Background and aim. In the fall of 2013, the US Centers for Disease Control and Prevention (CDC) published a preliminary report on a cluster of liver disease cases that emerged in Hawaii in the summer 2013. This report claimed a temporal association as sufficient evidence that OxyELITE Pro (OEP), a dietary supplement (DS) mainly for weight loss, was the cause of this mysterious cluster. However, the presented data were inconsistent and required a thorough reanalysis.
Material and methods. To further investigate the cause(s) of this cluster, we critically evaluated redacted raw clinical data of the cluster patients, as the CDC report received tremendous publicity in local and nationwide newspapers and television. This attention put regulators and physicians from the medical center in Honolulu that reported the cluster, under enormous pressure to succeed, risking biased evaluations and hasty conclusions.
Results. We noted pervasive bias in the documentation, conclusions, and public statements, also poor quality of case management. Among the cases we reviewed, many causes unrelated to any DS were evident, including decompensated liver cirrhosis, acute liver failure by acetaminophen overdose, acute cholecystitis with gallstones, resolving acute hepatitis B, acute HSV and VZV hepatitis, hepatitis E suspected after consumption of wild hog meat, and hepatotoxicity by acetaminophen or ibuprofen. Causality assessments based on the updated CIOMS scale confirmed the lack of evidence for any DS including OEP as culprit for the cluster.
Conclusions. Thus, the Hawaii liver disease cluster is now best explained by various liver diseases rather than any DS, including OEP.
A 2014 article in the New England Journal of Medicine provoked interest and controversy when it reported that epidemiologists at the US Centers for Disease Control and Prevention (CDC) had confirmed what a liver-transplant surgeon in Honolulu had suspected: OxyELITE Pro (OEP), a popular over-the-counter dietary supplement (DS) for weight loss, was responsible for a cluster of cases of severe hepatitis and acute liver failure (ALF) in the summer of 2013.1 OEP had been withdrawn from the shelves,1 and in September of 2013, the Hawaii Department of Health (HDOH), with the CDC and the US Food and Drug Administration (FDA), had initiated a public health investigation.2
Patients of this Hawaii cluster mostly became ill in the summer of 2013 - highly suggestive of a seasonal outbreak by zoonical, bacterial, or viral pathogens like hepatitis A virus (HAV) or hepatitis E virus (HEV);3 such geographic clusters are rarely caused by DS or drugs with national distribution. HEV genotypes 1–4 occur in humans, types 3 and 4 in animals.4–6 Infected animals may transmit HEV infections to humans,4–12 with farmers, hunters, and outdoor enthusiasts at high risk of contracting HEV from infected animals,4–13 including rats in their Hawaiian communities.13 A diagnosis of HEV can be easily missed because analyses by HEV polymerase chain reactions (PCR) are rarely employed in the US14 while results by US HEV antibody assays are heavily disputed and considered invalid due to lacking test sensitivity, specificity,4 and FDA ap-proval.4,14 Another Hawaiian dilemma exists since cases of drug induced liver injury (DILI) and herb induced liver injury (HILI) are not secure without appropriate testing for infections by HEV.4,15,16 These HEV related diagnostic problems in Hawaii may explain why HEV was not mentioned by regulatory assessors2 or clinicians of the Queen’s Medical Center (QMC) in Honolulu.2,17
The HDOH, CDC, and FDA did not disclose details of their interviews, chart reviews, or approaches to exclude alternative causes, nor did they specify the algorithm they used to assess causality.2 More critically, the procedural irregularities of the officials and physicians started with the supposed temporal association between the use of OEP and the liver disease in Hawaii.2,17 This assumption implicated OEP as the sole possible culprit, foreclosing a subsequent careful, unbiased case assessment.2,17 The unexpectedly high local and nationwide print press and TV publicity put assessors under enormous pressure to succeed. Such intense publicity on an unsettled clinical topic is unusual in the scientific community, the time pressure forces errors and is risky for regulators,2 physicians,17 and patients.18 The CDC report also noted that the investigation was ongoing and the data were preliminary,2 but the final CDC report has not been published.
The scientific community had high expectations that clinicians of the Honolulu QMC would provide more robust data on their mysterious liver disease cluster cases, but such expectations were not met by their report, which lacks transparency and is marred by ambiguity.17 These shortcomings led us to independently reassess a single particularly challenging and inconsistent case18 from the cluster,17 comparing the patient’s raw data18 to the published case data.17 Our reanalysis revealed that the claims made by the public officials2 and the Honolulu physicians17 are not sub-stantiated.18 For example, the patient was not “previously healthy” but instead was multimorbid and multimedicated by drugs and DS,18 not disclosed by officials2 and physicians.17 In retrospect, this patient’s liver disease is best explained as recurrent toxic hepatitis, likely caused by overdoses of naproxen.18 Additional possible diagnoses included DILI by other drugs and symptomatic acute cholecystitis.18 Convincing evidence is lacking for any of the four used DS as the culprit, including the OEP18 that was initially singled out.17 This case is no longer mysterious and best explained by alternative causes, such as naproxen overdoses.
Encouraged by these unexpected case findings18 and in search of the real culprits in additional cases of the disease cluster, we undertook a pragmatic and clinical approach to solve this Hawaii mystery. Therefore, we reassessed raw data of other patients of this Hawaii liver cluster and found numerous alternative causes and major confounders, which clearly dismissed the initial diagnoses of liver injury by OEP.2,17 Diagnostic workup requires careful and unbiased analyses especially in patients with specific treatment options. For example, acetaminophen (AAP) overdose can effectively be treated by N-acetylcysteine to restore hepatic glutathione levels,19 severe HEV infections by ribavirin,4,6,20 hepatitis B virus (HBV) infections by nucleos(t)ide analogues,21 herpes simplex virus (HSV) and varicella zoster virus (VZV) hepatitis infections by aciclovir,22 and cytomegalovirus (CMV) infections by intravenous ganciclovir.23 It appears that some cluster patients could have benefited from the correct diagnosis and appropriate therapy.
Material and MethodsInitially, anonymized raw data of the cases for re-analysis were kindly requested from the corresponding author17 but our request was declined; only brief case summaries were provided as well as details of the extremely high Council for International Organizations of Medical Sciences (CIOMS) scorings for each patient, allowing a further CIOMS analysis and comparison with documented data. Subsequently, we received redacted files that were obtained from the HDOH under Hawaii’s freedom-of-information act as well as few additional records filed as part of a public lawsuit in Hawaii. All medical files and raw data had patient names and other personal identifying information redacted prior to submission to us for reanalysis. Documents of patients of the Hawaii liver disease cluster included clinical files, patient’s records, files of their primary care provider (PCP), insurance reports, drug and DS information files, customer purchase records, and other information provided at trial. The clinical assessment focused on completeness of data, evaluation of documented clinical test results and the clinical conclusions of the treating physicians which were derived from the results documented in these files. Assessment also included consistency of documented data with published information.
Results and DiscussionAnalysis of regulatory assessments- •
Regulatory case assessments. In their preliminary report, CDC regulators correctly noted that attributing liver injury to a specific ingredient can be challenging because of multiple ingredients, product variability, and lack of tests to confirm specific exposure to a product. These and other obvious confounders of the suspected OEP cases were not appropriately handled by the CDC report2 or the clinical report.17,18 Assumed hepatitis outbreaks demand professional regulatory and clinical case management with sophisticated and structured approaches, broad clinical experience and expertise in hepatology - in addition to intuition, commitment, and empathy. Trained physicians with appropriate board certification should provide support and must be open to critical discussions of alternative explanations among all assessors to resolve the culprit(s) of this mysterious cluster phenomenon. A prerequisite is impartiality prior to any public statement; otherwise, public statements may predetermine one single explanation without the possibility of corrections. Risks are high for the involved regulatory agencies and physicians, if their public claims do not hold as promised. Without careful work, the correct clinical diagnosis and therapy may be missed. Finally, published claims that a product is potentially hepato-toxic may induce liver patients to report having used this product prior to their illness to claim financial compensation, although they have never consumed this product and lack proof of purchase.
