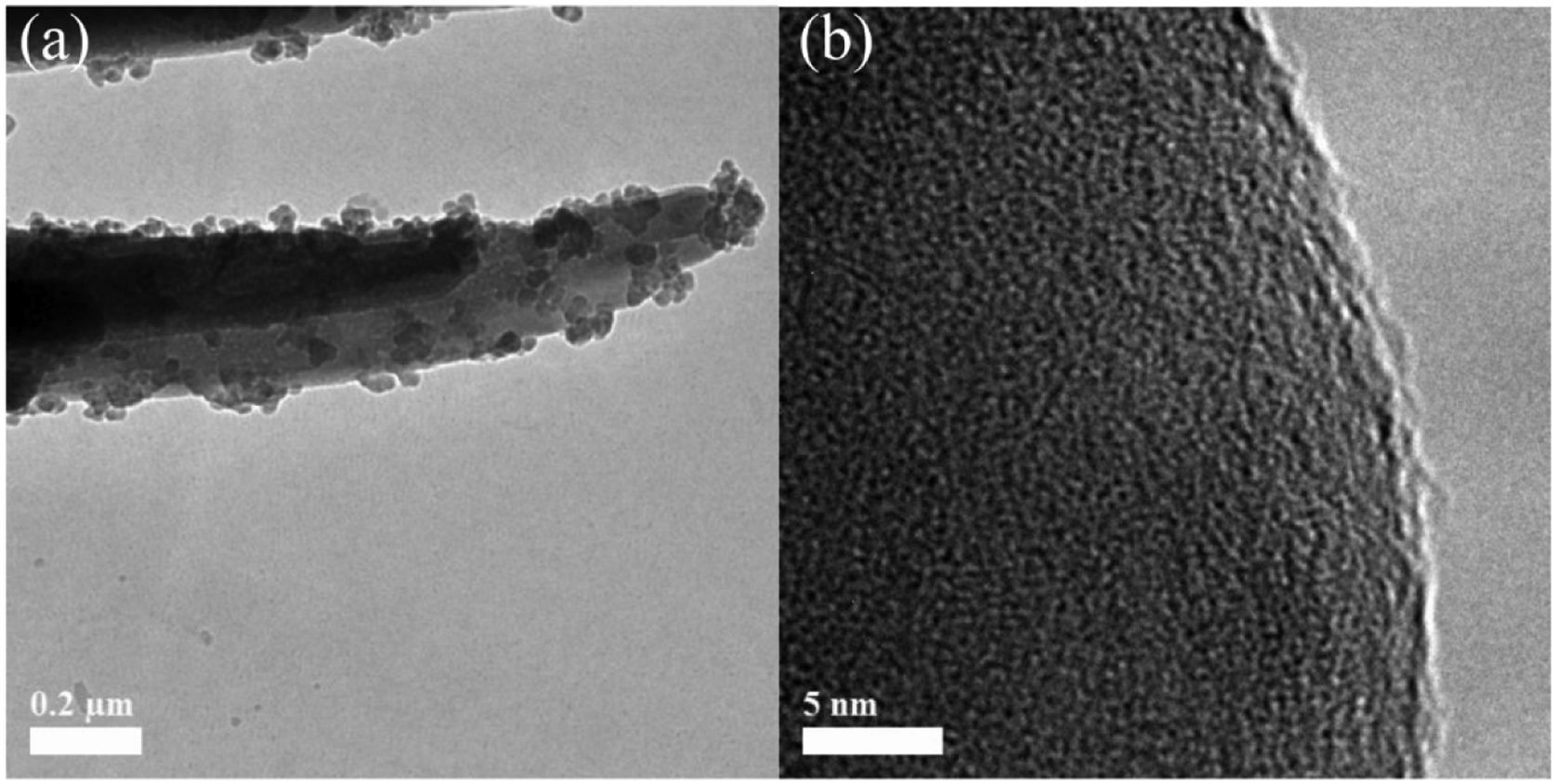
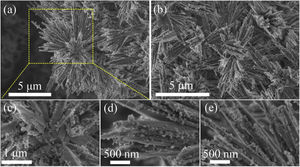
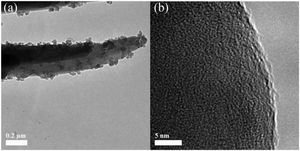
Fig. 2 shows the SEM micrographs of the as-prepared LDs. From the low-magnification micrographs of Fig. 2a and b, it could be observed that the final product was nearly monodisperse and composed of large uniform and branched particles with lengths in the range of 7–10μm. The enlarged SEM micrographs (Fig. 2c–e) of the center and end of the single particle in Fig. 2a revealed that the branching structures consisted of nearly symmetric and scattering pyramidal rods with lengths in the range of 3.5–5μm and widths of 156–312.5nm, and the surface of the rods was rough and bestrewn with secondary nucleated LDs nanoparticles with diameters predominantly in the range of 50–100nm. This result was extremely different from our previous work [36], in which the final product, without using CTAB or other surfactants in Fig. S1 (Support information), presented hollow microstructure composed of only Li2Si2O5·2H2O rods with smooth surfaces. What is most interesting was that most of the nanoparticles were aggregated and dispersed on the tips and edges of the rods. This kind of feature might be attributed to the higher curvature and surface energy of the rod tips and edges than the flat surfaces [37,38], which was favorable to the adsorption of the nanoparticles. The morphology of one corresponding building block is shown in Fig. 3(a). It could be seen that the nearly triangular pyramid rod had a solid structure due to an obviously dark-gray center and edge and the length was about 7μm. The nanoparticles deposited on the surface and tip of the pyramid rod presented disordered aggregated structure with diameter of 20–80nm. These were in good agreement with the SEM results. Interestingly, some pores arising from the internanoparticle space were found and perpendicular to the surface of rod, which would facilitate the mass transport and might render the LDs particles good adsorption performance. However, it is worth noting that lithium disilicates are not stable under electron irradiation and they are very sensitive to the electron-irradiation during TEM characterization. This led to that the lattices and SAED pattern were not formed as shown in Fig. 3(b) in spite of the well crystallized particles confirmed by XRD in Fig. 1. In addition, these LDs particles had excellent mechanical stabilities. After ultrasonication for at least 30min, they all preserved their original morphologies as shown in Fig. S2 (Support information). This also implied the tight integration between the building blocks.
