Several inorganic materials such as bioactive glasses, glass–ceramics and calcium phosphates have been shown to be bioactive and resorbable which make them suitable for coating bone implants. This study focuses only on bioactive glasses. These biomaterials are highly biocompatible and can form a strong chemical bond with the tissues. This review comprehensively covers bibliographic reports that have investigated bioactive glass deposition using different thermal spray techniques.
The main drawback for the glass coating deposition is the low adherence with the substrate. Some strategies can favour a good bond such as using bond coats, blends, pre-heating the substrate or modifying the glass composition.
The characteristics of the feedstock powders are determinant for the properties of the coatings obtained. Porosity and thickness of the coatings can be modulated by using different thermal spray techniques and varying parameters of the process.
The degradation rate of some bioactive glasses can achieve kinetics similar to the new bone formation. Taking advantage of its dissolution capacity, glasses can be doped with functional elements.
While several biological studies have been performed with bioactive glass materials, there has been relatively little research on the biological response of coated glasses by thermal spray techniques. Research studies have demonstrated the opportunities of this promising material to enhance the bioactivity of the implants.
In recent decades there is an increase of life expectancy which is associated to age-related diseases. Traumatic injuries and pathological diseases such as osteoporosis or osteoarthritis affect bone function causing pain to the patient and also damage and fractures to the bones.
Bone represents the second most common tissue implanted in the body after blood [1]. It has an excellent healing response when damaged, recovering both functional and structural properties. Notwithstanding severe damage to the bone implies the need of surgery to recover.
Most biomaterials and medical devices perform satisfactorily, improving the quality of life for the recipient. However, all manufactured devices have a failure rate affecting several patients annually [2,3]. The demand for primary and revision surgery related to bone diseases are increasing last decades and represent a high cost to the health system [4–6].
So bone repair remains an important challenge in the field of orthopaedic and craniofacial surgery.
When designing an implant is important to consider special requirements: geometry, mechanical properties, the tissue–implant interaction, the anatomical site of the implant, etc. Besides human tissue is very sensitive to foreign substances, and the body can promote a rejection response.
Biomaterials that provide the structural support are required for replace skeletal hard connective tissues. In 1890 the surgeon Themistocles Gluck implanted the first total joint replacement, a hinged ivory prosthesis for knee [7]. Lane introduced a metal plate for bone fracture fixation for the first time in 1895, however it was of current steel and corrosion occurred. It was not until 1926 that a stainless steel was discovered and used in the internal fixation of fractures which remain uncorroded for years [8,9]. In recent times, titanium alloy, cobalt–chromium alloy, stainless steel, zirconia and aluminium oxides are the main biomaterials used for orthopaedic implants [3,10,11].
Implant failure analysis studies of the devices have been performed in order to modify the designs. These have evolved much over the last century, getting reasonable long-term viability for the current devices in the market. Surgeons and researchers still work hand by hand to improve them.
The most common failure mechanism is due to loosening. A poor osteointegration is responsible of the undesired mobility that causes loosening. To obtain a good fixation is required biological and mechanical stability by the formation of a structural and functional interface between the device and the surrounding bone. Also the presence of pathogens can cause biological reactions after the implantation of the device [12].
Aseptic loosening occurs at long term and is due to the mechanical failure of the device, this mechanism represents the 75% of the failures. While septic loosening is due to pathogens such viruses and bacteria, this mechanism represents the 7% of the failures and occurs at early stage [13]. Other common causes of failure are the release of wear and corrosion particles into the body and fracturing of the device due to fatigue or creep (for polymeric components at early stage). The survivability of the implant also depends on the patient, for example wearing is more frequent in younger and more active patients.
To diminish these problems that can lead to failure surface devices can be modified.
The goal of the present article is to provide a literature review of the most relevant findings on the topic of bioactive glasses coatings obtained by thermal spray in order to clarify the current status of this strategy for improve biomedical implants. In this review, the effect of features related to the spray processes will be commented, such as the range of the particles sprayed and the raw material as powder or as suspension. Different approaches for achieve a good bond between substrate and coating will be introduced. The main features of the coatings will be discussed, with particular focus on coating thickness, porosity and bioactivity in simulated body fluid. Moreover, post treatments to modify the coating properties will be remarked. Finally, studies related to the biological behaviour of the coatings in vitro and in vivo will be presented.
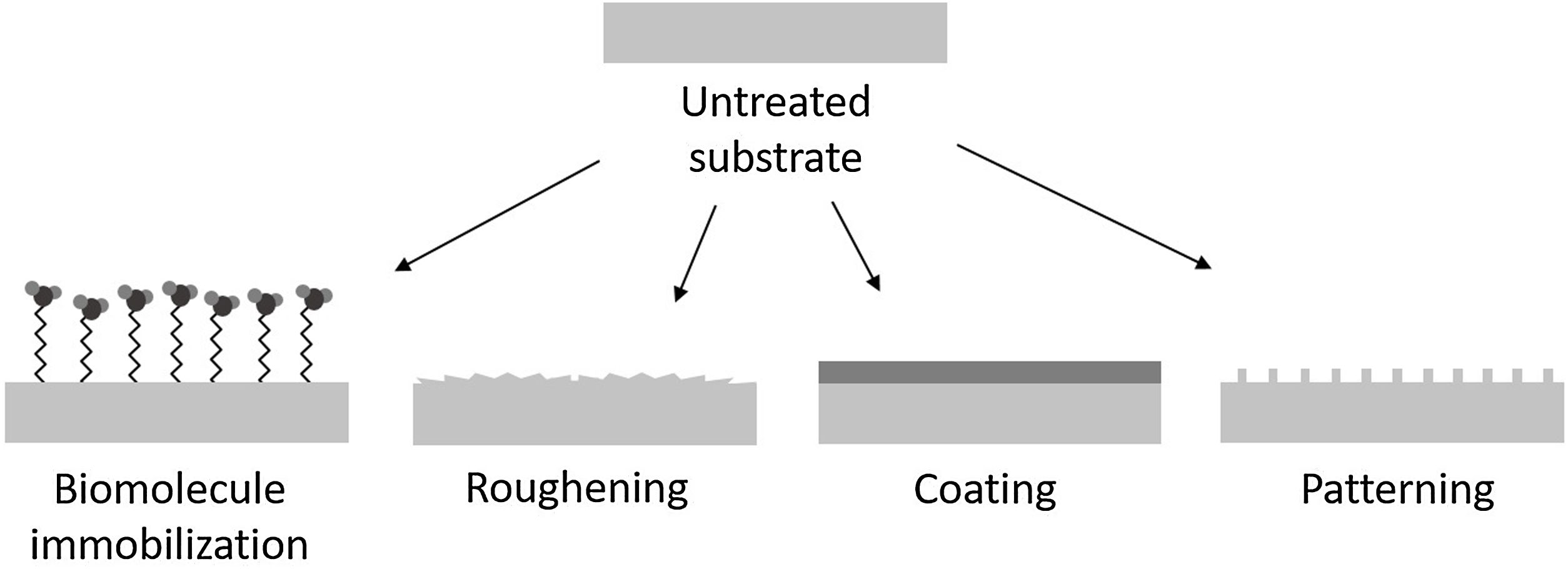
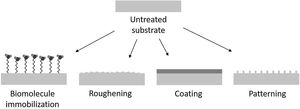
Functionalization via surface modificationsSurface modifications are used for improving biological response of the implants. With this strategy, the structural support provided by the substrate is maintained. Among the different possibilities the methods can be broadly divided into three categories: (1) chemically or physically altering the atoms, compounds or molecules in the existing surface (chemical modification, etching, mechanical roughening), (2) overcoating the existing surface depositing materials with a different composition (coating, grafting, thin film deposition) and (3) creating surface textures or patterns (Fig. 1).