Clearly, the case has to be defined and assessed under appropriate regulatory and clinical standards, which was attempted in this cluster.2 More importantly, no control group had been defined and assessed by regulatory and clinical standards.2,17
- •
Regulatory case criteria. According to HDOH, CDC, and FDA,2 a case was defined as acute hepatitis of unknown etiology, occurring after March 31, 2013, in a person who had consumed a DS within the previous 60 days and had a serum alanine aminotransferase (ALT) four times the upper limit of normal (ULN) and a total bilirubin level ≥ 2 x ULN; negative serologies for infections including viral hepatitis were mandatory.2 However, this case definition is imprecise and problematic for various reasons;2 it was not used in the clinical report.17 It disregards the period from cessation of DS use to disease onset, thus weakens the criterion of a strict temporal association; commonly, tentative hepatotoxicity is assumed only if liver disease is manifested within a short time window after the last use of the product, usually two weeks or less (slowly metabolized compounds, within a maximum four weeks).24–26 Also, hepatotoxicity commonly is not considered for ALT values < 5 x ULN24–26 to avoid inclusion of patients with unspecific ALT elevations. Furthermore, the agencies did not specify how they excluded preexisting liver diseases, nor how they evaluated infections including viral hepatitis.2 Thus, they ignored some crucial elements for assessing causality in suspected HILI and DILI.16,24–26 To exclude various viral hepatitis forms, anti-HAV IgM is tested for HAV; anti-HBc IgM and HBV-DNA for HBV; anti-HCV and HCV RNA for hepatitis C virus (HCV); PCR analyses for viral DNA and titer changes of specific IgM and IgG antibodies are mandatory to exclude infections by HEV, CMV, Epstein Barr virus (EBV), HSV, and VZV.16,24,25 The agencies reported exclusion of several etiologies such as autoimmune hepatitis, but they did not disclose the diagnostic exclusion criteria,2 as required.16,25 Regulators also interviewed patients, but again details of structured questionnaires to circumvent bias were not provided.2 In addition, regulators reported reviewing the medical charts of the patients.2 This commonly implies that the clinical diagnosis of hepatotoxicity by OEP is approved by the assessing regulators, being correct and well documented. The scientific community has high expectations for reports from health agencies, including the HDOH, CDC, and FDA. These should adhere to data transparency and impartiality. Instead, in the Hawaii liver disease cluster, the agencies have not made clear which viruses were actually excluded and whether they used a causality assessment method,2 like the updated scale of CIOMS,16 to be applied individually to each comedicated product. HEV exclusion is a key item of both the updated CIOMS scale and the checklist of alternative causes in hepatotoxicity cases.16 The reported cluster was not further specified,1 but obviously included at least the initial seven patients with assumed use of OEP,2 which later was reclassified as “new” OEP. As defined by the authors, “new” OEP refers to versions in which aegeline had replaced the DMAA (1,3-dimethylamylamine HCl) in the “old” OEP.17 The report did not consider the old OEP but only the new OEP as the assumed hepatotoxic product, which was used briefly for 2 weeks minimum and 6 weeks maximum by all 8 patients.17
- •
Missing control cohort. In the fall of 2013, the regulators2 and physicians1 missed the opportunity to define and evaluate an appropriate control cohort, possibly due to lack of funding. However, any case control study done later than the fall of 2013 would be inadequate because the proper data could not be collected. This neglect of epidemiological and clinical aspects invalidates the conclusions of their reports.2,17 A control cohort lacking DS use should have been defined at latest in the fall of 2013 or better earlier, consisting of patients with acute hepatitis or ALF of initially unknown etiology, occurring after March 31, 2013, and in care of the Honolulu QMC. Patients of this control group should not have reported any DS consumption within the previous 60 days but fulfil the regulatory criteria for hepatotoxicity, e.g. serum ALT > 4 x ULN and total bilirubin < 2 x ULN, with a valid diagnosis and established cause of the patients’ liver disease for comparison.
The present analyses of four patients of the mysterious Hawaii liver disease cluster is based on documents containing data that are condensed and presented as narratives for each patient in table 1. The aim of these case analyses is to retrieve and evaluate additional case data to identify possible culprit(s).2,17 Analytical approaches are similar to those applied in previous assessments of HILI and DILI cases.16 Due to uncertainties,2,17,18 the reanalysis of the raw data from these complex cases was focused on some important factors, including confirmed product purchase and consumption; the types of DS products used, possible temporal associations to liver disease; validity of case information; overall case data quality; confounding variables; complete exclusion of alternative causes; comorbidity; comedication; and firmness of causality. Due to lack of a valid diagnostic biomarker, the diagnosis of DILI or HILI is a diagnosis of exclusion.24–26 Genetic risk factors are important27 but are not specifically evaluated in this study.
Cases 1-4 and their narratives, case details and analyses.
| • CASE 1. |
| Narrative, case details and analyses |
| Male patient with liver cirrhosis and ascites of clinically unassessed etiology but possibly related to alcohol or acetaminophen use, or HEV. Significant outdoor activities with HEV risk: hunter, wild hog meat consumer, and coffee farmer. History of illicit drug and heavy alcohol use/ abuse some years ago, with present monthly use of 6-12 beers or a 6 pack of beer and 1-2 shots of hard liquor each time the patient drank. First symptoms of illness emerged within 24 hours after a restaurant meal. At hospital admission on 8/25/2013: ALT 1970 U/L, AST 1308 U/L, ALP 107 U/L, and bilirubin 33.6 mg/dL. PMH: asthma (drugs), morbid obesity, atopy, allergic hypersensitivity, impetigo, eczema, debilitating fungal infections on both feet until 2013 with open sores and scabbing: Vicodin (acetaminophen-hydrocodone); Bactrim. Percocet (acetaminophen-oxycodone) in early 2013 for pain in neck and paresthesia in the arm, possibly an early extrahepatic, neurological manifestation of HEV myelitis with polyradiculopathy,4,6 bilateral brachial neuritis,6 or peripheral neuropathy,4 which can overshadow the liver injury.4 Used overall 14 DS, with some information for four DS: old OEP use for one year, intermittently on an off and on basis and stopped 3 weeks prior to admission; subsequently, new OEP, Amplified Wheybolic Extreme-60, and Versa-1 were used for only one week until symptoms emerged two weeks prior to admission. According to purchase records and additional medical records, he consumed since March 2013 prior to liver illness these ten DS: Pro Performance AMP Wheybolic Extreme 60, Clk/Super Hd Combo Kit; Ripped Freak; Kre-Alkalyn EFX; Pump-HD; Green Coffee Bean; Super HD; CLK; and Beyond Raw Chocolate Re-Grow. |
| Clinical features and laboratory results are highly suggestive of HEV infection, but lacking results of HEV PCR and anti-HEV IgG; invalid negative anti-HEV IgM as assessed by a not FDA approved test. Valid HAV exclusion. Anti-HBs positive after HBV immunization. Negative anti-CMV IgM and anti-VZV IgM. Positive anti-CMV IgG and anti-VZV IgG without assessed IgG titer changes in the further course; possibly resolving acute hepatitis by CMV and VZV coinfection. Valid exclusion of EBV, HCV, and HSV. ALT decrease not continuously straight but variable and undulating with intermittent spikes, possibly caused by small and limited HEV episodes or intermittent acute cholecystitis bouts. Positive Murphy sign, imaging data with up to 1 cm thickening of the gallbladder wall and surrounding fluids, suggestive of acute acalculous cholecystitis with discussed surgical consultation. Liver histology without suggestion of possible culprit(s). No antiviral therapy (HEV). Patient required OLT: decompensated liver cirrhosis with ascites, confirming prior CT result. Explanted liver not assessed for HEV PCR. After OLT, increased LFTs by rejection or HEV episodes. |
| CIOMS assessments |
| Poor case data quality. CIOMS is basically applicable only to acute and not chronic liver injury such as decompensated liver cirrhosis. With the updated CIOMS scale,16,25 tentative causality is excluded for old and new OEP, all additional DS, and AAP (Table 2). Intermittent use of the old OEP impedes a valid scoring; the large interval of 2-3 weeks between old OEP stop and admission impedes a valid assessment of the natural dechallenge course of ALT. Alternative diagnoses include highly probable virus infection such as HEV, possible acute acalculous cholecystitis, or others. |
| Final diagnosis |
| Decompensated liver cirrhosis; highly probable hepatitis E. |
| Decompensated liver cirrhosis of clinically unassessed etiology, preexisting and likely due to alcohol, acetaminophen, or HEV. HEV highly probable, suggested by high ALT values, lack of ALT dechallenge, and patient’s specific HEV risks. Presumably, physicians missed the tentative HEV diagnosis and effective antiviral drug therapy by ribavirin prior to OLT. |