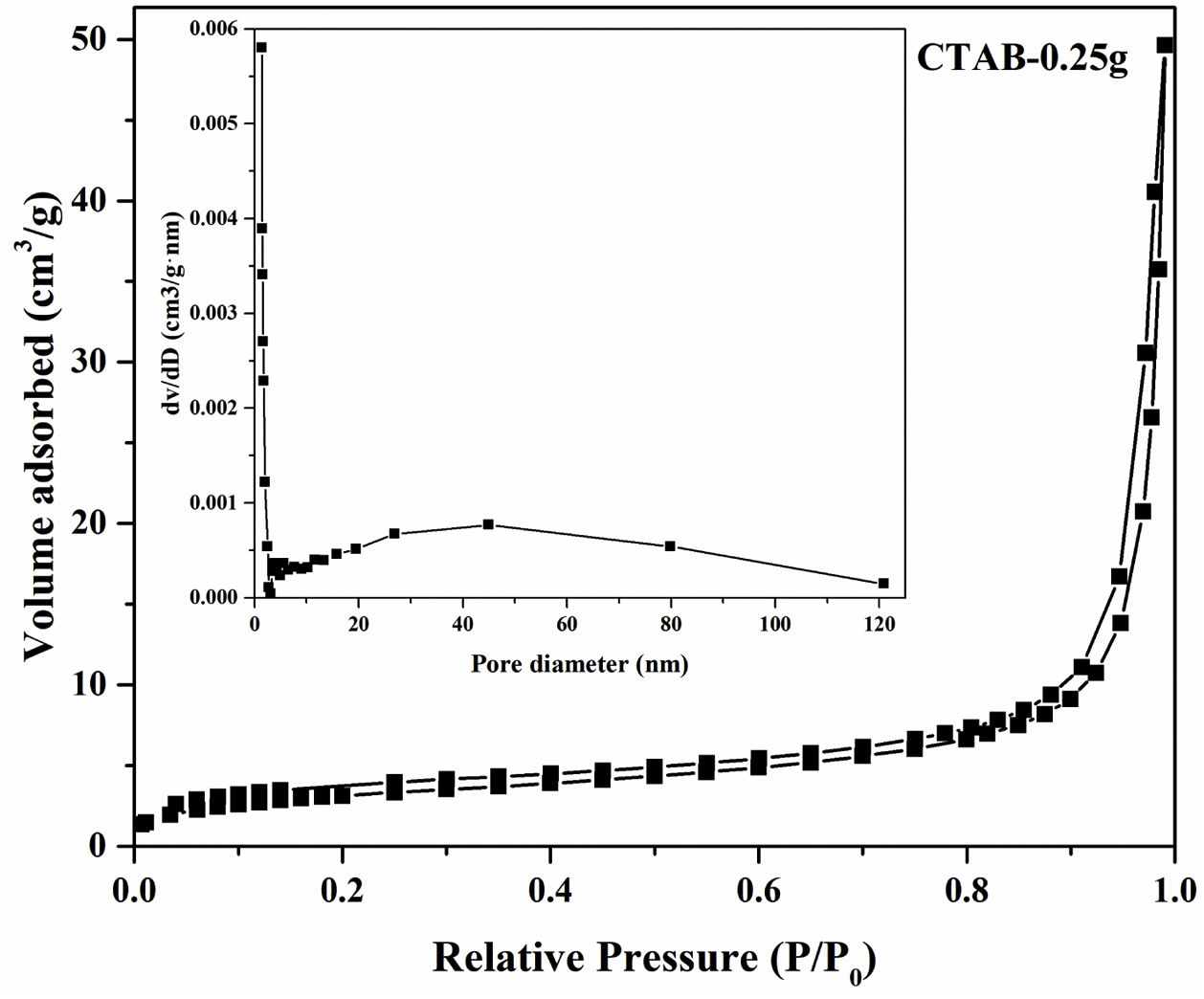
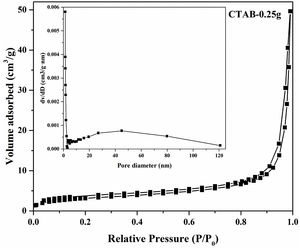
Moreover, the N2 adsorption–desorption isotherm and the corresponding pore size distribution (the inset) of the LDs particles is presented in Fig. 4. It could be found that the as-prepared LDs exhibited typical type IV isotherm with type H3 hysteresis loop [39] which was consistent with our previous work [40], indicating the mesoporous characteristics. Correspondingly, the Brunauer–Emmett–Teller (BET) surface area and pore volume were measure as 11.84m2g−1 and 0.077cm3g−1, respectively. The Barrett–Joyner–Halenda (BJH) pore size distribution showed the mesopores with an average diameter of 22.2nm, which was in accordance with the TEM results. It is noteworthy that lithium silicates, especially lithium metasilicate and disilicate had a nature of extremely low surface area because of the fast reaction processes to form their products [41]. While, this microscale mesoporous structure endows them still with a great potential as organic dye and heavy metal ions adsorbents used in wastewater treatment even their low surface area [36,42].