The similarity in composition, structure and morphology of the calcium orthophosphates to bone tissue make them good candidates for improve implants. Particularly, hydroxyapatite (HA) has been used for coating biomaterials due to the similarity with the inorganic mineral phase of the bone.
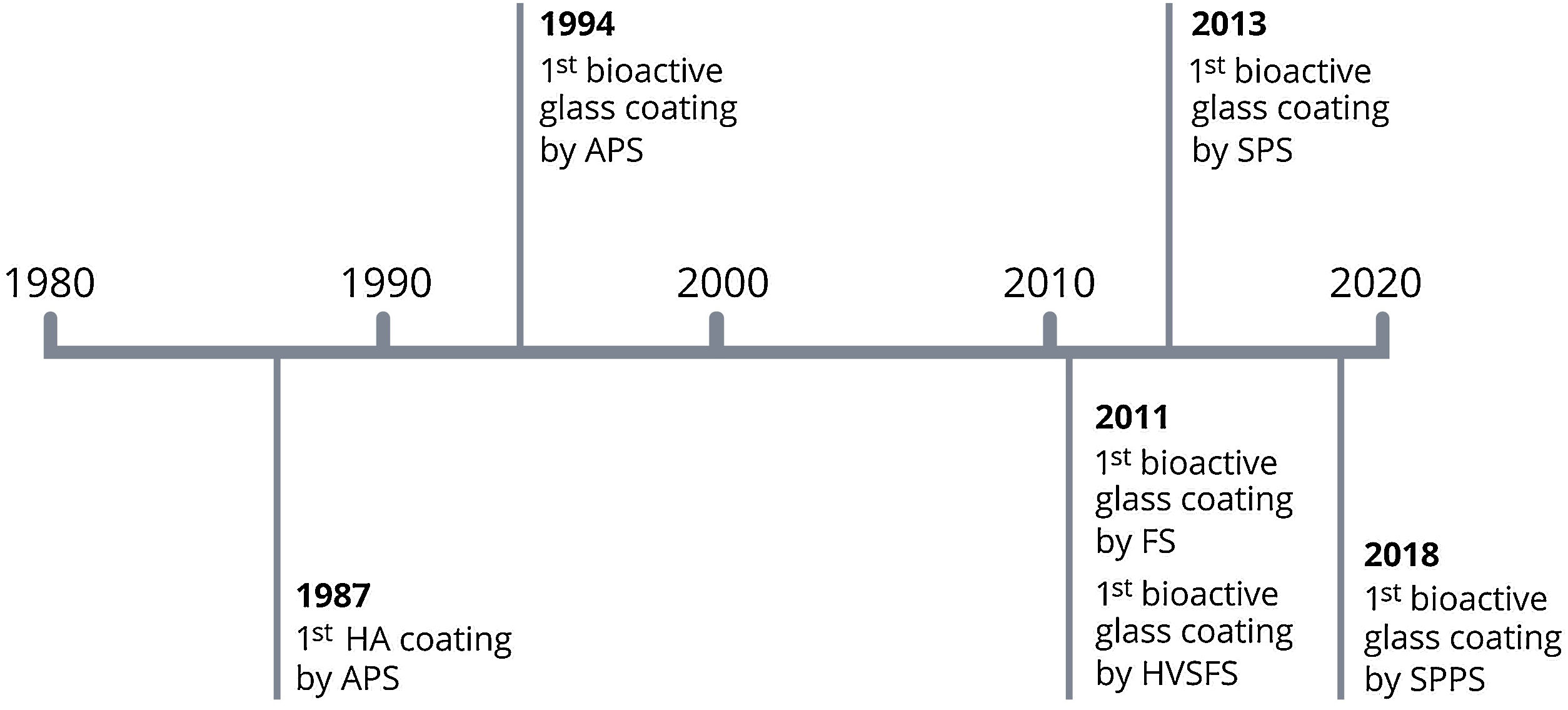
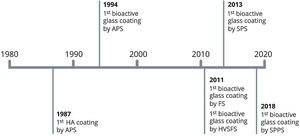
First studies of sprayed HA coatings started in the 1980s. In Netherlands, Geesink and co-workers reported some research studies with promising results [14–16], while Furlong and Osborn in the United States started also that research at the same period [17,18]. The first clinical trials with HA coated implants started in Europe in 1987 and some months later, in 1988, in the United States with a coated femoral component (Omnifit-HA, Osteonics Corporation, Allendale) [19–25].
HA coatings enhance the bone formation on orthopaedic implants [26–28]. First generation of HA coatings were thick and some adverse events were reported [29–31], current coatings are thinner and more uniform.
Hydroxyapatite coatings can be prepared by different techniques such as thermal spray, sol–gel, dip coating, electrophoretic deposition, dip coating, pulsed laser deposition, etc. [32]. Nowadays only plasma spray is commercially accepted by the Food and Drug Administration (FDA) for producing HA coatings. The coatings should accomplish specific requirements, such as the tensile adhesion strength that shall have a value not less than 15MPa, the Ca/P atomic ratio between 1.67 and 1.76, a crystallinity ratio major or equal to 45% or the content of harmful metals below 50mg/kg, with a lower value for some specific elements (arsenic, cadmium, mercury, lead) [33].
HA offers a good bonding to the bone, however other bioactive materials can provide osteoinductive properties and a strongly osteointegration with the implant surface.
In the late 1960s bioactive glasses were developed by L. Hench, a particular range of glass compositions that react in physiological environment [34]. These glasses bond to the bone by the formation of a hydroxyl carbonate apatite (HCA) layer and also promote bone cell growth along its surface. Moreover, the dissolution products of bioactive glasses can stimulate cellular differentiation [35].
In the last decades researchers have studied several compositions inspired in the first one developed, the 45S5, a highly reactive glass in the SiO2–Na2O–CaO–P2O5 system. Some of the most common constituents used for developing formulations are Al2O3, B2O3, MgO, K2O or CaF2, which have been added with particular purposes in any case [36–41]. The bioactivity of a glass largely depends on its composition and surface reactivity, modifications should be analyzed carefully since small variations can affect notably its properties.
The connectivity of the silicate network affects directly the dissolution rate of the glass. More disrupted networks make glasses more susceptible to degradation and then more bioactive. If the connectivity network is too high glasses are not bioactive, as the reactivity is promoted by the non-bridging oxygens of the open silicate network. Then connectivity can be diminished adding network modifiers such as sodium and calcium.