| Alternative and other relevant diagnoses |
| 1. Suspected acetaminophen hepatotoxicity. Acetaminophen hepatotoxicity and liver cirrhosis with alcohol as predisposing factor remained unconsidered by regulatory2 or clinical assessments,17 as was the possible specific treatment by N-acetylcysteine to circumvent ALF and OLT. |
| 2. Possible acute acalculous cholecystitis. Clinical symptoms emerged 24 hours after a restaurant meal, compatible with this diagnosis and supported by thickening of the gallbladder wall and surrounding fluids, reported as suggestive of acute acalculous cholecystitis. |
| 3. Possible resolving acute hepatitis by CMV and VZV coinfection. |
| 4. Exclusion of hepatitis A-C, EBV, and HSV. |
| 5. Excluded liver injury causality for old OEP. |
| 6. Decompensated liver cirrhosis with excluded causality for new OEP and 13 other DS New OEP was used for 1 week as documented in the files or for 2 weeks as published.17 |
| 7. Morbid obesity. |
| 8. Significant asthma under therapy with synthetic drugs, causally unrelated to liver disease. |
| 9. Suspected HEV related cervical myelitis or cervical spinal syndrome, treated by acetaminophen-oxycodone. |
| 10. Fungal infection on both feet, treated by acetaminophen-hydrocodone and Bactrim. |
| 11. Previously diagnosed with ringworm, unrelated to liver disease. |
| • CASE 2. |
| Narrative, case details and analyses |
| Female patient with initial admission on 8/25/2013 and later transferal to the liver transplantation center due to ALF with liver transplantation on 9/9/2013. The day prior to admission: ALT 1416 U/L, AST 936 U/L, ALP 107 U/L, bilirubin 7.9 mg/dL. Two week history of vomiting and inability to eat prior to admission, yellowing of her eyes. Significant PMH: asthma (as child), hypertension, kidney stones left side, ovarian cyst right, sphincterotomy for choledocholithiasis, cholecystolithiasis, laparoscopic cholecystectomy in 2008, migraine, fracture right ring finger, treatment with ibuprofen, disc bulges, gastric vertical sleeve in 2012 that resulted in a 90-pound weight loss. The patient mentioned multivitamins prescribed by her PCP for vertical gastric sleeve as a consequence of morbid obesity, but denied despite multiple specific questionings use of any synthetic drug, DS, herb, or illicit drug at numerous occasions prior to OLT, as documented in the files. However, her statements are at variance to those of her partner, who informed the hospital staff that she has been taking a lot of medications for headaches. He found an empty bottle of migraine medications (in the car) that had Tylenol (i.e. acetaminophen), caffeine and something else. He also said that she has been taking a large amount of ibuprofen for migraines. There is also the note that she might have used new OEP for 3 or 6 weeks or a couple of months, possibly also since beginning of July 2013 and until two weeks before admission (vomiting, inability to eat) and phentermine for weight loss. Consumers purchase documents for OEP were not provided. The purchase records and medical records only support the consumption of the following DS prior to liver illness: Mega Men Perform & Vitality; Mega Men Sport Vitapak; Amino Energy; Stby Isopure; and Alph Isopure. The information on this case is inconsistent, fragmentary, and difficult to assess since it appears that the patient did not tell correct details; results of this assessment are therefore tentative. |
| Firm exclusion of hepatitis A - C and hepatitis by CMV, HSV, and VZV. High titers for anti-EBV IgG, suggestive of resolving acute EBV infection with already normal IgM; IgG titers not assessed in the further course to confirm the resolving EBV infection. Anti-HEV IgM and IgG negative, but applied antibody tests not described with their characteristics of sensitivity and specificity. HEV PCR not done in blood, stool, and liver. Upon ultrasound examination, initially some intrahepatic biliary duct dilatation in the right hepatic lobe and about the gallbladder fossa, but not persisting. Liver histology: Massive confluent necrosis associated with ballooning hepatocytes and cholestasis; periportal and perisinusoidal fibrosis. Final comment: The sinusoidal fibrosis is indicative of an underlying chronic process, likely NASH associated with patient’s prior BMI of 47. The etiology of the acute liver failure is uncertain, but pathologist’s report of liver histology: consideration of acetaminophen (AAP) toxicity is warranted given the patient’s history of gastric sleeve/gastric bypass procedure – a known risk factor for such toxicity. |
| CIOMS assessments |
| Insufficient data quality. Causality assessment with the updated CIOMS scale16,25 provides excluded causalities for the new OEP, phentermine, multivitamins, and various DS, a probable causality for acetaminophen, a possible causality for ibuprofen, and a likely causality for the multiple unidentified headache medicines (Table 2). |
| Final diagnosis |
| Acute liver failure by accidental chronic acetaminophen overdose. |
| This diagnosis was clinically missed, and timely specific therapy by N-acetylcysteine was withheld which could have prevented OLT. In his liver histology report, the pathologist reminded the physicians that consideration of AAP toxicity is warranted, a suggestion ignored by the physicians. Mainstream opinion suggests this kind of treatment in any case of acute liver failure, independent of the cause. Updated CIOMS score with a tentative probable causality for AAP. |
| Alternative and other relevant diagnoses |
| 1. Acute liver failure by the known potentially hepatotoxic nonsteroidal antiinflammatory drug ibuprofen. Ibuprofen is a known hepatotoxic drug, with a tentative possible causality level for the case according to the scale of the updated CIOMS (Table 2). Some key features are poorly documented. |
| 2. Possible resolving acute EBV infection. |
| Documented are high anti-EBV IgG titers, suggesting a resolving acute EBV infection in which IgM has vanished. Titers of anti-EBV IgG were not followed in the further course to confirm or disprove the assumed resolving acute EBV infection. |
| 3. Nonalcoholic steatohepatitis due to morbid obesity and following bariatric surgery. |
| Diagnosis based on liver histology result. Fatty liver (2008). |
| 4. Uncertain hepatotoxicity by “a lot of unidentified medications for headaches”. |
| The partner of the patient reported that she used a lot of unidentified medication for headaches (UMH); mainly due to poor data, CIOMS score provided a tentative excluded causality. |
| 5. Possible, not sufficiently excluded hepatitis E. |
| High ALT values and clinical features are compatible with and suggestive of HEV infection. HEV exclusion is fragmentary and likely done using antibody tests that are not FDA approved due to overt problems of specificity and sensitivity. HEV PCR was not assessed in blood, stool, and the explanted liver. |
| 6. Excluded hepatotoxicity causality for multivitamins. |
| Details of the used multivitamins are lacking: composition, daily dose, cumulative dose. Causality excluded, except in vitamin A overdose. |
| 7. Excluded hepatotoxicity causality for phentermine and multiple DS. |
| 8. Excluded hepatotoxicity causality for new OEP. |
| CIOMS causality for the new OEP is excluded (Table 2). As for the other products, cessation of the new OEP use likely was 2 weeks prior to admission, since the patient experienced severe abdominal symptoms including intractable vomiting and inability to eat. Therefore, ALT decline after OEP cessation cannot be validly scored. In addition, new OEP use by the patient is more than questionable and not substantiated by consumer purchase proof. |
| 9. Firm exclusion of hepatitis A, hepatitis B, hepatitis C, CMV, HSV, and VZV. |
| Appropriate exclusion was done. |
| 10. Migraine (2006). |
| Medications are documented, but not all are specified. |
| 11. Choledocholithiasis, sphincterotomy, cholecystolithiasis, laparoscopic cholecystectomy (2008). |
| 12. Hypertension (2006). |
| Specific drug therapy is likely but not documented. |
| 13. Kidney stones left side (2009). |
| Lack of relationship to acute liver failure. |
| 14. Disc bulges (1999). |
| Specific drug therapy is likely but not documented. |
| 15. Asthma as child. |
| • CASE 3. |
| Narrative, case details and analyses |