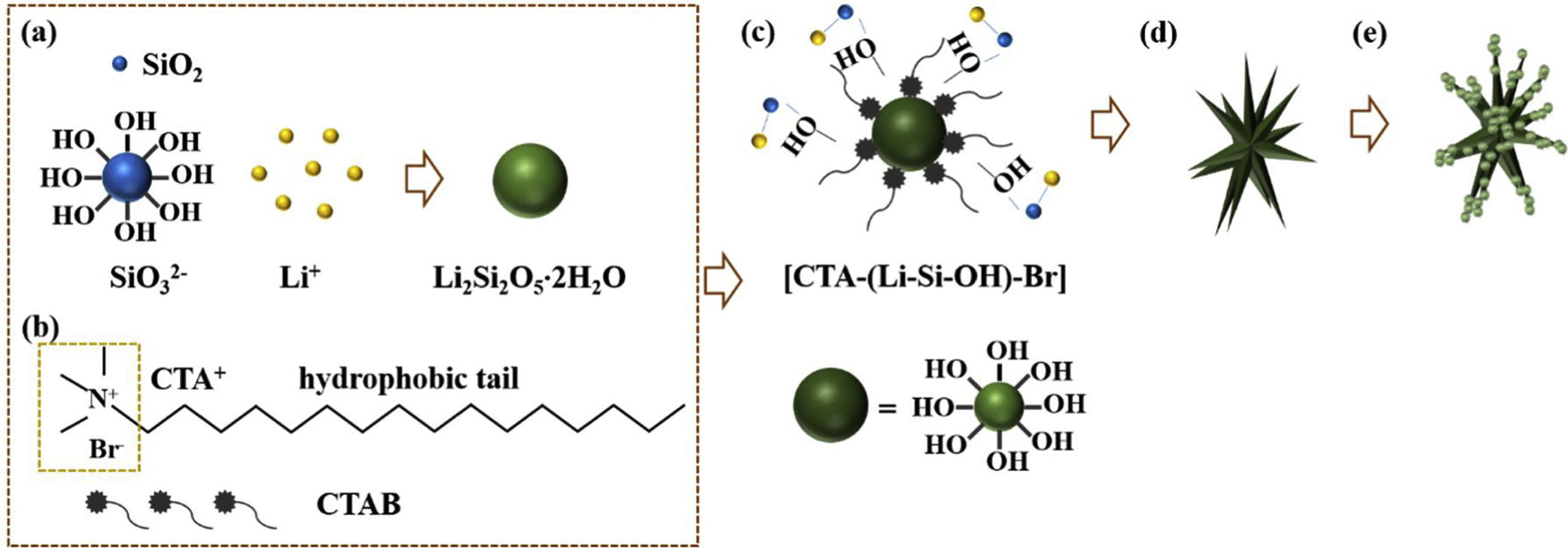
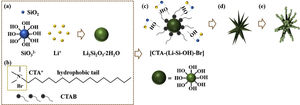
Based on the results aforementioned and results from our previous works and others [22,36,43–46], a speculative formation mechanism for the as-synthesized LDs particles was given as follows. Fig. 5 illustrates the corresponding procedure for the preparation of LDs particles. First of all, when TEOS was injected into the basic solution, SiO2 colloids were formed attributable to the fast hydrolysis and condensation of TEOS in the high pH alkaline environment that was provided by the dissolution of LiOH H2O. But the released OH− groups could break the silicon-oxygen chains of the SiO2 colloids so that the silicate ions were generated by the dissolved or etched silica displayed as Equation 4. Then LDs primary nanoparticles were quickly formed once the silicate ions met Li+ under the strong alkaline hydrothermal condition (Eq. (5)). These two procedures occurred almost simultaneously (Eq. (6)) and very fast due to the initial highly supersaturated degree, indicating rapid crystal seeding (Fig. 5a). After the rapid reactions, the concentration of OH− groups dropped caused by the consumption for the formation of primary LDs nanoparticles, and the crystal growth proceeded. CTAB is a cationic surfactant which could ionize completely into positively charged CTA+ with a tetrahedral head and along hydrophobic tail in water (Fig. 5b). As is known, silicates were negatively charged in water due to the surface consisting of broken bonds exist in the form of silanol (SiOH) groups [24,47]. So the [CTA-(LiSiOH)-Br] complexes were formed by a combination of the electrostatic attraction between negatively charged LDs nanoparticles and CTA+, or from the intermolecular H-bonding between SiOH and amino groups (Fig. 5c) [48–51]. Since