Bioactive glasses have gained a place in the market, mainly as bone grafts for orthopaedic and dental uses to regenerate and heal bone defects from trauma or tumour removal [42–45]. But they also can be found as an attractive active component in toothpaste for reducing sensitivity in teeth [46–48].
Currently, there are many researchers investigating their use as scaffolds because of their osseous regenerative capacity, but further studies are still required before the translation to clinical trials [49].
Both bioactive glasses and HA are fragile materials, therefore their use as a bulk is not suitable. One more interesting application for bioactive glasses is the coating deposition in a similar approach to HA coatings, in this way can be used for load bearing applications.
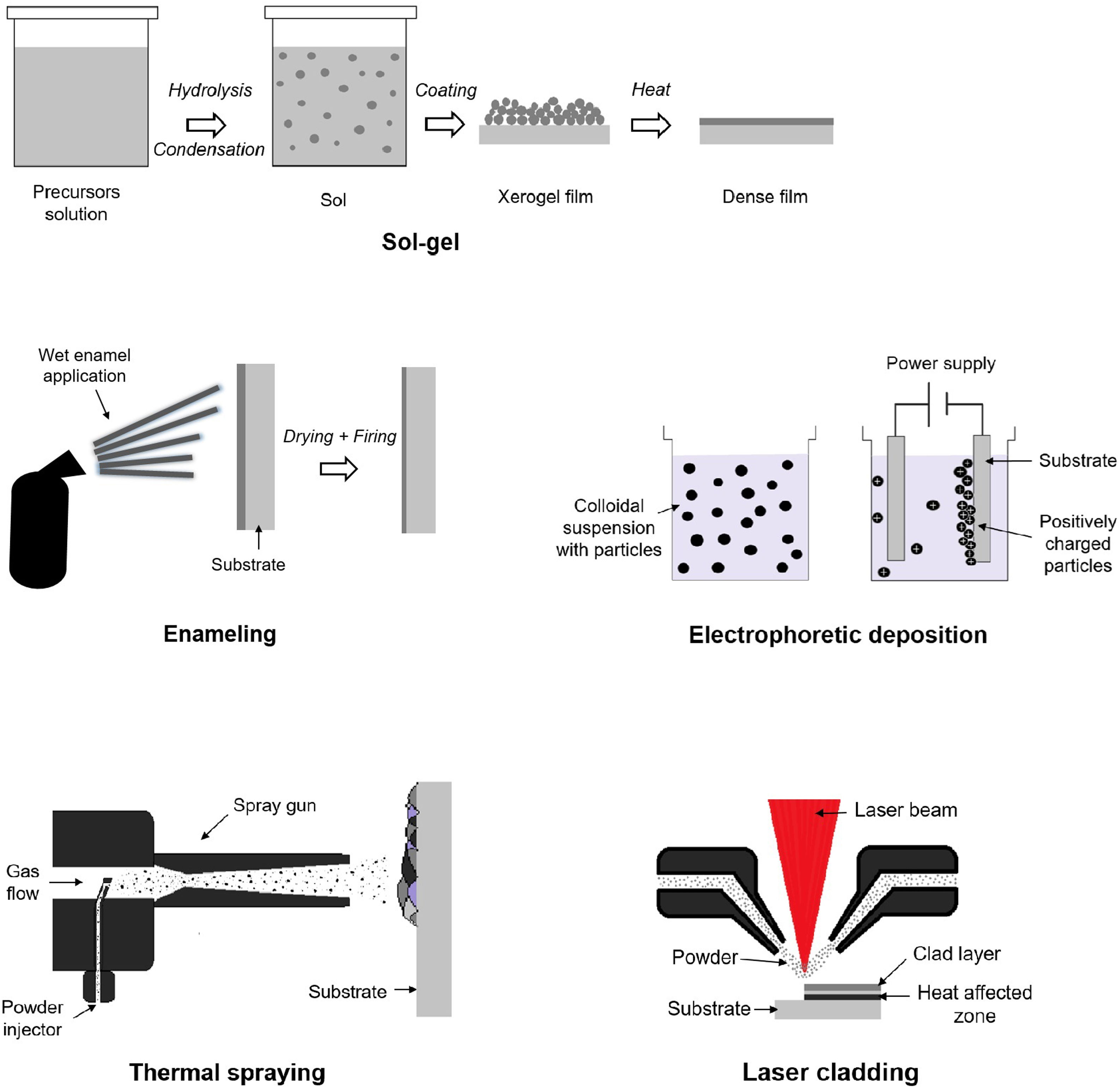
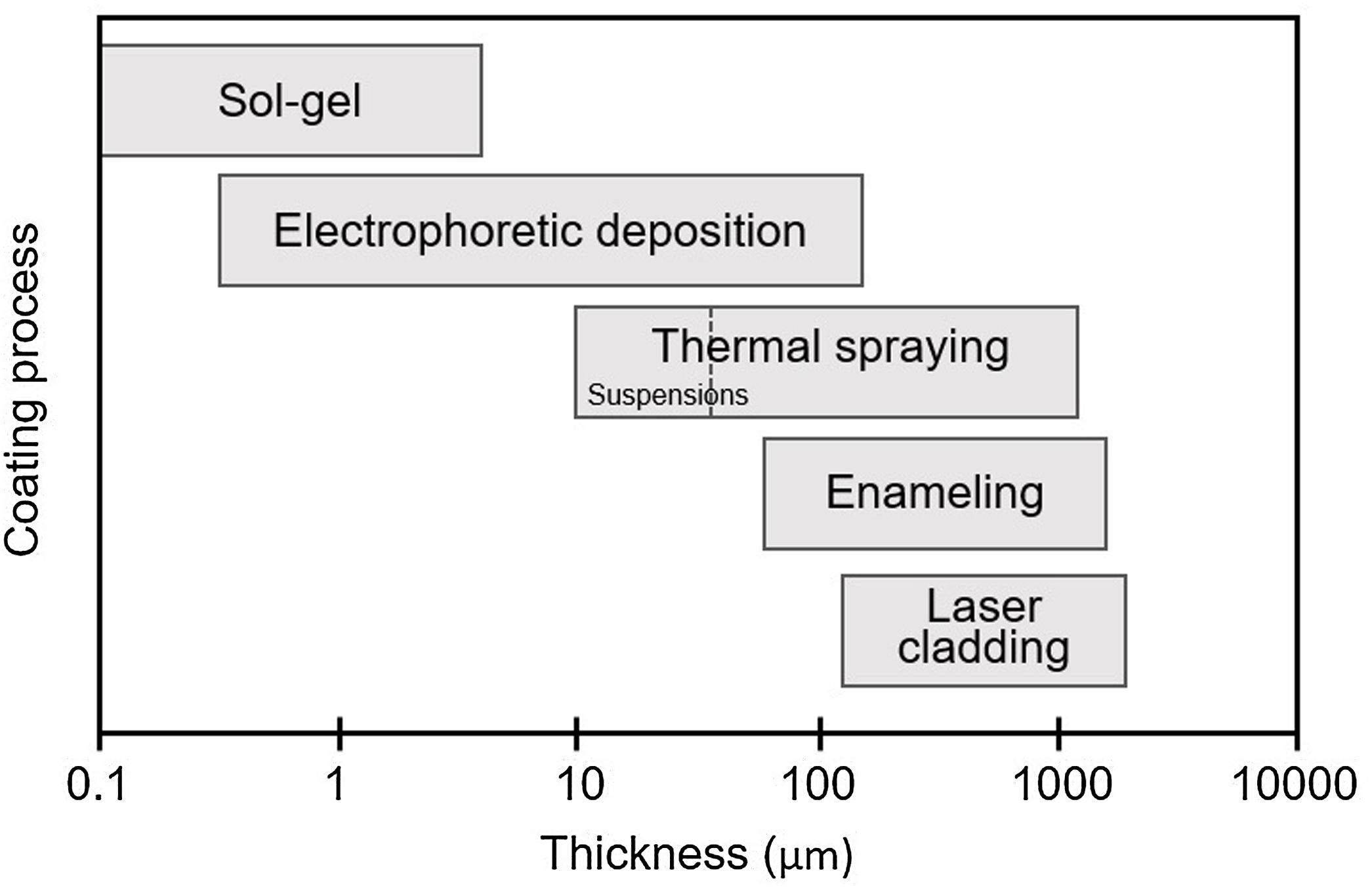
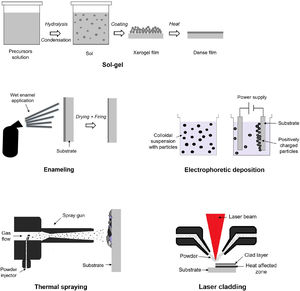
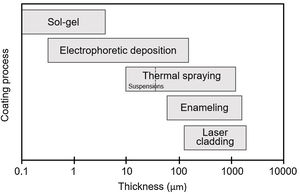
Different strategies can be used for obtain the bioactive glass coatings, like sol–gel, laser cladding, enamelling, electrophoretic deposition and thermal spraying (Fig. 2). The main disadvantage of all these methods is the poor adhesion strength of the coating to the metallic substrate, in part because of the mismatch of the thermal expansion coefficients. Being one of the main challenges, achieving coatings that comply with the regulations to be inserted into the body. In addition, each of these techniques is capable of producing coatings in a different thickness range (Fig. 3). In particular, thermal spraying provides a wide and interesting range for this application.
Functionalization by thermal sprayThermal spraying are coating processes where particles are melted (or partially melted) and deposited onto the substrate in the form of flattened drops that pile on each other to produce a layered coating [50–53].
The conventional techniques atmospheric plasma spraying (APS) and flame spraying (FS) have been used for produce coatings with bioactive glass materials [54–57] (Fig. 4).
In the APS process a high temperature ionized plasma gas acts as heat source. The raw material, usually in powder form, is carried in an inert gas into the plasma jet where is heated and accelerated towards the substrate. The high temperature achieved during the process allow spraying materials with high melting points. Moreover, the high cooling rate of the particles can preserve the amorphous nature of the feedstock. The features of the APS process make it suitable for manufacture coatings with bioactive glasses.
FS is a process in which the heat from the combustion of a fuel gas (acetylene or propane) with oxygen is used to melt the feedstock material, the material is heated and propelled onto a substrate. The flame temperature and velocity is lower than for APS. Few research of bioactive glass coatings involving this process can be found in the literature [56,57].
Suspension spraying is a particular group of thermal spray processes that differs from conventional ones by the use of liquid suspensions instead of dry powders as feedstock material while using the spray torches of the conventional techniques [58].
Suspension plasma spraying (SPS) was developed before the high velocity suspension flame spraying (HVSFS), for this reason there is more research done with SPS process [59]. While HVSFS has been investigated by only a few research groups. By contrast more research with bioactive glass materials has been performed by HVSFS as can be appreciated in this review. In a comparative study of the development of bioactive glass coatings by both techniques, which will be discussed later, SPS was found to produce less suitable coatings for orthopaedic applications than with HVSFS [60]. In addition, nanostructured coatings can be produced with solution precursor plasma spraying (SPPS), which could achieve better biological properties due to higher reactivity. In that case, precursor solutions are used instead of traditional feedstock (powders and suspensions) [61].