| The female patient used ferrous sulfate in 6/-7/2013, which is not hepatotoxic in normal doses: prescription is well documented, but not the indication, likely blood loss anemia of unknown etiology. On 6/12/2013, start with 50 doses of trama-dol-acetaminophen with unknown daily dose and duration, likely for pain relief, which may have emerged as initial symptoms of the later established acute hepatitis by HBV and VZV coinfection. Normal blood tests in 7/2013 claimed, but details not documented. On 8/12/2013, admission with diagnosis of obstructive jaundice, anemia, and abnormal LFTs. Subsequent diagnoses included acute liver failure (lacking criteria), hepatitis, or simple transaminitis. Treatment prior to admission documented for amoxicillin, clarithromycin, omeprazole, ibuprofen, and promethazine, all prescribed 8/1-3/ 2013, but not mentioned for tramadol-acetaminophen or OEP; OEP use was mentioned only late in the clinical course. Inconsistently described use of new OEP for 3 or 6 weeks, or a couple of months, possible prior use of old OEP for 3 and 2 years; proof of purchase not documented, also no documentation in the PCP files. On admission, AST 736 U/L, ALT 636 U/L, ratio AST/ALT constantly > 1, ALP 156 U/L, and bilirubin 6.5. Hgb 9.9 g/dL, cholesterol 272 mg/dL, BMI 33 kg/m2. Without specific treatment, her liver values failed to decline, as ALT was with 867 U/L on 11/30/2013 even higher than at admission, suggesting ongoing hepatitis. Unclear outcome in face of missed diagnosis and lacking specific therapy. Imaging report suggests acute versus chronic gallbladder disease. Immunization records: empty. Hepatitis B serologies obtained after admission and later establish acute hepatitis B virus infection: anti-HBs were twice positive within 4 weeks, while anti-HBc undulated and was positive, negative, and finally negative/positive. Hepatitis B surface antigen was already negative. Coinfection with acute VZV infection, confirmed by positive anti-VZV IgM and IgG. Firmly ex-cluded: hepatitis A - C, EBV, CMV, and HSV. Anti-HEV IgM negative by a kit from Focus Diagnostics that has not been approved by the FDA. HEV PCR assessment was not done, neither in blood nor in stool. Liver histology includes microgranulomata and shows acute confluent necrosis, analyzed by the pathologist with the knowledge of prior OEP use, evaluation thereby possibly biased: findings are described as consistent with DMAA supplement use - although DMAA supplement use by the patient is questionable and DMAA is not known as a hepatotoxic chemical, thereby lacking any typical histology features. |
| CIOMS assessments |
| Challenging evaluation by the updated CIOMS scale16,25 due to poor data, but provides hepatotoxicity causality gradings of excluded for old and new OEP, of unlikely for ferrous sulfate, of possible for AAP, and of excluded for additional drugs (Table 2). |
| Final diagnosis |
| Acute hepatitis by HBV and VZV coinfection. |
| The medical records substantiate the diagnosis of acute HBV and VZV coinfection: anti-HBs and anti HBc were positive; HBV immunization is not documented. Acute VZV hepatitis is ascertained by positive tests for anti-VZV IgM and IgG; microgranulomata are described in virus hepatitis including VZV. Both infections might have been occurred around or after June 2013. In June, the patient was on a trip in California for one week. Both diagnoses were missed, also potential therapy; prolonged clinical course under lacking therapy. Recovery was not documented in the files and not published.17 |
| Alternative and other relevant diagnoses |
| 1. Acute cholecystitis. |
| Ultrasound and abdomen MRT presented results of a thickening of the gallbladder wall, according to assessors suggestive of cholecystitis with pericholecystic fluids and consistent with acute versus chronic gallbladder disease. Clinical signs including prolonged abdominal pains and prolonged increase of LFTs are compatible with chole-cystitis. No therapy is documented in the files. |
| 2. Possible DILI by acetaminophen. |
| The use of acetaminophen-tramadol is well documented with an appropriate temporal association, suggesting a clinically possible hepatotoxicity by AAP. Associated clinical data are poor and impede a realistic CIOMS based evaluation (Table 2). |
| 3. Excluded DILI by medications such amoxicillin, and clarithromycin, omeprazole, ibuprofen, and promethazine. |
| The CVS record provides abundant medications that have been prescribed in the past years for the patient who was obviously not healthy. |
| However, the above listed medications were used after symptoms emerged, lacking a temporal association. |
| 4. Excluded hepatotoxicity by old and new OEP. |
| Inconsistent, poorly documented data of old and new OEP use, lacking purchase proof on the patient’s name. Possible actual use of new OEP for 3 weeks, 6 weeks, or a couple of months. Possible prior intake of old OEP for 3 and 2 years. Excluded causality for old and new OEP (Table 2). |
| 5. Insufficiently excluded hepatitis E. |
| Hepatitis E was insufficiently excluded by an anti-HEV IgM test that is not approved by the FDA. HEV PCR assessment was not done, neither in blood nor in stool. |
| 6. Firm exclusion of hepatitis A and C, EBV, CMV, and HSV. Through appropriate valid analyses, all five infections have been excluded with the required degree of certainty. |
| 7. Obesity, hypercholesterolemia. |
| BMI 33 kg/m2, obesity. Cholesterol 272 mg/dL. |
| 8. Blood loss anemia of yet not assessed etiology. Hgb 9.9 g/dL. |
| 9. Slight claustrophobia. |
| • CASE 4. |
| Narrative, case details and analyses |
| The symptoms of the female patient started with pruritus on 5/18/2013 and progressed to jaundice, dark urine, nausea, stomach pain, and rash, before LFTs were assessed. On 6/12/2013: ALT 1750 U/L, AST 1847 U/L, ALP 190 U/L, bilirubin 14.1 mg/dL. ALT declined slowly, undulating and with spikes, suggesting some hepatitis or cholecystitis episodes. Morbidities include hypertension, hypercholesterolemia, anxiety, insomnia, mild cataracts, polyp in rectum, pseudomelanosis coli. Her PCP’s medical files also documented problems with hot flushes and revealed multimorbidity treated by numerous drugs at various times, but scattered documentation prevails and impedes realistic associations between drug use and liver disease. Documented drug use includes phentermine and azithromycin, and in May 2013 one month before liver illness, she received prescriptions for Hydroxyzine hydrochloride, Kenalog ointment, methylprednisolone, diazepam, and clindamycin. Earlier in the year, the patient was prescribed Lisinopril every other day, betamethasone-clotrimazole, and acetaminophen-hydrocodone (Vicodin). Regarding documented use of herbs and DS, she has been on numerous undefined herbal supplements for months. This patient’s medical history states that the patient was taking the DS Amberen, Garcinia cambogia, Cellucor Super HD, Raspberry Ketone Chews, Vita Chews, and HCA Supreme. The medical records also noted that the patient may have taken some other DS, but she does not know the names of all of these. At the time of illness, the patient reported being on multiple undefined herbal supplements for months. The purchase history of DS before the liver illness includes not only OEP but also Vitamin Code Raw Calcium, Mega V products energy products, Hydro Pure, Amp 100% Whey Protein, Pre-Diet Cleanse, Meta Ignite, R3 Extreme Chrome, C4 Xtreme Blue, CLK, Compound 20, and many others. After the liver illness, this patient continued to purchase dietary supplements, including new OEP in 9/2013 as well as Hydroxycut, which also might have been used before, Total Body Rapid Cleanse Renew, Keto-Xt, and Premium Detox 7 day Cleanser. Therefore, DILI or HILI cannot be established as diagnosis due to lack of a temporal association and poor documentation. Prolonged and high dosed aciclovir therapy for genital herpes. |
| Abdominal ultrasound with contracted gallbladder and wall thickness of 8 to 9 mm, multiple stones, or sludge. Negative Murphy sign. MRCP: spleen 10.8 cm, trace periscystic fluid and ascites. Autoimmune parameters negative. Negative results for anti-HAV IgM, anti-HAV total, Monoscreen EBV antibody test, HBs Ag, anti-HBc IgM, and anti HCV; HBV and HCV PCR not done. Anti-CMV IgM negative, anti-CMV IgG positive but titer changes in the further course not assessed. HEV, HSV, and VZV not assessed. Liver histology report mentions severe cholestasis and that the most likely etiologies include adverse drug reaction, acute viral hepatitis, and AIH, testing for HEV can be considered. |
| CIOMS assessments |
| Using the updated CIOMS scale,16,25 causality for the old and new OEP, and many additional DS is excluded, and unlikely for drugs including AAP (Table 2). |
| Final diagnosis |
| Acute HSV hepatitis; acute cholecystitis with multiple gallbladder stones. |
| Clinical symptoms, high ALT values, liver histology, and antiviral HSV treatment by prolonged high dosed aciclovir therapy for genital herpes are suggestive of acute HSV hepatitis. |
| Alternative and other relevant diagnoses |
| 1. Resolving acute CMV hepatitis. |
| Anti-CMV IgG titers increased with normal IgM titers, a constellation compatible with a resolving acute CMV infection where IgM had already disappeared. It was forgotten to determine IgG in the further clinical course with assessment of quantitative titers to evaluate titer changes. |
| 2. Acute hepatitis, preferentially hepatitis E (not yet excluded). |
| Liver histology suggests acute virus hepatitis and testing for HEV, but clinicians ignored this advice. |
| 3. Insufficiently excluded HBV, HCV, and VZV. |
| PCR analyses were forgotten for HBV and HCV, their exclusion is incomplete. VZV infection was not excluded. Firm exclusion of EBV. |
| 4. Liver injury with excluded causality for drugs, including acetaminophen, diazepam, lisinopril, and clindamycin. Various drugs or DS are good candidates, but basic documentation is insufficient and does not allow a firm conclusion. There was lack of a tox-screening regarding acetaminophen. OEP use may have been started before symptoms such as pruritus. |
| 5. Hepatotoxicity with excluded causality for dietary supplement(s) such as Garcinia cambogia or multiple others. |
| 6. Hepatotoxicity with excluded causality for old and new OEP. |
| 7. Pseudomelanosis coli. |
| The endoscopic diagnosis of pseudomelanosis coli implies a constipation problem, substantiated by the documented use of cleansing products with possible hepatotoxic adverse effects. |