most of the CTAB molecules were concentrated on the LDs surfaces, the growth of LDs primary nanoparticles mainly occurred along a certain direction, thus in this process, the cationic CTAB played a key role as a structure director facilitating the heterogeneous nucleation process on their surfaces while inhibiting homogeneous nucleation process in the bulk solution (Fig. 5d). Along with prolonging the reaction time, LDs particles composed of scattering pyramidal rods were formed by consuming most of the ions in bulk solution. At the same time, fast growth would bring surface defects on the pyramidal rods, so that some nanoparticles precipitated on their surface, especially on their edges (Fig. 5e) where offering high surface energy. Additionally, when the amount of CTAB increased to 0.5g, much more CTAB micelles formed in the bulk solution than those adsorbed on the surfaces of LDs and SiO2 nanoparticles. The increase of free CTAB proportion gave rise to the steric hindrance and the halide effects of bromide ions Br−[50], inhibited the growth of Li2Si2O5·2H2O and the diffusion of Li+ making Li+-rich region formed that resulted in the formation of Li2Si2O5·2H2O spheres and nanosheets aggregated flower like Li2SiO3 (Fig. S3a-b, support information) by Eq. (7)[42]. This result was in good agreement with the XRD results in Fig. S3c, showing that the crystal content of Li2SiO3 and Li2Si2O5·2H2O were 42.25 and 57.75wt.%, respectively. Therefore, LDs particles could be obtained by an appropriate CTAB surfactant-mediated formation process.
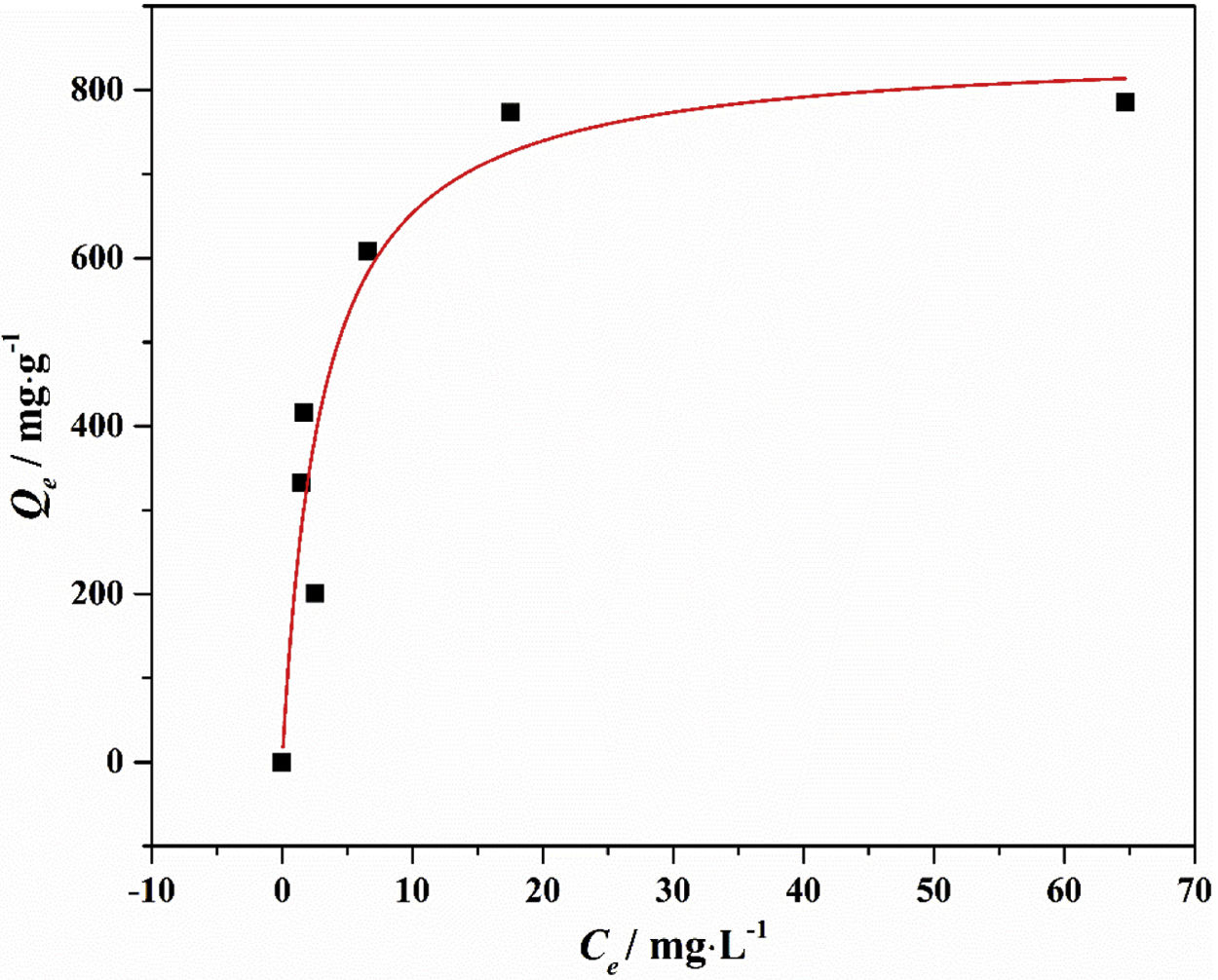
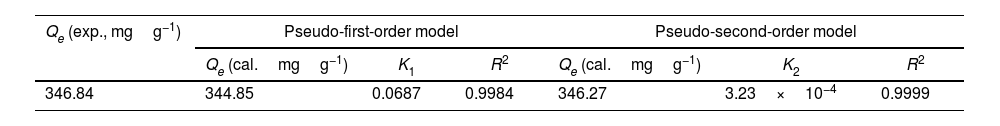
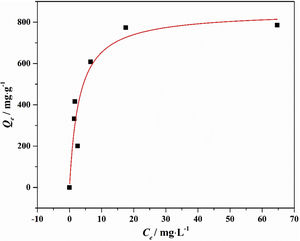