Influence of raw materialThe main particularity of working with suspensions is that allow spraying very fine particles which tend to clog in the conventional powder feeders due to their low flowability.
In 2015 Bolelli et al. [60] published an interesting study comparing bioactive glass coatings sprayed by both suspension spraying above-mentioned techniques. The glass composition sprayed onto a TiO2 bond coat applied by APS was in the system SiO2–Na2O–CaO–K2O–P2O5. The SPS process produced highly porous and rough coatings where the particles are incompletely flattened and partly attached among them. These coatings had a thickness ≤50μm and were highly reactive in SBF due to its high specific surface area and porosity.
However, the HVSFS process produced denser bioactive glass coatings, containing few rounded pores and transverse microcracks. The thickness of the coatings achieved were between 20 and 50μm. The HVSFS coatings reacted slower in SBF due to a much lower specific surface area than SPS ones. The microstructure and properties of the coatings developed by HVSFS in this study were more suitable for use in metallic implants.
Narrow particle size distribution favour the coating homogeneity. Furthermore, due to the low thermal conductivity of the glasses for the smaller particles is easier to reach the complete melting during the process. Then spraying fine particles results in more regular coatings.
In 2016 Cañas et al. [62] presented a work related to the effect of the particle size of the powder feedstock on the final coatings, for this purpose 45S5 glass was plasma sprayed onto stainless steel AISI 304L. For the fractions higher than 200μm no coating was obtained because the melting of the particles was not achieved. For the intermediate fractions 200–63μm coatings were obtained but not all the particles were fully melted. As a consequence of the insufficient melting, the coarser the particle size the more irregular the coating. Finally the finest fraction <63μm needed a fluidiser (hydrophobic fumed silica) to be sprayed, more regular coatings were obtained with this fraction.
Adhesion strengthThe main problem of thermal spraying bioactive glass materials is the poor adhesion to metal substrates. The effect of a large coefficient of thermal expansion (CTE) mismatch of the dissimilar materials create stress concentration in the glass near the metal. Furthermore, the rapid cooling of the particles characteristic of the thermal spray processes causes severe temperature gradients, which results into residual stresses across the coating–substrate interface.
A suitable coating must meet tensile strength values to be used in metallic implants. Depending on the coating material, this minimum required value may vary between 15 and 22MPa according to the applicable regulations (ASTM F1147-05, ASTM F1185-03).
Many authors sandblast substrates before spraying for rise its roughness, which improves the mechanical adhesion; but this is not enough and other actions should be carried out. Several strategies have been studied in order to increase the abovementioned bonding strength of the coating with the substrate.
The first solution presented is the use of a bond coat, as was reported by Goller in 2004 [63]. 45S5 bioactive glass was plasma sprayed onto titanium with and without Amdry 6250 (60% Al2O3 and 40% TiO2) bond coat. The results show a uniform coating layer with 20μm of bond coat and 80μm of top coat with a tensile strength of 27.18MPa. While the coating without bond coat has a thickness of 110μm and a tensile strength of 8.56MPa. ASTM C633 was followed to obtain the strength values. In this study the application of the bond coat increase the bonding strength about three times, and the adhesive bonding observed at the bioactive glass metal interface turned into cohesive bonding.