| 8. Essential hypertension under drug therapy. |
| Drug treatment by lisinopril every other day, unknown duration. |
| 9. Menopause: drug therapy (?). |
| Documented at one place is the wish of the patient to get drug therapy, but it is not documented whether this was provided. |
| 10. Hypercholesterolemia; drug therapy (?). |
| Poor documentation. |
| 11. Anxiety. |
| Possible therapy with diazepam. |
| 12. Migraine: drug therapy (?). |
| Therapy not documented. |
| 13. Cancer of left breast, with local simple mastectomy. |
| No association to liver disease. |
AAP: acetaminophen. ALF: acute liver failure. ALP: alkaline phosphatase. ALT: alanine aminotransferase. AST: aspartate aminotransferase. CIOMS: Council for International Organizations of Medical Sciences. CMV: cytomegalovirus. EBV: Epstein Barr virus. HAV: hepatitis A virus. HBV: hepatitis B virus. HBs: hepatitis B surface. HBc: hepatitis B core. HCV hepatitis C virus. HSV: herpes simplex virus. NASH: non-alcoholic steatohepatitis. OEP: OxyELITE Pro. PCR: polymerase chain reaction. PCP: primary care provider. VZV: varicella zoster virus.
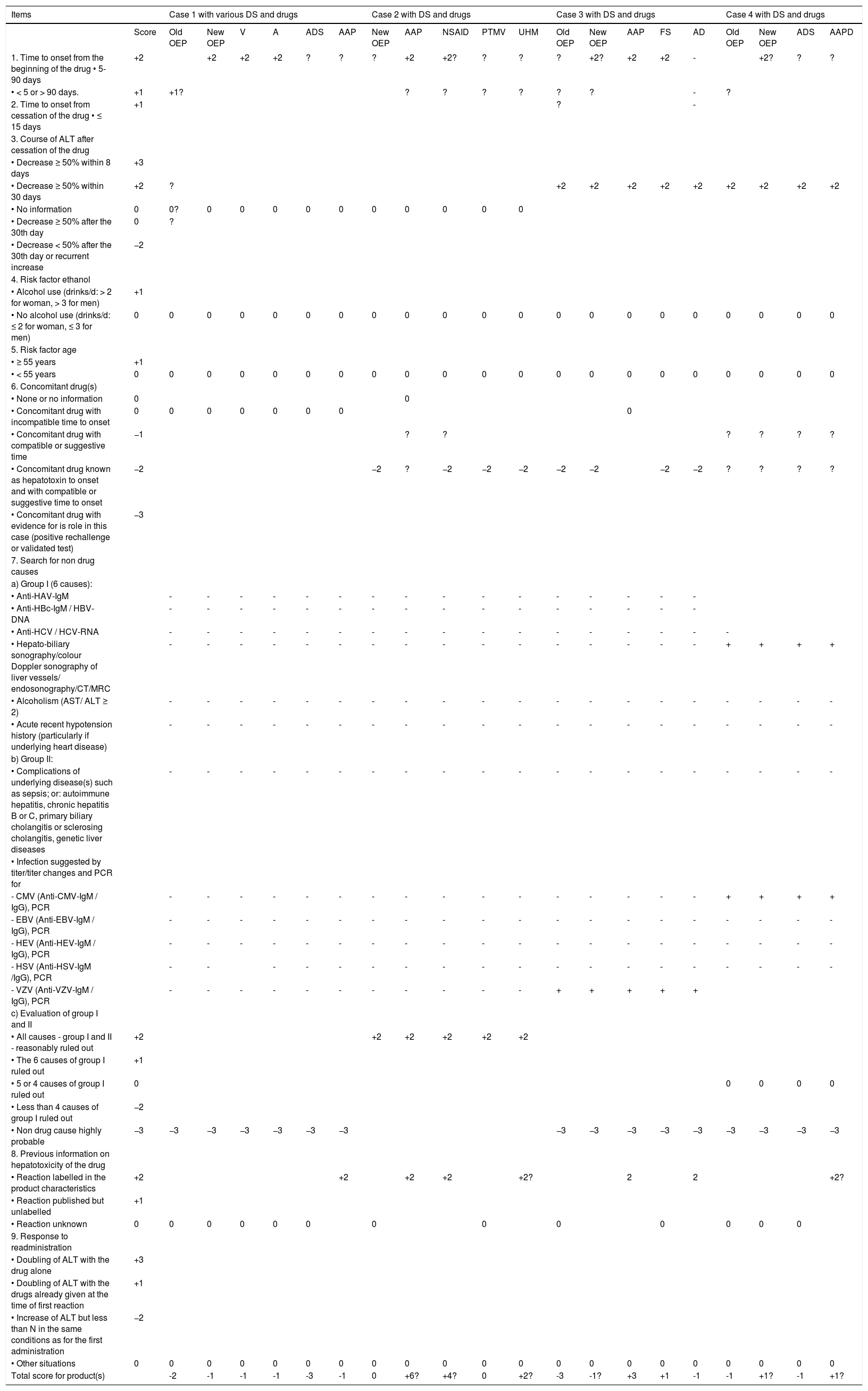
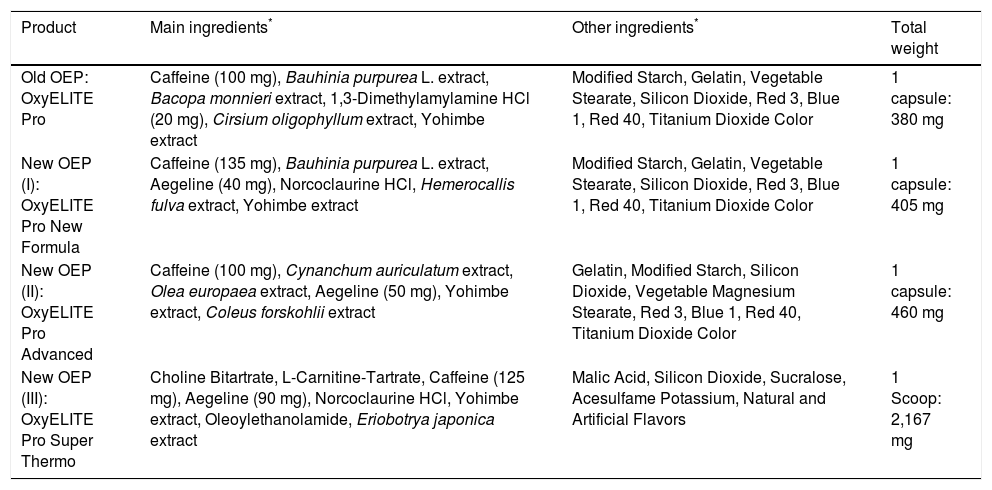
Similar to our first analyzed single Honolulu case,18 in all four cases of this analysis, patients had multiple diseases and medications (Tables 1 and 2). In other words, they were not “previously healthy” as claimed.2,17 For comparison, case narratives of these four patients (Table 1) followed the order of the recent report.17 All case data are submitted to a causality assessment using the updated CIOMS scale (Table 2), also called Roussel Uclaf Causality Assessment Method (RUCAM), which is quantitative, structured and liver specific.16 Detailed consideration was given to the different OEP formulas, containing DMAA or aegeline (Table 3).17
Cases 1-4 with causality assessments for various DS and drugs using the updated CIOMS scale.
| Items | Case 1 with various DS and drugs | Case 2 with DS and drugs | Case 3 with DS and drugs | Case 4 with DS and drugs | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Old OEP | New OEP | V | A | ADS | AAP | New OEP | AAP | NSAID | PTMV | UHM | Old OEP | New OEP | AAP | FS | AD | Old OEP | New OEP | ADS | AAPD | |
| 1. Time to onset from the beginning of the drug • 5-90 days | +2 | +2 | +2 | +2 | ? | ? | ? | +2 | +2? | ? | ? | ? | +2? | +2 | +2 | - | +2? | ? | ? | ||
| • < 5 or > 90 days. | +1 | +1? | ? | ? | ? | ? | ? | ? | - | ? | |||||||||||
| 2. Time to onset from cessation of the drug • ≤ 15 days | +1 | ? | - | ||||||||||||||||||
| 3. Course of ALT after cessation of the drug | |||||||||||||||||||||
| • Decrease ≥ 50% within 8 days | +3 | ||||||||||||||||||||
| • Decrease ≥ 50% within 30 days | +2 | ? | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | ||||||||||
| • No information | 0 | 0? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| • Decrease ≥ 50% after the 30th day | 0 | ? | |||||||||||||||||||
| • Decrease < 50% after the 30th day or recurrent increase | −2 | ||||||||||||||||||||
| 4. Risk factor ethanol | |||||||||||||||||||||
| • Alcohol use (drinks/d: > 2 for woman, > 3 for men) | +1 | ||||||||||||||||||||
| • No alcohol use (drinks/d: ≤ 2 for woman, ≤ 3 for men) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5. Risk factor age | |||||||||||||||||||||
| • ≥ 55 years | +1 | ||||||||||||||||||||
| • < 55 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6. Concomitant drug(s) | |||||||||||||||||||||
| • None or no information | 0 | 0 | |||||||||||||||||||
| • Concomitant drug with incompatible time to onset | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||
| • Concomitant drug with compatible or suggestive time | −1 | ? | ? | ? | ? | ? | ? | ||||||||||||||
| • Concomitant drug known as hepatotoxin to onset and with compatible or suggestive time to onset | −2 | −2 | ? | −2 | −2 | −2 | −2 | −2 | −2 | −2 | ? | ? | ? | ? | |||||||
| • Concomitant drug with evidence for is role in this case (positive rechallenge or validated test) | −3 | ||||||||||||||||||||
| 7. Search for non drug causes | |||||||||||||||||||||
| a) Group I (6 causes): | |||||||||||||||||||||
| • Anti-HAV-IgM | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| • Anti-HBc-IgM / HBV-DNA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| • Anti-HCV / HCV-RNA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| • Hepato-biliary sonography/colour Doppler sonography of liver vessels/ endosonography/CT/MRC | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | |
| • Alcoholism (AST/ ALT ≥ 2) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| • Acute recent hypotension history (particularly if underlying heart disease) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| b) Group II: | |||||||||||||||||||||
| • Complications of underlying disease(s) such as sepsis; or: autoimmune hepatitis, chronic hepatitis B or C, primary biliary cholangitis or sclerosing cholangitis, genetic liver diseases | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| • Infection suggested by titer/titer changes and PCR for | |||||||||||||||||||||
| - CMV (Anti-CMV-IgM / IgG), PCR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | |
| - EBV (Anti-EBV-IgM / IgG), PCR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - HEV (Anti-HEV-IgM / IgG), PCR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - HSV (Anti-HSV-IgM /IgG), PCR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| - VZV (Anti-VZV-IgM / IgG), PCR | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | |||||
| c) Evaluation of group I and II | |||||||||||||||||||||
| • All causes - group I and II - reasonably ruled out | +2 | +2 | +2 | +2 | +2 | +2 | |||||||||||||||
| • The 6 causes of group I ruled out | +1 | ||||||||||||||||||||
| • 5 or 4 causes of group I ruled out | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||
| • Less than 4 causes of group I ruled out | −2 | ||||||||||||||||||||
| • Non drug cause highly probable | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | −3 | |||||
| 8. Previous information on hepatotoxicity of the drug | |||||||||||||||||||||
| • Reaction labelled in the product characteristics | +2 | +2 | +2 | +2 | +2? | 2 | 2 | +2? | |||||||||||||
| • Reaction published but unlabelled | +1 | ||||||||||||||||||||
| • Reaction unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 9. Response to readministration | |||||||||||||||||||||
| • Doubling of ALT with the drug alone | +3 | ||||||||||||||||||||
| • Doubling of ALT with the drugs already given at the time of first reaction | +1 | ||||||||||||||||||||
| • Increase of ALT but less than N in the same conditions as for the first administration | −2 | ||||||||||||||||||||
| • Other situations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total score for product(s) | -2 | -1 | -1 | -1 | -3 | -1 | 0 | +6? | +4? | 0 | +2? | -3 | -1? | +3 | +1 | -1 | -1 | +1? | -1 | +1? | |
Clinical case documentation is extremely poor, impeding substantially a sophisticated evaluation. Causality assessment was provided for cases 1-4, with case details presented in table 1. Causality for various DS and drugs is accomplished by using the updated scale of Council for International Organizations of Medical Sciences (CIOMS), considering the items for the hepatocellular type of liver injury according to published criteria16,25 and using the updated CIOMS scale available for this specific type of injury. In the above section 6 of concomitant drug(s), the following products were considered: synthetic drugs, dietary supplements including herbal ones, and polyherbal products. In the section 7 (search for non drug causes), the symbol of - denotes that the obtained result was negative and that of + was positive, whereas lack of a symbol indicates that assessment was not or only partially performed. HEV antibody test results are invalid since tests are not FDA approved. A: amplified wheybolic extreme-60. AAP: acetaminophen. AD case 3: additional drugs (use after onset: amoxicillin, ibuprofen, clarithromycin, omeprazole, and promethazine). AD case 4: additional drugs (aciclovir high dosed, lisinopril, hydroxyzine hydrochloride; obviously numerous drugs, insufficiently documented); ADS case 1: additional DS (Clk/Super Hd Combo Kit; Ripped Freak; Kre-Alkalyn EFX; Pump-HD; Green Coffee Bean; Super HD; CLK; and Beyond Raw Chocolate Re-Grow); ADS case 4: additional DS (Amberen; Garcinia cam-bogia; Cellucor Super HD; Raspberry Ketone, Vita Chews; HCA Supreme, and many others). ALT: alanine aminotransferase. AST: aspartate aminotransferase. CMV: cyto-megalovirus. CT: Computed tomography. EBV: Epstein Barr virus. FS: ferrous sulfate. HAV: hepatitis A virus. HBV: hepatitis B virus. HBc: hepatitis B core. HCV: hepatitis C virus. HEV: hepatitis E virus. HSV: herpes simplex virus. MRC: Magnetic resonance cholangiography. MV: multivitamin (section includes various additional DS such as Mega Men Perform & Vitality; Mega Men Sport Vitapak; Amino Energy; Stby Isopure; and Alph Isopure). New OEP: OEP Super Thermogenic New Formula or other types; NSAID: non steroidal antiinflammatory drugs. OEP: OxyELITE Pro. PCR: polymerase chain reaction. PT: Phentermine. UHM: unidentified headache medications. V: Versa-1. VZV: varicella zoster virus. Total points/causality: ≤ 0 = excluded; 1-2 = unlikely; 3-5 = possible; 6-8 = probable; ≥ 9 = highly probable.