Drinking water contaminated by or rich in Mn2+ ions beyond the limit of 0.1ppm is regarded as a severe environmental problem that threatens public health. The nanostructured silicates have great potential in the treatment of wastewater by virtue of their low cost, stable and environmental benignity. In this way, LDs may be suitable for extracting Mn2+ ions from wastewater. So MnSO4·H2O was employed as the model pollutant and added to water. The LDs samples were then introduced as the adsorbents to remove Mn2+ ions without adjusting pH value. To investigate their adsorption capacity, the adsorption isotherm as a function of Mn2+ concentrations (Ce values) was measured. The adsorption data fitted the Langmuir adsorption isotherm well (Eq. (8)[52]) according to the correlation coefficient R2=0.8125, the fitting curve and related parameter were shown in Fig. 6 and Table 1. The established Langmuir model certified that Mn2+ ions were homogeneously adsorbed in mono-layer by the surface of LDs. The adsorption capacity increased from 200.9873 to 785.2542mgg−1 with the increase of initial concentrations due to that the higher concentration could provide more adsorbate to adsorbent. The capacity values were substantially higher than the ones of other reported silica (4.2mgg−1) or biochar (163.194mgg−1) adsorbents without pH [52,53]. Apparently, the high capacity Qm and b value (related to the binding strength) indicated that Mn2+ was favorably adsorbed onto LDs. Hence, LDs particles were appropriate for Mn2+ adsorption.
where Qm (mgg−1) is the maximum adsorption capacity; Qe (mgg−1) is the equilibrium adsorption capacity; Ce (mgL−1) is the equilibrium solution concentration; b (Lmg−1) is the Langmuir adsorption constant related to the interaction bonding energy.Adsorption performance of LDs particles at different Mn2+ concentrations and its Langmuir isotherm parameters.
| C0 (mgL−1) | Ce (mgL−1) | Qe (exp., mgg−1) | Langmuir parameters | ||