A preceding study use a titanium bond coat to enhance adhesion reported by Lee et al. in 1996 [64]. A bioactive glass in the system SiO2–Na2O–CaO–P2O5 was plasma sprayed onto Ti6Al4V substrates with a titanium bond coat. The thickness of the titanium bond coat and bioactive glass top coat are 130μm and 50μm respectively. The titanium bond coat was used to ensure adherence between the substrate and the bioactive glass coating.
Another study using a bond coat for improve adhesion strength was published by Bellucci et al. in 2012 [65]. Bioactive glass composition based on the K2O–CaO–P2O5–SiO2 system, named “Bio-K”, was deposited by HVSFS onto titanium. The effect of deposit a TiO2 bond coat by APS was investigated. In this study 5 different bioactive glass compositions in the system mentioned previously were used, the TiO2 bond coat improve the adhesion for three of these compositions. Particularly in the Bio-K 5 reaches the higher tensile strength value of 17MPa with bond coat, while presents 8MPa without bond coat. The bond strength was measured following the ISO 4624 method. Besides the microstructure of the coatings and their bioactivity are not affected by the presence of the bond coat.
Blends are also used in order to improve the bonding strength as presented by Chern et al. in 1994 [54]. A bioactive glass in the system SiO2–Na2O–CaO–P2O5 mixed with HA was deposited by APS onto Ti6Al4V. The aim of this study was enhance the bioactivity and the bonding strength of the common HA coating. The adhesion strength was measured following the ASTM C633 method, the values for HA, bioactive glass/HA (1:1 in powder weight) and bioactive glass coatings were 33.0MPa, 39.1MPa and 52.0MPa respectively.
Ding et al. in 2000 published another study working with bioactive glass and HA blends [66]. A series of HA mixed with a bioactive glass in the system SiO2–Na2O–CaO–P2O5 (10:3:5:2 in weight) was plasma sprayed on Ti6Al4V substrates. Blends with 2.5, 5, 10 and 25wt.% of bioactive glass were prepared by both sinter-granulation and direct mixing methods. The majority of coatings had a thickness in the range of 90–140μm and the tensile strength values vary in the range 50–60MPa. Getting the coating with higher amount of bioactive glass 55MPa of bond strength. The values were measured following the ASTM C633. High bond strengths were obtained from all coatings. The different methods used for mixing the powders does not show significant differences in bond strength of the coatings.
Another study working with bioactive glass blends was reported by Nelson et al. in 2014 [57]. Blends of bioactive glass 45S5 with pure titanium or with Ti6Al4V were flame sprayed onto titanium. These blends are done with the 15wt.% of bioactive glass in both cases. Besides the blends are prepared with different particle size distribution for glass and metallic powders. ASTM C633 was followed for measure tensile strength. Blends with Ti6Al4V show an increase of strength as the size particles of bioactive glass is higher, while the blends with pure titanium present less variation of tensile strength with increasing the powder size of bioactive glass. The higher value of tension obtained for the blend with pure titanium is 13MPa, and for Ti6Al4V 20MPa. Concluding that the use of the mix of Ti6Al4V and 45S5 is a better choice.
In 2013 Cattini et al. designed various bioactive glass/hydroxyapatite (HA) functional coatings by SPS [67]. The different designs included: composite coating with randomly distributed constituent phases, duplex coating with glass top layer onto HA layer and graded coating with a gradual changing, starting from pure HA at the interface with the substrate up to pure glass on the surface. The functionalized coatings were mechanical characterized using the scratch test. The critical load for the composite coating is 27.1N, the lower 21.2N for the duplex design, caused likely for the abrupt interface between the glass and the HA. While the graded design resists the maximum load of the test without reaching the substrate, concluding that the stresses could be progressively reduced with this design. With the graded coating, that provides better mechanical results, the authors continued the research to improve the functional coating [68].
An alternative presented by Altomare et al. in 2011 is pre heating the substrate to improve the adhesion [69]. 45S5 bioactive glass was deposited by HVSFS on titanium substrates. This study was performed to understand the deposition mechanisms during the process. In fact, pre heating to 100°C the substrate was crucial to deposit a homogeneous coating. If the substrate was not preheated or was allowed to cool before spraying the deposition was highly impaired. The most important role of pre heating is the mitigation of the rapid cooling of glass droplets in the first layer which hinders their adhesion.
Bolelli and co-workers reported in 2012 two studies [65,70] were bioactive glasses were deposited by HVSFS using a pre-heating step to enhance adhesion, in that occasion arriving to higher temperatures. The pre-heating of the substrate generally improves splat–substrate wetting and results in better adhesion. In these studies Bio-K was sprayed onto titanium substrates after pre-heating with two torch cycles with no suspension injection, coating deposition started when the substrate temperature was about 230–260°C.
Influence of the thicknessAccording to the ASTM F1854-15 there is no specification for the thickness of thermal sprayed medical implant coatings. However, is a critical feature for achieving long term stability and avoid the implant mobility. The coating thickness is a compromise between mechanical properties and its dissolution, thus is a parameter to analyze carefully when manufacturing a coating. Excessive thickness can favour delamination and fragmentation of the coating, by contrast very thin coatings can be degraded before achieving a good bonding with bone tissue. Most of the commercial HA coatings for orthopaedic implants have a thickness between 50 and 75μm [71]. So for bioactive glass coatings the value should be on near values.
The thickness can be modulated by controlling parameters of the process such as stand-off distance, number of passages or the melting of the particles. But also characteristics of the powder as powder size, glass composition or density. As can be seen in Table 1 the thickness for coatings produced with dry powder as feedstock material, by APS, vary from 40 to 150μm while the coatings obtained by suspension spraying are in the range of 10–83μm. This difference is due to the fine particle size used with the suspension spraying techniques.
Summary of most relevant results of bioactive glass coatings without blends obtained by means of thermal spray.