Formulas of OEP products used by the patients of the present analysis (cases 1-4).
| Product | Main ingredients* | Other ingredients* | Total weight |
|---|---|---|---|
| Old OEP: OxyELITE Pro | Caffeine (100 mg), Bauhinia purpurea L. extract, Bacopa monnieri extract, 1,3-Dimethylamylamine HCl (20 mg), Cirsium oligophyllum extract, Yohimbe extract | Modified Starch, Gelatin, Vegetable Stearate, Silicon Dioxide, Red 3, Blue 1, Red 40, Titanium Dioxide Color | 1 capsule: 380 mg |
| New OEP (I): OxyELITE Pro New Formula | Caffeine (135 mg), Bauhinia purpurea L. extract, Aegeline (40 mg), Norcoclaurine HCl, Hemerocallis fulva extract, Yohimbe extract | Modified Starch, Gelatin, Vegetable Stearate, Silicon Dioxide, Red 3, Blue 1, Red 40, Titanium Dioxide Color | 1 capsule: 405 mg |
| New OEP (II): OxyELITE Pro Advanced | Caffeine (100 mg), Cynanchum auriculatum extract, Olea europaea extract, Aegeline (50 mg), Yohimbe extract, Coleus forskohlii extract | Gelatin, Modified Starch, Silicon Dioxide, Vegetable Magnesium Stearate, Red 3, Blue 1, Red 40, Titanium Dioxide Color | 1 capsule: 460 mg |
| New OEP (III): OxyELITE Pro Super Thermo | Choline Bitartrate, L-Carnitine-Tartrate, Caffeine (125 mg), Aegeline (90 mg), Norcoclaurine HCl, Yohimbe extract, Oleoylethanolamide, Eriobotrya japonica extract | Malic Acid, Silicon Dioxide, Sucralose, Acesulfame Potassium, Natural and Artificial Flavors | 1 Scoop: 2,167 mg |
The quality of case data for completeness and details included was insufficient (Tables 1 and 4). This applies also to Wilson disease that was not validly excluded by laboratory analyses and clinical assessments, considering that ceruloplasmin alone is not an appropriate diagnostic parameter. For transparency, case data included in the present study (Tables 1, 2, and 4) are compared to case data and their interpretations in prior publications,2,17 as presented for each individual case (Tables 5-8). This approach provides for each case a final main diagnosis and a list of alternative and other relevant diagnoses. To overcome the shortcomings of the previous reports by regulators and physicians,2,17 we have presented as many details as possible (Tables 1 and 5-8).
Certainly, alcohol and hepatotoxic drugs including AAP and ibuprofen are key components for a critical discussion (Tables 1–8). Since these liver diseases occurred in the summertime, were clustered geographically and localized to Hawaii, infectious and toxic causes should be considered, including hepatitis A,3,29 hepatitis E,2–15 leptospirosis,30,31 aflatoxicosis,32–40 pyrrolizidine alkaloids (PA),41–44 green tea extracts,45 noni juice,46 and kava.48–52
- •
Hepatitis A. HAV infection is a well-known problem in Hawaii, and health officials have alerted the public about this disease.28 This possibility was not specifically addressed and publicized by the regulators2 or physicians.17 In the updated CIOMS scale, HAV is to be excluded during clinical assessment of patients with suspected liver injury, at least by anti-HAV IgM analysis.16 This test certainly detects infection with short incubation times29 but may miss longer and latent disease courses like those in the cluster cases due to very broad time frames allowed as inclusion criteria by the regulators.2 For these long times,2 HAV IgM and IgG antibodies29 would have been better suited to exclude HAV infections (Tables 5-8).2,17 In the four assessed cluster cases, HAV was likely excluded by anti-HAV IgM in cases 1 and 2, by total anti-HAV in case 3, and by both parameters in case 4 (Tables 5-8).
- •
Hepatitis E. With well-documented HEV reservoirs in infected animals in Hawaii,13 exposed persons are prone to acquire HEV infections, a possibility at least in some summertime cluster cases.2,17 However, attempts to verify or disprove HEV infections in the cluster patients were not discussed or published by regulators2 or clinicians.17 After analysis of five cases from the Hawaii cluster, which comprized the four cases (Tables 5-8) and the fifth case published recently,18 in two out of these five analyzed cases (40%), neither anti-HEV IgM, anti-HEV IgG, nor HEV PCR was assessed, namely in case 4 (Tables 1 and 8) and the single case published earlier.18 In none of the remaining three patients (cases 1-3) was HEV PCR assessed (Tables 5-7). Anti-HEV IgM was determined as negative in cases 1-3, and anti-HEV IgG was tested negative in case 2 but not in the others (Tables 1 and 5-8).
Neglecting to perform HEV PCR testing to quantify virus RNA copies4 for all five patients, namely the cases 1-4 (Tables 5-8) and the published case,18 is a regulatory and clinical disaster, as it prevents a thorough evaluation of this summertime cluster by ignoring an important alternative cause.2,17 The patients’ files do not reveal a stringent and transparent regulatory or clinical protocol to resolve the HEV issue in the cluster patients. It appears that HEV antibody tests were ordered only haphazardly. Anti-HEV IgM analyses, which have only a short diagnostic window,4 were sometimes performed during the late phase of liver illness when HEV IgM antibodies may have already vanished. Additionally, HEV antibody tests in the US lack FDA approval4,14 and are plagued by poor sensitivity and specificity.4 It is unclear whether these test characteristics are related to the various HEV genotypes,4–6 raising the question whether all relevant HEV genotypes are detected by the available HEV antibody tests. For one patient (case 3) (Table 4), the patient’s files identify a kit from Focus Diagnostics used for antiHEV IgM testing, with the information source available in the legend of table 4; since all patients were cared for at a single medical center, this kit likely was used in all other patients, although not approved by the FDA. The manufacturer recommends that this test should not be used for diagnosis without confirmation by other medically established means; this restriction specifically applies to tests for hepatitis E antibody types IgM and IgM, IgG. Consequently, all HEV antibody test results in the cluster cases are insecure and are to be questioned (Tables 1, 2, and 4-8).
In other countries, validated HEV antibody tests are approved and marketed.15 These antibody tests should be used in the Hawaii cluster patients to retrospectively test retained patients’ biological material of blood, obtained at various time intervals to investigate HEV infections; concomitantly, PCR analyses should be done in retained blood, stool and liver specimens. Actual testing is also essential in the patient with the clinically suspected HEV infection (case 1) who still has increased liver function tests following orthotopic liver transplantation (OLT), which might be ascribed to rejection events or still ongoing HEV infection episodes. Missing a HEV diagnosis might result in delaying or withholding an effective therapy in severe HEV infections beyond the point of self-limitation and complete cure4,6,20 and may further harm the affected patient.
Of particular diagnostic importance but likely not yet recognized by regulators2 or physicians17 are extrahepatic neurological symptoms prior to or early in a HEV disease.4,6 Neurological manifestations and complications of HEV are described in detail as polyradiculopathy,4,6 bilateral brachial neuritis,6 or peripheral neuropathy.4 These neurological findings likely are caused by a localized cervical myelitis by hepatitis E viruses and may overshadow the liver injury symptoms.4 Among the analyzed cluster patients, the case files for one patient (case 1) (Tables 1 and 5) documented a prescription of Percocet (acetaminophen-oxycodone) in early 2013 for neck pain and paresthesia in the arm, but this severe neurological symptomatology was not further investigated and later not considered in connection with a possible HEV infection.
For most cluster cases, hepatitis is well documented as initial diagnosis, based on clinical symptoms and supporting laboratory values. For instance, ALT values of 1,970 U/L are extremely high and suggestive of an acute infection by a hepatitis virus such as HEV in case 1 (Tables 1 and 5). This unusually high ALT value certainly is not suggestive of DILI by OEP as assumed before.2,17 For established DILI, mean serum ALT values of 398 ± 442 U/L (SD) were published as opposed to mean serum ALT values of 1410 ± 799 U/L (SD) in patients with proven acute HEV.15 These liver enzyme patterns may dissociate DILI from HEV and should have been recognized early in the actual cluster management.2,17 Misidentifying HEV as DILI has been reported for various countries,14,15 including the US.14 Therefore, it seems justified to demand that DILI4,14,15 and HILI cases16,24 are not liver injury cases unless HEV is firmly excluded, a requirement also for the present Hawaii cluster patients.2,17
- •
Leptospirosis. Leptospirosis is a common problem in Hawaii due to infected rats and mice.30,31 It usually affects the liver and the kidneys but was not explicitly discussed or considered2,17 and rarely excluded in the cluster cases by appropriate tests (Tables 1 and 5-8).