|---|---|---|---|---|---|
| Qm (mgg−1) | b (Lmg−1) | R2 | |||
| 50 | 2.567 | 200.9873 | 851.9594 | 0.3295 | 0.8125 |
| 80 | 1.482 | 332.7034 | |||
| 100 | 1.71 | 416.4831 | |||
| 150 | 6.577 | 607.7246 | |||
| 200 | 17.51 | 773.2627 | |||
| 250 | 64.68 | 785.2542 | |||
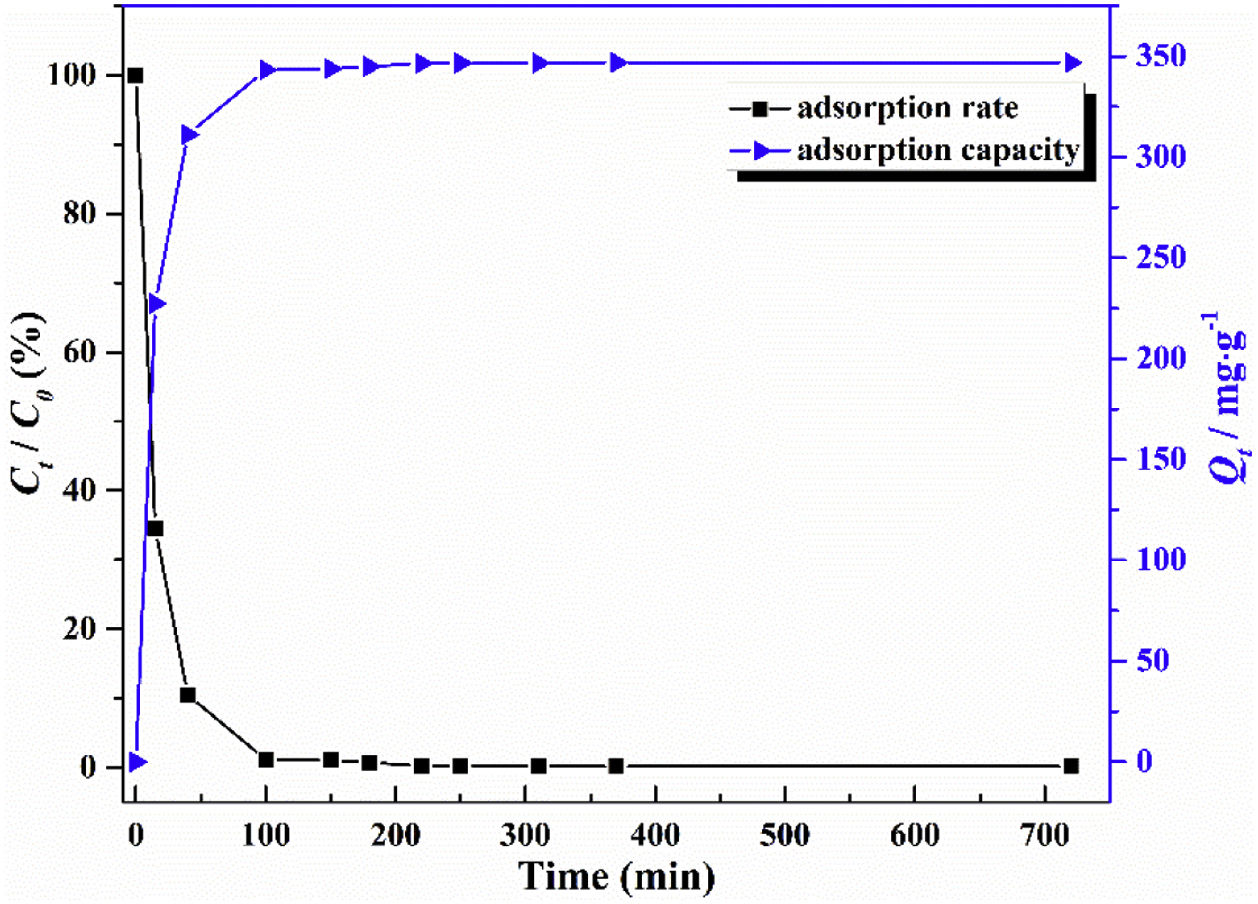
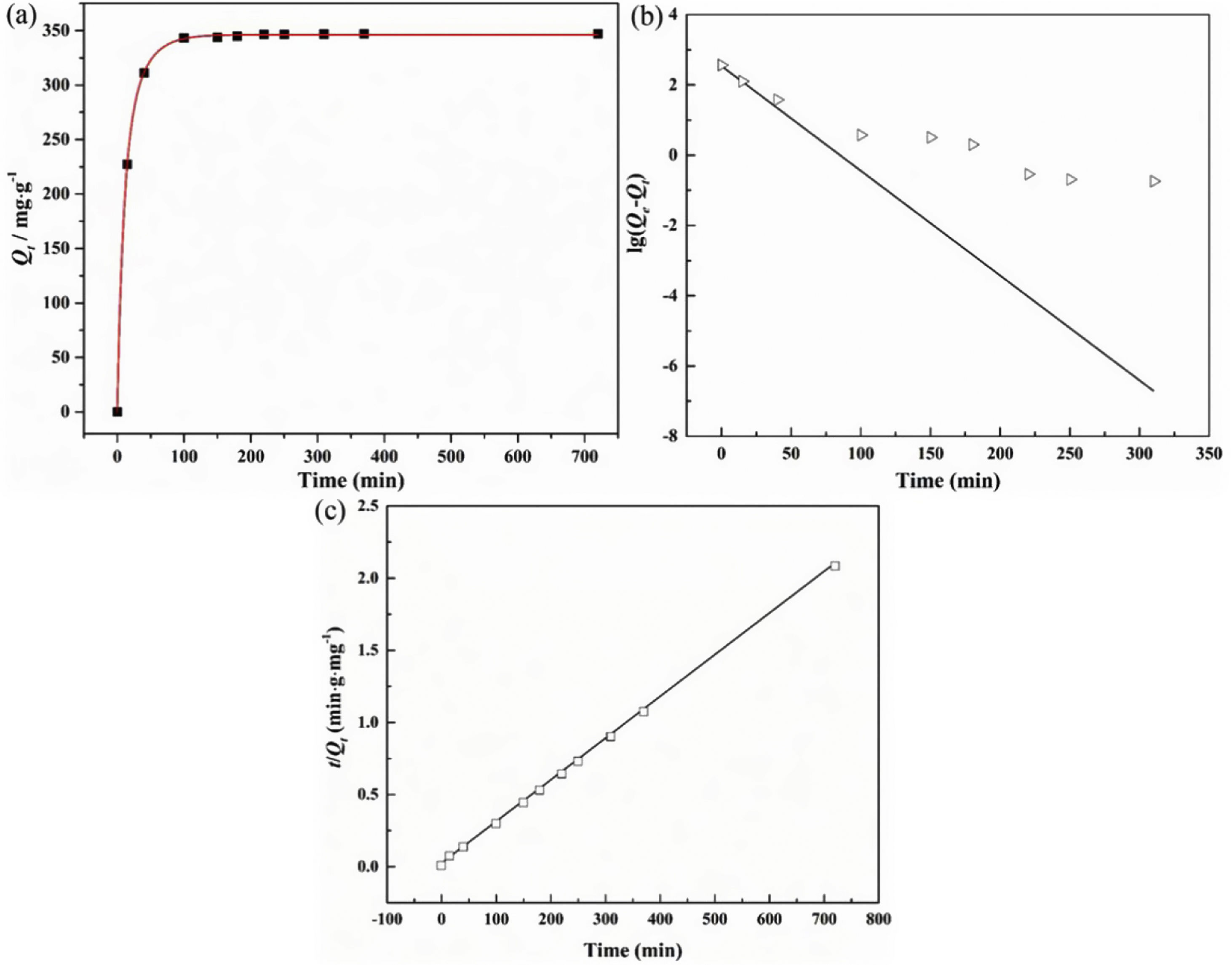
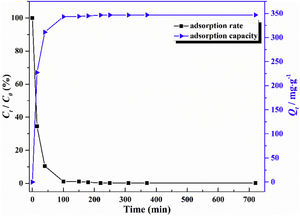
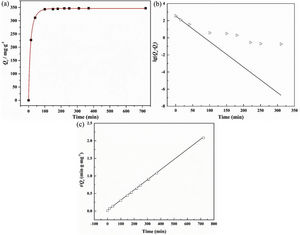
The adsorption capacity and rate of LDs toward 82mgL−1 Mn2+ solutions were measured with contact time, as shown in Fig. 7. During the first 100min, the adsorption process was very rapid, after which the adsorption rate and capacity toward Mn2+ achieved equilibrium. This tendency was likely to be attributable to the fast decrease of available binding sites on the surface of LDs. The removal efficiency at equilibrium reached up to 99.82% and its corresponding adsorption capacity attained 346.84mgg−1. The kinetic pseudo-first-order and pseudo-second-order models (Eqs. (9) and (10)) were used to describe the adsorption kinetics. The related fitting curves of corresponding models and kinetic adsorption parameters were displayed in Fig. 8 and Table 2, respectively. The pseudo-first-order model was used to simulate the adsorption process in liquid and the solid phase on the adsorbent surface, while the pseudo-second-order model described the chemical adsorption process involving chemical bonding between metal ions and functional groups on adsorbent surface showing a rate-controlling [22,54].
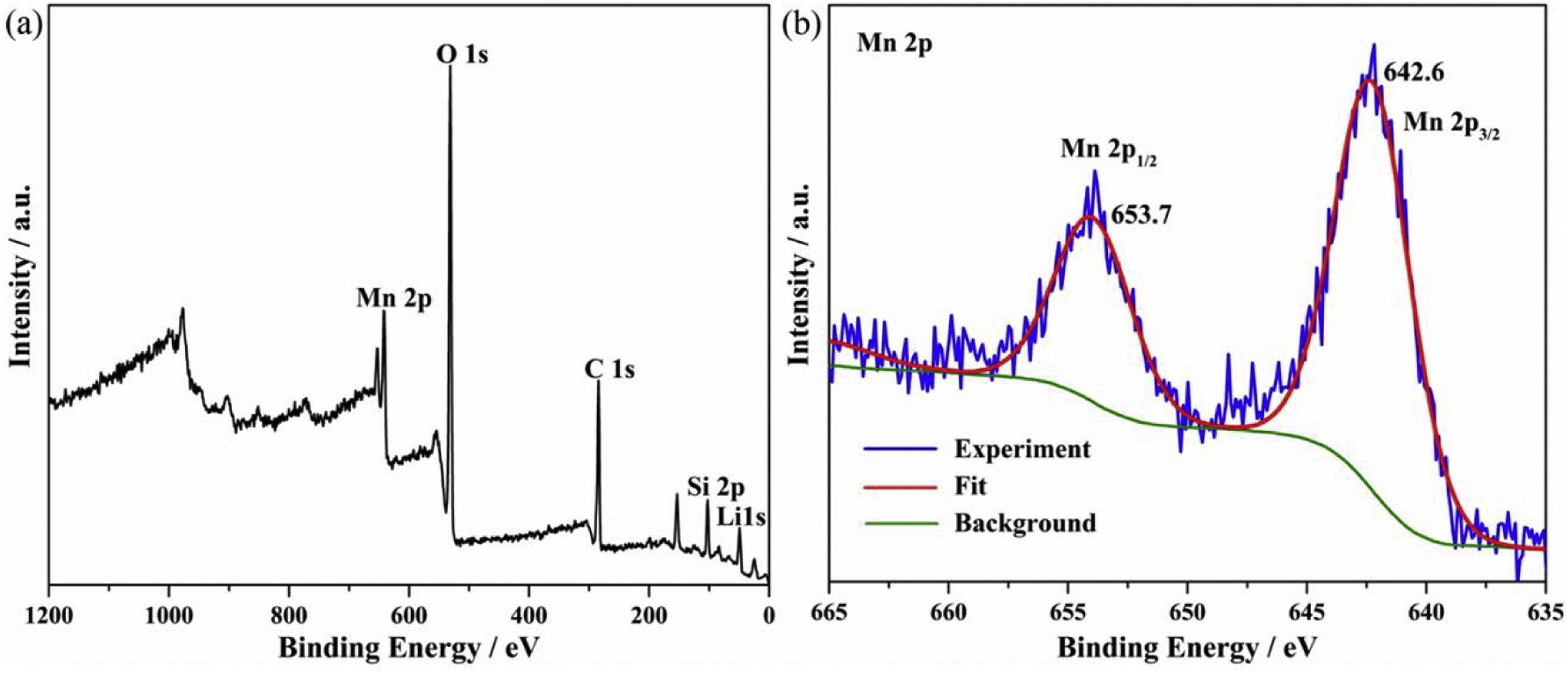
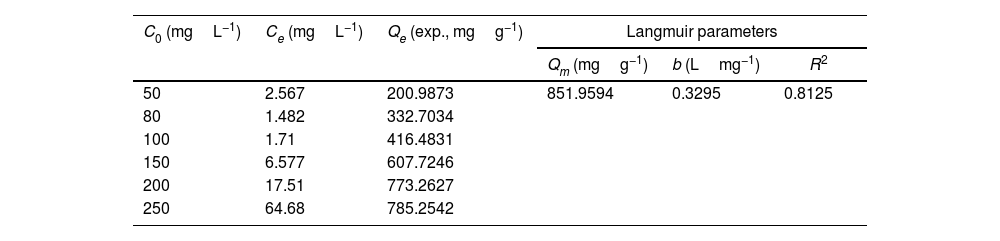
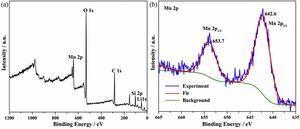