| Raw material | Glass composition | Spraying process | Coatings phases | Thickness | Ref. |
|---|---|---|---|---|---|
| Powder | SiO2–Na2O–CaO–P2O5 system | APS | Amorphous | 70μm | [54] |
| Biovetro (SiO2–Na2O–K2O–CaO–MgO–P2O5–Al2O3 system) | APS | Amorphous | 80μm | [78] | |
| 45S5 | APS | Amorphous | 130μm (titanium bond coat)50μm (top coat) | [64] | |
| Biovetro | APS | Not reported | 80μm | [35] | |
| SiO2–Al2O3–CaO–Na2O system | APS | Amorphous | 80μm | [40] | |
| 45S5 | APS | Not reported | 20μm (Al2O3–TiO2 bond coat)80μm (top coat)110μm (without bond coat) | [63] | |
| 45S5 and Bio-K (K2O–CaO–P2O5–SiO2 system) | APS | Na4Ca4(Si6O18) and CaSi2O5 phase(45S5)Amorphous (Bio-K) | 150μm | [81] | |
| SrBioactiveGlass (K2O–CaO–ZnO–MgO–Na2O–P2O5–SiO2 system) | APS | Amorphous | 50–100μm | [37] | |
| 45S5 | APS | Some amorphous coatings and some with Ca2–SiO4 phase | 40–100μm | [77] | |
| 45S5 | APS | Some amorphous coatings and some with Na6Ca3Si6O18 | 150μm | [55] | |
| Suspension | 45S5 | HVSFS | Amorphous | 41–83μm | [69] |
| Bio-K | HVSFS | ZrO2 (contamination) and other crystalline peaks with much lower intensity barely distinguishable | 10–15μm | [65] | |
| 45S5 | HVSFS | Amorphous | 10–25μm | [80] | |
| SiO2–Na2O–K2O–CaO–P2O5 system | HVSFS and SPS | Mainly amorphous, some coatings present Ca3(Si3O9) phase | 20–50μm | [60] | |
| SiO2–Na2O–CaO–P2O5 system | SPS | Ca2SiO4 phase | 20μm (top coat) | [105] | |
| 45S5 | SPPS | Amorphous | 35μm | [106] | |
Has long been known that material properties, such as chemical surface, porosity and surface finishing have a great influence in the biological response of the cells with the coating. High porosity is able to mimic biological structures, it plays an important role in tissue ingrowth through the pore size [72] and, more critical, the interconnected porosity [73]. Some authors have reported that a minimum pore size about 100–150μm was needed for the continued health of bony ingrowth [73,74], but smaller porosity can contribute also to cellular attachment [75]. In vivo results on porous titanium implants showed that increase of porosity and pore size positively influence their osteoconductive properties [76]. But porosity not only supports tissue adhesion, growth and vascularization, it also reduces the elastic modulus mismatch of the coating and substrate reducing the stress shielding associated. Therefore it is a very interesting parameter to take into account in the manufacture of these coatings.
In the aforementioned study by Chern et al. in 1994 a detailed description of the coating porosity is indicated [54]. Blends of HA with bioactive glass in the system SiO2–Na2O–CaO–P2O5 were deposited by APS onto Ti6Al4V. The presence of bioactive glass increases the surface roughness of the coatings. When adding bioactive glass on HA coatings large open pores are formed because the glass particles went through a low viscosity stage, not being totally melted and flattened when impacting with the substrate and other particles. Chern et al. suggested that the porosity achieved could provide bone ingrowth.
A remarkable study about porosity was presented by Rojas et al. in 2020 where parameters of the APS process affecting the coating porosity are analyzed [77]. 45S5 bioactive glass is plasma sprayed onto stainless steel AISI 304L. The cross sectional structure of the coating reveals a significant amount of inter and intralamellar circular porosities produced by volatilization of chemical components, in this case P2O5 and Na2O, from the feedstock powder. This phenomenon occurs at high temperatures as reported by Gabbi et al. [78] and Pawlowski [53]. The wide particle size distribution of the feedstock causes a non-homogeneous heating of the in-flight particles during the manufacture of this coating. As a result a weak interlamellar interaction and a low spreading generate irregular porosity.
In the same study, the stand-off distance was analyzed and could be observed that circular porosity increases with decreasing spray distance. Furthermore, it was observed that the porosity of coatings decreases when using air jets forward the samples to cool the coating during the process. This can be explained because the particles are cooled before their impact resulting in less volatilization. Finally, the amount of porosity can also be controlled by the plasma enthalpy being lower at high enthalpy. Control of the porosity can be achieved by adjusting the plasma enthalpy, the spraying distance and the air jet used to cool the substrate, in this study the variation of porosity was between 4 and 16%.
López et al. in 2014 reported a study of bioactive coatings obtained from feedstocks prepared by different routes [79]. 45S5 was plasma sprayed onto stainless steel AISI 304L. The 45S5 frit was milled using two different routes: dry milling followed by sieving and wet milling followed by spray drying to obtain a powder comprising porous agglomerates. The coatings produced with spray dried powder reveal a quite heterogeneous microstructure with high porosity and a marked variation of pore sizes. The coated samples prepared with dry route feedstock present less porosity than the previous ones, however large and round pores are also observed in this coating. Furthermore, in both cases the particles with a size range higher than 63μm are few deformed due to the low thermal conductivity of the glass resulting in high roughness and heterogeneity. The characteristics of the feedstock strongly impact on the final coating microstructure. The spray-dried agglomerates present a high porosity. During the spraying process the low conductivity of the glass particles prevent the core from melting and maintain its high porosity. Thus particles arrive to the substrate with low melting degree and deformation, giving as a result a high porous coating.
Nelson et al. studied the deposition of a bioactive glass-titanium alloy composite in 2011 [56]. Flame spray was used to manufacture porous composite coatings of 45S5 and Ti6Al4V with the aim of improve the bioactivity of the coatings. The amount of bioactive glass to the blend represent the 15wt.% and 38wt.%, but in the latter the glass distribution through the coating was not homogeneous. So the blend with lower content of glass was selected. The porosity of the coatings was increased with the presence of bioactive glass achieving 33% while the coatings without glass reach 26% of porosity.
In 2014 Nelson et al. published another work with titanium and bioactive glass composites [57]. Blends of 15wt.% 45S5 glass with pure titanium or with Ti6Al4V were flame sprayed onto titanium. Porosity was characterized following the ASTM E2109. Blends with pure titanium get higher porosity (8–29%) than the ones with Ti6Al4V (5–18%). In pure titanium and bioactive glass composites, pores are localized around the glass particles suggesting that some interactions could occur between the materials. Some possible interactions could be poor stacking, viscous flow of molten glass, localized regions evolving gas or splashing of molten particles. In addition higher porosity was achieved when increasing the glass powder size. The larger particles result in lower particle temperatures and hence insufficient deformation of the particles when impact with the substrate or other particles.
Bolelli et al. in 2012 presented the comparison of a bioactive glass Bio-K and a tricalcium phosphate (TCP) bioactive ceramic deposited by HVSFS onto pre-heated titanium substrates [70]. Cross-sections confirm more porous microstructure in Bio-K than TCP coatings. Bio-K particles are less flattened and often containing spherical central cavities which can be responsible for most of the fine porosity appreciated.
In 2019 Bano et al. presented a work where analyze the microstructure of glass coatings obtained by HVSFS [80]. 45S5 glass was deposited onto stainless steel AISI 304L at three different flame powers (low, medium and high). Well adhered coatings with a thickness of 25μm were obtained at medium and high flame powers. Coating with higher porosity, 16%, correspond to the medium flame power. The coating produced with high flame power was 10% porous and present a higher roughness surface. With high flame there is more heat transfer to the particles and these are more melted resulting in a denser microstructure.
Influence of post-treatmentsA post deposition heat treatment can be used to modify the microstructure of the coatings as reported by Cannillo et al. in 2010 [81]. Two different bioactive glass powders Bio-K and 45S5 were plasma sprayed onto titanium substrates.