- •
Aflatoxicosis. Aflatoxins may cause epidemic toxic hepatitis by contaminated food in humid and hot areas.32–40 It was not considered in the cluster cases by epidemiologists, regulators,2 or clinicians.17
- •
Pyrrolizidine alkaloids. PAs were not discussed although they have caused toxic hepatitis epidemics described following consumption of PA containing plants.41–44
- •
Green tea. Green tea extracts also are potentially hepatotoxic,45 not adequately considered as possible causes for the cluster patients by the regulators2 and clinicians.17
- •
Noni juice.Morinda citrifolia, a typical Hawaiian plant, and the Noni juice prepared from the fruits of this tree is potentially hepatotoxic,46 not yet considered as possible cause.2,17
- •
Kava. Awa is the Hawaiian name of kava (Piper methysticum), a shrub grown in the South Pacific region and Hawaii. Kava prepared from its rhizomes is a popular traditional beverage in Hawaii47 with potential rare hepatotoxicity,48–52 which remained unconsidered in previous evaluations by the epidemiologists, regulators,2 and clinicians.17
Clinicians used for their analysis of the cluster cases17 the old CIOMS scale, published in 199353,54 rather than the updated, current CIOMS scale with specific items including HEV evaluation (Table 2).16,25,55–57 Case data were presented incompletely (Table 4) in their report17 as shown in the present study (Tables 5-8). CIOMS based case assessment likely was done retrospectively17 rather than prospectively as recommended; for this purpose the CIOMS method was updated to support clinicians with a robust assessment framework for causality evaluation early in the clinical course.14,24 We also strongly recommended early prospective clinical evaluation by the updated CIOMS scale, ideally when the diagnosis is suspected and the symptoms are unfolding, rather than retrospectively.14 This also ensures collection of complete case data sets, to be presented as itemized CIOMS key points that allow individual scores and avoid nontransparent summarized scores as those published.17
The published CIOMS-based analyses erroneously attributed total CIOMS scores of 6-7 for their cases 1-4.17 Such high scores commonly are a privilege of cases with excellent and complete data sets but are not achievable with poor case data as reassessed (Tables 1, 2, and 5-8). Multiple unpublished and ignored potentially hepatotoxic drugs as comedication17 reduced the scoring, as do incomplete case data and poor dechallenge data (Tables 1, 2, and 5-8). Patients’ files also commonly fail to document valid ALT decreases by ≥ 50% within 8 days after cessation of drug or DS use and not just after admission (Table 2), or exclusion of most likely alternative causes was insufficient (Tables 1, 2, and 5-8), which again reduces considerably individual and total scores (Table 2). The incorrect or even lacking transfer of basic data from the medical records and files (Tables 1, 2, and 5-8) into the publication17 is indeed remarkable.
The incomplete case data presentation (Tables 1 and 4-8) is not expected from reputed regulatory agencies or medical centers. Problems focus on case data retrieving, selection, documentation, interpretation, and public presentation.2,17 Major shortcomings also are evident for the documented clinical case management that was not appropriately stringent. In particular, there was no documented procedural approach about how to verify or exclude alternative and treatable liver diseases such as CMV, HEV, HSV, VZV, and acetaminophen overdose. Ordered arbitrarily, most test results were presented inconsistently. Pathologists were informed on OEP use prior to their evaluation of liver specimens, possibly leading to biased assessment and a tendency not to seek thoroughly for alternative causes.17 Nevertheless, two exemptions are documented, one pathology report suggested hepatotoxicity by acetaminophen overdose for case 2 (Tables 1 and 6); the other report judged features compatible with HEV and recommended exclusion of HEV in case 4 (Tables 1 and 8). These suggestions would call for additional diagnostic efforts to consider possible effective treatments but were disregarded by the physicians17 who were focused on OEP as culprit (Table 1). Other major discrepancies between the published account and the facts documented in the medical records are summarized (Tables 2 and 5-8).
Among the Hawaii patients, comedication was frequent, with > 10 drugs or > 17 DS; this is a major confounder, and particularly scary for the cluster cases (Tables 5-8). The documented use of synthetic drugs and DS was likely for 5 drugs and 14 DS in case 1 (Table 5), > 10 drugs and 7 DS in case 2 (Table 6), 7 drugs and 0-2 DS in case 3 (Table 7), and 10 drugs and > 17 DS in case 4 (Table 8). Regulators failed to report the use of any of the comedicated synthetic drugs in any of their published case,2 in line with the clinical report.17
Final diagnosesThe four cluster patients studied in this report had an extraordinary high frequency of other diseases; all were classified as “previously healthy” by regulators2 and physicians,17 but this was not corroborated by the analyzed documents (Tables 5-8). Contrary to the diagnoses of OEP hepatotoxicity,2,17 various OEP-unrelated diseases may be responsible in these cluster cases following this analysis (Tables 1, 2, and 5-8).
- •
Case 1. Decompensated liver cirrhosis; highly probable hepatitis E. The decompensated liver cirrhosis (Tables 1 and 5) remained clinically unassessed regarding its possible causes and was not disclosed or discussed in the published regulatory and clinical reports2,17 but can hardly be attributed to a two-week use of the new OEP as claimed;17 instead, this patient was at risk for HEV infections (Table 1), may have had a prior alcohol problem, and had used AAP. Potential treatment of low-dosed ribavirin for severe HEV infections4,6,20 or N-acetylcysteine for AAP hepatotoxicity19 remained unconsidered prior to OLT.2,17 In early 2013, he experienced pain in neck and paresthesia in the arm, possibly early extrahepatic, neurological manifestations of HEV as viral cervical myelitis in line with well described neurologic complications such as polyradiculopathy,4,6 bilateral brachial neuritis,6 or peripheral neuropathy.4 These neurologic findings can overshadow the liver injury.4 ALT values were extremely high and suggestive of an acute infection by a hepatitis virus, including HEV. In support of hepatitis as cause, ALT values showed only a slow decrease, which was not continuously straight down but undulating; short HEV episodes may explain undulation and some intermittent spikes of ALT. Overall, clinical assessment and documentation of hepatitis E was insufficient.
- •
Case 2. Acute liver failure by acetaminophen overdose. In this patient, AAP hepatotoxicity with ALF is the most probable diagnosis according to the analyzed clinical documentation and was suggested as likely by the liver pathologist (Tables 1 and 6) - but not considered or discussed by regulators or clinicians.2,17 Treatment options of N-acetylcysteine for AAP overdose remained unconsidered prior to OLT.17
- •
Case 3. Acute hepatitis by HBV and VZV coinfection. Acute hepatitis B and acute VZV hepatitis (Tables 1 and 7) were not recognized, neither by regulators nor clinicians.2,17 Evidence of self-limiting clinical course is not provided, specific antiviral therapy by nucleos(t)ide analogues21 and aciclovir22 may still be required because of the prolonged clinical course (Tables 1 and 7) as reported.17
- •
Case 4. Acute HSV hepatitis; acute cholecystitis with multiple gallbladder stones. The liver pathologist suggested acute hepatitis and testing for HEV, which remained unconsidered by clinicians (Tables 1 and 8) and in publications.2,17 Although the initial regulatory and clinical diagnosis of OEP hepatotoxicity was incorrect,2,17 antiviral treatment of HSV by prolonged and high-dose aciclovir22 was finally correctly done (Tables 1 and 8).
Reevaluations of the complete clinical data and other documents reveal that the Hawaii liver cases are not mysterious any more. The published diagnoses of OEP hepatotoxicity for the Hawaii liver disease case cluster2,17,58,59 are not supported by their reevaluation; other, more likely alternatives are identified in at least 5 patients by the present analysis (Tables 1, 2 and 5-8) and the previous evaluation.18 Related to the liver disease cluster in Hawaii, it is now clear that we are dealing not any more with a case mystery but more likely with both a medical center mystery of the QMC and a regulatory mystery of the HDOH, CDC, and FDA.
It is unclear how clinical and regulatory assessments could have resulted in a multiplicity of erroneous results and conclusions. Obviously, confounding variables prevail at different levels including clinicians, regulators, and patients, that merit further attention.
- •
Clinicians and regulators. Equating a temporal association with a causal association obviously was the initial error that led QMC clinicians to assign OEP as the sole culprit of all cluster cases, both initially2 and consecutively.17,58,59 After the initial cases were seen as “OEP hepatitis”, this clinical misconception was sustained and culminated in biased clinical and diagnostic conclusions, which are by no means acceptable (Tables 1, 2 and 5-8). This first misinterpretation led to additional confounding variables and resulted in the clinical QMC mystery in Honolulu,58,59 due to its inability to provide solid, impartial work that would have allowed correct diagnoses and appropriate therapies in the cluster cases. Close analysis of the complete clinical data provides insight how additional or misfitting data were neglected, misinterpreted, or simply deleted from further assessment to support the initial OEP concepts (Tables 1, 2 and 5-8). Most irritating is also the clinical upgrading of CIOMS scores,17 not corroborated by documented data (Tables 2 and 5-8). It remains a mystery why the published and publicized statements2,17,58,59 did not receive the benefit of prior internal and external critical assessments by qualified external peers or experienced superior(s) to ensure impartiality and valid work - a prerequisite to solving controversial and/or mysterious clinical situations. Physicians should have been prepared to cope pragmatically with complex diseases, otherwise additional confounders may emerge.