where K1 (Lmin−1) and K2 (gmg−1min−1) are the adsorption rate constants for pseudo-first-order and pseudo-second-order models, respectively.According to the higher R2=0.9999 and the closer values between the theoretical adsorption capacity and the experimental ones, the kinetic data fitted better in the pseudo-second-order model, revealing that the Mn2+ adsorption process was controlled by chemisorption. This could be also confirmed by XPS analysis. Fig. 9 shows the XPS survey spectrum of LDs after Mn2+ adsorption and its high-resolution spectrum toward Mn 2p, the binding energies obtained in XPS analysis were corrected for specimen charging by the C 1s peak to 284.6eV. From the survey spectrum in Fig. 9a, elements of Mn, O, Si, C (standard peak) and Li were clearly observed, confirming the adsorption of Mn2+. In Fig. 9b, Mn 2p3/2 and Mn 2p1/2 peak signals were observed in binding energies of 642.6 and 653.7eV, respectively. The fitted Mn 2p3/2 peak suggested that the manganese was completely in the Mn4+ oxidation state because the adsorbed Mn(OH)2 was oxidized to MnO(OH)2[55–57]. As previously mentioned, the surfaces and the edges of LDs particles were negatively charged with SiOH groups, which had superior affinity to Mn2+ ions due to the electrostatic attraction between negatively charged OH− and Mn2+. Undoubtedly, the mesoporous structure of LDs increased the amount of surface active adsorption sites and facilitated the chemisorption process toward Mn2+ in spite of its low surface areas. Thus, it was inferred that the CTAB-mediated synthesized LDs particles with mesoporous structures could be used to remove Mn2+ or other cation metal ions in wastewater. Meanwhile, since surface area and pore structure were two critical factors for adsorbents, we would keep trying to fabricate lithium disilicates with good pore structures and high surface areas in future research.