Bio-K was derived from the 45S5, just replacing all the sodium oxide with potassium oxide to reduce its tendency to crystallize at high temperature. In this study the sprayed coatings were treated at 700°C for 1h, above the glass transition temperature of both glasses. This treatment maintains the Bio-K coating amorphous, while in the 45S5 coating two crystalline phases were detected, sodium–calcium silicates and calcium silicates. These phases were also identified in the 45S5 as-sprayed coatings, which were generated through the spraying process. After the heat treatment the peaks of the 45S5 coating were more intense meaning an increase in its crystallinity. As a result, the thermal treatment may be helpful to reduce the defectiveness of the glass coatings. Exceeding the glass transition temperature, it can soften and adapt between particles and to the substrate. Consequently, the mechanical properties are enhanced.
Another post treatment but in this case with a multifunctional approach was reported in 2009 by Verné et al. [82]. Bioactive glass coatings were doped with silver to provide them antibacterial properties. A glass in the system SiO2-Al2O3-CaO-Na2O was plasma sprayed onto titanium alloy and stainless steel substrates. Amorphous coatings with a thickness of 80μm were obtained.
Coated samples were treated by a patented ion-exchange treatment to introduce silver ions in the surface in two different solution concentrations 0.5M and 0.05M. Leaching tests revealed that in both conditions, silver is rapidly released during the first day of immersion in SBF, this feature is interesting due to the incidence of infections just after surgical procedures. The in vitro test in SBF confirmed the low bioactivity of the coatings before and after the silver-doping, with no variation due to the silver. The low degree of bioactivity was expected due to the glass reactivity.
Antibacterial tests showed a marked bacteriostatic behaviour of Ag-coated samples, proportional to the silver content. The doped coatings inhibited the proliferation of most of the adherent bacteria on the coatings surfaces but not kill them. According to the good results of the antibacterial tests only the samples with less silver concentration were tested for biocompatibility. Cell culture tests for 6 and 24h confirmed the safety of the coatings for fibroblast cells.
In vitro evaluation for apatite-forming abilityWhen bioactive glasses are in contact with simulated body fluids a HCA layer, that allows the chemical bond to bone, is developed on its surface. The formation of the HCA starts at the surface of the glass and moves inward.
The development of the HCA layer starts with the formation of a silicon-rich layer almost instantaneously, this is covered in few minutes with a layer of amorphous calcium phosphate, which subsequently crystallizes with an apatite-like structure. L. Hench described in detail the interactions and reactions that take place in the formation of the HCA layer [83–86].
The bioactivity of a glass coating can be affected by many factors such as crystallinity, composition, porosity or specific surface area. So it is important to evaluate the apatite-forming ability of new formulations and processed coatings, due to alterations produced during the development.
Despite the fact that bioactive glasses exhibit an amorphous structure the deposition process or post-treatments can generate crystalline phases. These can affect mechanical properties and also bioactivity, which tends to decrease with the level of crystallization, however some crystalline phases are not affecting its bioactivity, as can be checked in Table 1, most of the coatings obtained by thermal spray preserve its amorphous structure.
The variations on glass composition to adjust some properties can also affect the glass reactivity, which is specially linked to the network connectivity. Furthermore, high porosity and high specific surface area can accelerate the HCA layer formation process.
It is important to keep in mind that not always having a high degradation rate is beneficial, since if the coating degrades very quickly and a good bond with the bone has not been formed, it can negatively affect the mechanical stability of the implant. Therefore, the degree of reactivity must be adjusted to the specific application.
For bioactivity assessment many types of simulated body fluids can be used, which consist of similar ion concentrations to physiological plasma. SBF solution defined by Kokubo [87] has become the main used in current experiments as can be seen in Table 2. In many studies the solution is refreshed after certain time points (2–3 days), especially when longer time points are tested. Some studies have evaluated among other factors the role of the solution chosen, the frequency of the renewal of the solution, suggesting that the results can be affected depending on the testing solutions and conditions selected [41,88].
Summary of most relevant results of apatite-forming ability of the bioactive glass coatings obtained by means of thermal spray.
| Sprayed material | Spraying process | Solution conditions | Apatite formation | Ref. |
|---|---|---|---|---|
| 45S5 | APS | SBF solution.Soaking times: 1, 2, 4, 8, 16, and 32 days. | After 1 day, the concentration of Ca2+ was enough to precipitate HCA on the surfaces of the coatings.The thickness of the Ca–P rich layer was about 10μm after 16 days. | [64] |
| Blend: HA+SiO2–Na2O–CaO–P2O5 system(10:3:5:2 in weight) | APS | SBF solution, agitated daily.Immersion time: 30 days. | Apatite thickness between 20±30μm after 30 days. | [66] |
| 45S5 | HVSFS | SBF solution.Soaking times: 1, 3, 7, 14 and 28 days. | Only 1 day soaking is needed to develop a continuous hydroxyapatite layer onto the surface of the samples. | [69] |
| Blend: Ti6Al4V+45S5 (15wt.%) | FS | SBF solution, changed every 3 days.Soaking times: 1, 7 and 14 days. | No apatite layer was formed on the Ti6Al4V alloy control after 14 days.The bioactive glass-alloy shows evidence of crystalline HA formation after 14 days. The primary XRD peaks were observed at low intensities after 7 days of exposure to SBF. | [56] |
| 45S5 | APS | SBF solution.Soaking times: 1, 2, 3, 5 and 7 days. | An apatite layer was developed after 7 days of SBF exposure, but some areas are formed by silica gel which has not evolved yet to apatite. | [79] |
| 45S5 | SPPS | SBF solution.Soaking times: 1 and 7 days. | The coating exhibits the formation of a HCA layer after 1 week immersed. | [106] |
| 45S5 | HVSFS | SBF solution.Soaking times: 1, 2, 3 and 7 days. | After 7 days, no apatite precipitation on 25kW coatings. Apatite layer of 24μm on 50kW coatings and apatite layer of 17μm on 75kW coatings. More degradation occurs on the coating produced at 50kW likely for the higher porosity. | [80] |
The aim of the test is to determine the mineralization process by observing the apatite nucleation upon a surface over a period time. Moreover, is important consider performing a mechanical evaluation of the samples after the SBF immersion to detect alterations in coating properties.
Biological behaviour of the bioactive glass coatingsBiomaterials designed to be implanted inside the body should integrate with host tissue and not become encapsulated by a dense layer of fibrous connective tissue [89]. The success of the implant integration involves the formation of a bone-like interface that integrates the implant surface with the surrounding bone.
Implant materials are designed to promote osteoconduction and osteoinduction, essential features for osseointegration [90]. The first is related to the capacity of the surface to allow bone growth, the latter refers to the process by which cells are guided to become differentiated osteogenic cells.
The basic reactions of osteoblasts on material surfaces involves the following phenomena: protein adsorption at the material surface, followed by the cellular attachment which occurs rapidly, then adhered cells migrate, proliferate and differentiate [91].
Some surface properties of the implanted materials can affect biological responses on the cell-material interface, such as composition, ion release, topography or chemistry [92]. For this reason cell culture tests and in vivo models are essential for validate the obtained coatings, ensuring the capability of the material to interact properly.