Regulators of HDOH, CDC, and FDA claimed having reviewed the medical charts of the cluster patients.2 Their reviews obviously overlooked incomplete or incorrect clinical data and accepted uncritically the unproven clinical concepts from the Honolulu QMC2 that OEP was causing toxic hepatitis.17 Although the numerous conceptional and clinical flaws are clearly documented in the clinical records (Tables 1, 2 and 5–8), regulators failed to recognize these flaws.2 This represents another confounder and a problem of regulatory bias, remaining a regulatory mystery. Consequently, neither the external regulatory cluster case review nor a tentative internal review of the medical center worked properly, a poor situation in an attempt to search for an impartial solution of a regulatory and clinical issue. These conditions may call for an official hearing to investigate the overt problems, to provide clarity, and to avoid similar problems in the future. Regarding the incriminated OEP products, discrepancies are evident since the CDC report considers globally all OEP products as hepatototoxic,2 whereas the clinical report only focused on the new OEP that contains aegeline,17 a herbal chemical found in the tree Aegle marmelos (bael), a plant with a historical use in Ayurvedic medicine and Traditional Chinese Medicine. The QMC physicians did not consider aegeline as hepatotoxic,17 in support of their earlier statement that they are still unsure about the cause, favoring causal connections more with race, ethnicity, and genetics, but this assumption again is not proven by a corresponding control group.58 However, their statements17,58 are inconsistent with other conclusions of the same medical center that aegeline is identified as harmful and as cause for their liver diseases, but evidence for this new claim again was not provided.59 In fact, regulators of the CDC, HDOH, and FDA did not publicly claim any ingredient of OEP as causative; rather, they correctly stated that attributing liver injury to a specific ingredient can be challenging because of multiple ingredients and product variability.2 Based on present evidence and the confirmed lack of hepatotoxicity by any OEP product shown previously18 and in the present analysis (Tables 5-8), causation discussions around any putative chemical ingredient are less promising. All ingredients of the various OEP products are listed (Table 3). None of these has been considered validly as hepatotoxic based on an internet search. We have not attempted to present the ingredients of the other DS used by the cluster patients (Tables 1 and 5-8).
- •
Patients. Confounders also affect the cluster patients and substantially influence the overall assessment quality. The information presented by the patients as documented in their files often is inconsistent (Tables 1 and 5-8); some details appear fictitious and may belong to wilful overreporting of OEP use. This likely is a consequence of the publicity associated with OEP at the time their assumed disease emerged, recognized by patients as a chance to improve their financial situation. As an example, one patient (case 2) vigorously denied any use of DS despite multiple questionings and only “admitted” OEP use when the OEP issue was discussed publicly and with their QMC physicians (Tables 1). For only a few patients, OEP purchase was retrospectively verified through consumer purchase records or documentation in the files of their PCPs (Table 1). In support of this assumption, the CDC report correctly stated that attributing liver injury can be challenging because product intake cannot be proven since tests are lacking.2 The analyzed documents also suggest an unusually straight-forward strategy of physicians at QMC to “collect” as many cases as possible to bolster their initial claims of OEP hepatotoxicity. Well documented are discussions in which physicians repeatedly informed the patients that only OEP is to be considered as cause of their liver disease, alternatives were not discussed and also were not assessed, assuring the patients of OEP as the single explanation. This approach may have encouraged the patient to seek legal assistance.
Aggravating risk factors as confounders in the cluster cases include preexisting liver disease such as non-alcoholic fatty liver disease (NAFLD), which is commonly associated with overweight, obesity, and morbid obesity, well described as a risk factor for DILI by additional synthetic drugs,24,60 and possibly relevant for some analyzed cases (Tables 1 and 5-8). Similarly, alcohol use predisposes to AAP hepatotoxicity,61 multimedication due to multimorbidity is a risk factor for DILI,62 and preexisting liver diseases including alcoholic liver cirrhosis for HEV.6
Other major confounding variables are uncovered; among these are inappropriate diagnostic and clinical evaluations, poor data quality, unverified DS and drug use with unknown latency periods and daily dose, unassessable dechallenge characteristics in the analyzed cluster cases, and lacking search for therapeutical options by effective drugs to avoid OLT (Tables 1, 2 and 5-8).
- •
Regulatory agencies. In future cases, a regulatory statement by officials of HDOH, CDC, and FDA like having reviewed medical charts should not be published unless this has been done with the required scrutiny. None of the analyzed files documented a regulatory assessment, nor were clinical results and conclusions questioned, or regulatory recommendations based on their review documented. Scientifically, this CDC report seemed to erroneously imply regulatory approval of the clinical files despite insufficient case data documentation and to suggest both completeness of case evaluations and correctness of the resulting clinical conclusions. The lack of an efficient regulatory medical chart review protocol and the overlooking of major documented clinical flaws (Tables 1, 2 and 5-8) will invalidate any official statement or document.2 In the future, a thorough well documented regulatory review of medical charts should be mandatory, including documentation of data gaps and inconsistencies.
- •
Liver transplantation center. The present analysis indicates major shortcomings of case management in the medical center (Tables 1, 2 and 5-8), calling for substantial improvements in the future. Transplantations are unquestionably the domain of the surgeons, but the prior diagnostic work-ups clearly belong to qualified hepatologists and other internists, also to minimize conflicts of interests of transplant surgeons in favor of high numbers of transplantations. Hepatol-ogists likely will easily find initial therapeutic options to make some OLTs unnecessary and can improve their case analysis by using the updated CIOMS scale; they also will include all and not only selected data from the clinical documents in potential manuscripts prior to submission. Basically, their goal will be to prepare an initial diagnostic program for all incoming patients with suspected DILI and HILI or other diseases, since this seems not to be available or not used at the QMC. Structured protocols will facilitate completeness of data and avoid diagnostic flaws and confounding variables. Case data might be documented as suggested previously.16 With this approach, we can expect solid work more in line with mainstream medicine.
- •
Primary care providers. PCPs should be aware that some DS are associated with rare hepatotoxic risks.63–70 During patient care, they should question about the use of DS and synthetic drugs and express concern if the number of used products is unusually high. For DS it has not yet been investigated whether they are specific risk factors of DILI by synthetic drugs, but their continuous use over years is irresponsible and should be discouraged. Combined use of several DS appears less advisable due to possible accumulation of specific ingredients derived from any of the used DS.
- •
Patients. For many individuals, being overweight or obese is a serious concern. In search for additional ways for weight loss, they often consider the use of DS as slimming aid and will sometimes use several DS concomitantly. The combined use of several DS for weight loss should be discouraged, unless the manufacturers have presented compelling evidence for their higher effectivity compared to a single DS. Concomitant use of several DS may lead to possible unknown interactions between their numerous ingredients. Safety aspects may be sufficiently considered by restricting the DS use to two months with subsequent cessation for at least one month. Drug therapy duration should also be limited, strictly observing drug indications to reduce potential health hazards.
- •
Manufacturers. DS manufacturers are obliged to follow all regulations to ensure consumer safety; they should be encouraged to provide additional evidence of product efficacy and safety in support of a positive benefit/risk profile. Published evidence suggests that some DS may be hepatotoxic in predisposed individuals but underlying mechanisms mostly remain unclear; this impedes valid individual suggestions for preventive measures.63–70 As for regulators, physicians, and patients, we propose several pragmatic steps for manufacturers. First, we recommend that manufacturers include any known rare hepatotoxic risks in their product labelling or leaflets. Second, manufacturers should provide information on their DS bottles about their specific ingredients, and that a physician’s clearance before use is mandatory. As suggested by our current analysis of the Hawaii liver disease cluster from 2013, clinicians and regulators may otherwise not be prepared to quickly prove or disprove a causal attribution in cases related to DS, calling for a close cooperation.
- •
Updated RUCAM. The recently published updated RUCAM should now be used for future cases, replacing previous scales.71
The present analysis is retrospective, as is the original report.17 We relied on published data,17 raw data, and the conclusions of the physicians as documented, which were considered as facts without need of verification at this stage of the analysis. We also did not interview directly any of the patients, whose identities remained undisclosed. To avoid the impression of bias inherited with our analysis, we presented all details of our analysis and conclusions for reasons of scientific transparency, ready to be re-evaluated and discussed.
Concluding RemarksThe mysterious Hawaii liver cases in the summer of 2013 received much publicity and caused major inconsistencies in regulatory and clinical statements, associated with an initially undetermined causality and biased conclusions, though file analyses of 5 cases uncovered other liver diseases as likely causes rather than DS or OEP. For assessment, all available case data were screened, in addition to selective data used for prior regulatory and clinical publications. It turned out that previous regulatory statements on the cluster cases resulted from biased clinical conclusions, based on invalid and impartial work. This situation calls for substantial improvements of regulatory and clinical assessments to prevent future similar cases.
Conflict of Interest DisclosureRolf Teschke received an honorarium for lectures at scientific meetings by Merz Pharmaceuticals and Max Zeller Pharmaceuticals and for consultation by Covington & Burling LLP Washington DC. None of the other authors received honoraria or has a conflict of interest.
Abbreviations- •
AAP: acetaminophen.
- •
ALF: acute liver failure.
- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI: body mass index.
- •
CDC: Centers for Disease Control and Prevention.
- •
CIOMS: Council for International Organizations of Medical Sciences.
- •
CMV: cytomegalovirus.
- •
CT: computed tomography.
- •
DILI: drug induced liver injury.
- •
DMAA: 1,3-dimethylamylamine.
- •
DS: dietary supplement.
- •
EBV: Epstein Barr virus.
- •
FDA: Food and Drug Administration in Washington DC, USA.
- •
HAV: hepatitis A virus.
- •
HBV: hepatitis B virus.
- •
HBc: hepatitis B core.
- •
HBs: hepatitis B surface.
- •
HCV: hepatitis C virus.
- •
HDOH: Hawaii Department of Health.
- •
HDS: herbal dietary supplement.
- •
HEV: hepatitis E virus.
- •
HILI: herb induced liver injury.
- •
HSV: herpes simplex virus.
- •
LFTs: liver function tests.
- •
MRC: magnetic resonance cholangiography.
- •
N: normal range as multiple of its upper limit.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NSAID: non-steroidal antiinflammatory drugs.
- •
OEP: OxyELITE Pro.
- •
OLT: orthotopic liver transplantation.
- •
PCP: primary care provider.
- •
PCR: polymerase chain reaction.
- •
QMC: Queen’s Medical Center in Honolulu.
- •
R: ratio.
- •
RUCAM: Roussel Uclaf Causality Assessment Method.
- •
ULN: upper limit of normal.
- •
VZV: varicella zoster virus.