ConclusionsIn summary, we developed a facile cation surfactant CTAB-mediated strategy to fabricate mesoporous LDs with a hydrothermal method. In this approach, CTAB was used as a morphology agent to control the growth of LDs. The LDs particles exhibited excellent performance as adsorbents of Mn2+ in wastewater treatment. The adsorption capacity was up to 346.84mgg−1 with corresponding removal efficiency up to 99.82% when Mn2+ concentration was 82mgL−1, and the maximum adsorption capacity reached up to 785.25mgg−1 toward Mn2+ of 250mgL−1. The isotherm and kinetic fitting results indicated that the adsorption process could be well followed the mono-layer chemisorption due to the electrostatic attraction between negatively charged SiOH groups on the surfaces and cation Mn2+ ions, which was well confirmed by the XPS results. It could be concluded that the LDs showed great potential in the remedy of Mn2+-containing wastewater. The approach is facile, effective and green without requiring intricate procedures, instruments and harmful chemicals. With a rational improvement, we expect that a modified CTAB-mediated strategy can be extended to prepare lithium disilicates with higher BET surface area rendering them wider applications in environmental protection.
Conflicts of interestWe confirm that there is no conflict of interest for submitting this manuscript and that the manuscript has been approved for publication by all authors. The work described is original research and has not been published before, nor has it been considered for publication in whole or in part elsewhere. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors thank the Research Laboratory of Advanced Powder Metallurgy at Xi’an University of Technology, for providing the preparation equipment. This work was supported by the innovation of Xi’an science and technology project (Project No. 21XJZZ0042).