Gabbi et al. [78] published in 1995 an in vivo and in vitro study performed to evaluate the biological results of Biovetro (Na2O–K2O–CaO–MgO–Al2O3–SiO2–P2O5 system) coatings obtained by APS on Ti6AI4V plates. For in vitro assays cells were seeded at a density of 1×105cellscm−2 and incubated for different intervals. A decrease in cell proliferation is observed for the samples coated with Biovetro but not for control samples, which is associated to the ionic release of the glass. For in vivo testing a group of rabbits was selected, for each animal a sample coated with Biovetro was introduced in one tibia and an uncoated sample in the other. The reabsorption of the Biovetro layer is confirmed at 180 days, which is replaced by newly formed bone thus preventing fibrous tissue from filling the gap between the implant and the bone tissue. This study confirms the biodegradability and osteoconductivity of the Biovetro glass.
To perform cell culture studies with bioactive glasses a preconditioning step for the materials is required to avoid cytotoxicity caused by the rapid pH increase. Different strategies have been proposed to avoid this problem, consisting of immersing samples in physiological solutions [93].
The second cellular study with thermal sprayed bioactive glasses is reported in 1998 by Oliva et al. [35]. For this study two bioactive glasses from the Biovetro family, in the Na2O–K2O–CaO–MgO–Al2O3–SiO2–P2O5 system, were used. As control were chosen a third composition without P2O5 resulting in a non-bioactive glass and the titanium alloy substrate. The glasses were sprayed by APS onto Ti6Al4V specimens.
Primary cultures of human osteoblasts were used in this research. The samples were preconditioned before the cellular assay, to stabilize the pH and avoid the cytotoxicity. This procedure consists of soaking the samples for 24h in phosphate-buffered saline (PBS) and process was repeated other three times refreshing the PBS to reach the stabilization of the pH. To evaluate the biological response on the different materials cells were seeded with a density of 2×104cellscm−2. A similar adhesion for the different samples was assessed after 24h of incubation, except for one of the bioactive glass coatings that recorded lower adhesion. After 4 days the MTT test revealed higher amount of osteoblasts on the control surfaces rather than the bioactive ones. However for long periods of incubation, 8, 16 and 24 days, the results changed and the bioactive coated samples presented a higher proliferation than the control samples. Scanning electron microscopy was carried out for samples at 24 days of incubation, and the micrographs revealed that on bioactive glass coated samples the cells were fully spread forming a very close layer of osteoblasts. In contrast the micrographs related to bio-inert glass surfaces revealed not completely spread of the cells and these were less close among them.
In 2011 Altomare et al. reported an in vitro study on 45S5 coatings sprayed by HVSFS onto titanium substrates [69]. A human osteosarcoma cell line was used for the tests. Coated and acid-etched titanium samples (commonly used in bone-contact dental applications) were seeded with a cell density of 1×104cellscm−2. After 7 days of incubation cells were able to proliferate in a similar way on both studied surfaces, confirming the ability of the coated samples to support adhesion and proliferation of the human osteoblast-like cells. Moreover, the morphological observation by scanning electron microscopy confirm no adverse changes in cell morphology. The in vitro tests corroborate that 45S5 coatings maintain the biocompatibility characteristic of bulk glass. Consequently, the results suggest that bioactive glass coatings are an alternative to thermally-sprayed hydroxyapatite.
Related to the in vivo studies, two works more have been published. In 1998 Lopez-Sastre et al. reported a comparative study between HA and Biovetro coatings onto titanium implants [94]. APS technique was used to coat the implants with a thickness of 80μm.
The cylinders were implanted in the distal femoral epiphysis of six sheep, on the right side the bioactive glass implants, on the opposite the HA coated. The results were assessed at 6 different times. The implants coated with Biovetro present larger pore size and four times more porosity than HA ones. These bioactive glass coatings were less integrated into the bone, as was observed on histological examination of the interface. The authors attribute these results to the amount of aluminium oxide in the composition. It was demonstrated an accumulation of aluminium at the interface by aluminon staining. Above 3% its capacity to bond the bone is lost. These results are according to the literature, where is reported than the presence of alumina in the composition can inhibit the bone bonding. Up to 1.5–2% of alumina can be included in a glass formulation without significantly diminish the glass bioactive capacity [86,95,96].
More than a decade later, in 2014 Newman et al. reported another in vivo study with bioactive glass coatings [37]. The glass, in the system SiO2–Na2O–K2O–CaO–MgO–ZnO–P2O5–SrO, was applied to Ti6Al4V implants by APS.
Glass and HA coated implants were inserted into the distal femur and proximal tibia of twenty-seven New Zealand White rabbits for the periods of 6, 12, or 24 weeks. The bioactive glass composition used in this research was designed to achieve a CTE similar to HA. Also with an amorphous structure and an appropriate network connectivity for bond to bone. Furthermore, the use of strontium has been used as treatment for osteoporosis [97,98]. Degradation test reveals the rapid release of the Sr2+. At 6 weeks an increase of the early bone formation around bioactive glass coated implants was observed comparing with HA and the fixation of implants by 24 weeks is superior with bioactive glass coated specimens.
Summary and future outlookThere are key factors to be accomplished to obtain a successful coating.
Firstly, the good adhesion at the interface between the glass and substrate, which is likely the main drawback for the glass coatings deposited by thermal spray techniques. Different approaches can improved that bonding, as using a bond-coat between glass and substrate, the use of blends (mainly with HA), pre-heating the substrate or modifying the glass composition in order to achieve a closer CTE of the glass and the metallic substrate.
Secondly, to achieve a homogeneous coating a narrow particle size distribution is recommended. The low thermal conductivity of the glasses can result in irregular microstructures due to non-homogeneous heating of the particles. Moreover, the characteristics of the feedstock strongly impact on the final coating microstructure.
Thirdly, by using different thermal spray techniques and varying the parameters of the process we can control porosity and thickness of the coatings. The plasma enthalpy and the spraying distance seem to have an important paper in modulating the porosity, while thinner coatings can be achieved using suspension spraying processes because of the possibility of work with finer particles.
Keep the apatite forming ability of the coatings is a key point for the final coatings. Specific attention must be paid when varying glass compositions or when introduce crystalline phases during the deposition or the post-deposition processes. However not all the crystalline phases affect negatively the degree of bioactivity, and by other side can enhance the mechanical properties [55]. In fact, the bioactive glass–ceramics are partially crystallized glasses with a similar degree of bioactivity than bioactive glasses and improved mechanical properties, some of them have a clear presence in clinical use [85,99].
Another key factor is the assessment of the coating stability and its biological response. Few studies involving cell culture or in vivo tests have been performed with bioactive glass coatings obtained by thermal spraying and some of them are not concluding. Further research is necessary to corroborate the response of the materials for long-term applications.
Besides the improvement of bonding to the bone, other functionalities can be achieved for the coatings. Taking advantage of its ability to dissolve, it could be possible to add functional elements and get a release of this elements over time to provide an improvement. For example, enhance angiogenesis capacity [100,101], osteostimulation or antibacterial activity [102–104].
It is clear that bioactive glasses have a promising role in the future of medicine. In recent years most of the research with these materials is going towards scaffolds, however the future as coating is encouraging and should not be neglected. Further research need to be developed to determine the applicability of the coated implants.
This work was supported by Generalitat de Catalunya (2017SGR-1777) and Spanish Government (MAT2016-76928-C2-1-R).