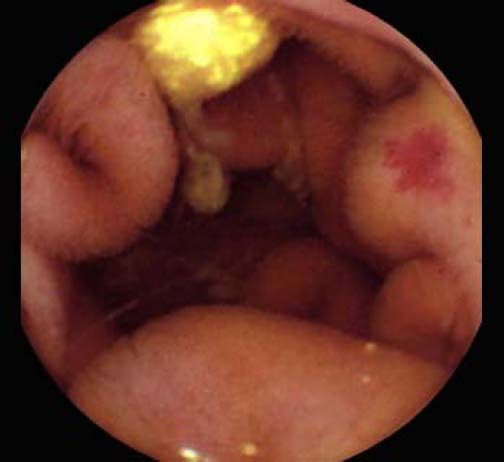
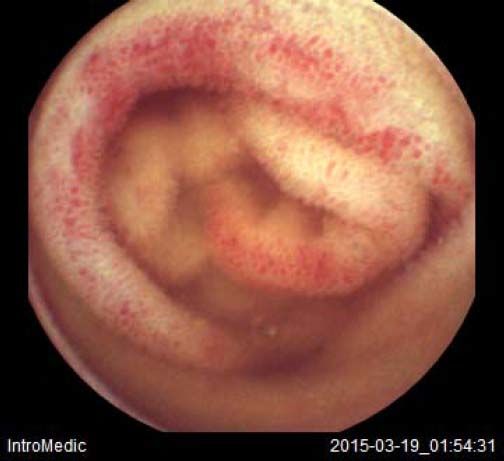
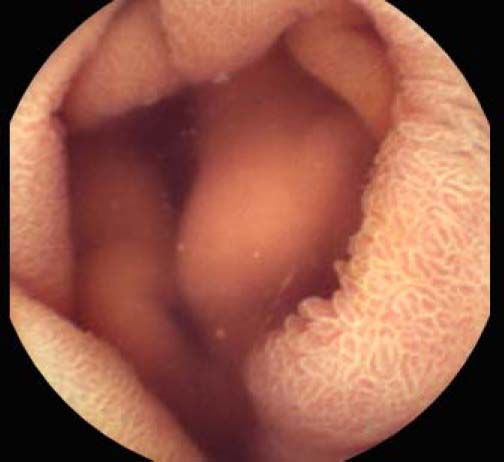
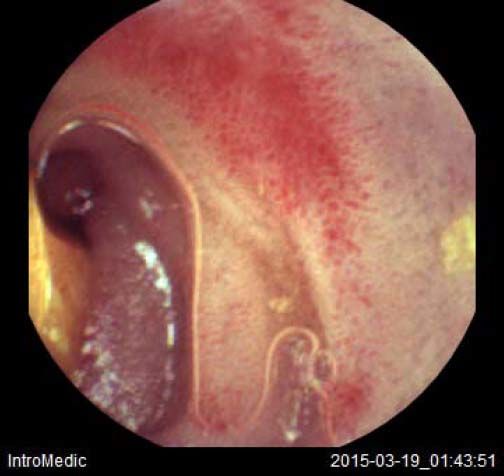
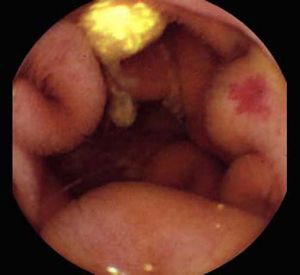
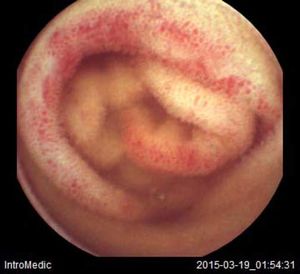
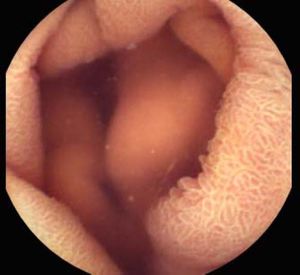
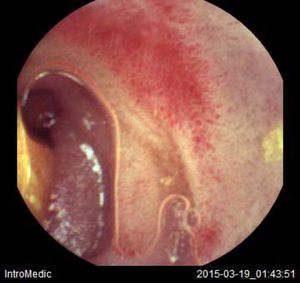
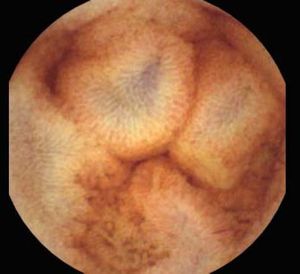
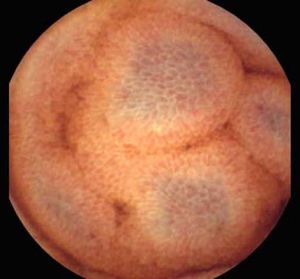
Background and rationale. Portal hypertensive enteropathy (PHE) remains difficult to diagnose in patients with cirrhosis and portal hypertension. Limited test choices exist for the inspection of the small bowel in these patients. Small bowel capsule endoscopy (SBCE) is ideal in this situation but rarely performed. We aimed to determine the prevalence of PHE using SBCE in a cirrhotic patient population and correlate its presence with clinical and CT imaging findings.
Material and methods. We retrospectively analysed data from cirrhotic patients who underwent SBCE at our unit. Studies were evaluated for the presence of cirrhosis-related findings in the oesophagus, stomach and small-bowel. The relationships between PHE and patients’ clinical characteristics were evaluated.
Results. 53 patients with cirrhosis underwent SCBE. We used PillCam®SB on 36 patients and MiroCam® capsule on 17. Thirty patients were referred for iron deficiency anaemia, 15 for obscure gastrointestinal bleeding, and 4 for other indications. Four data sets were not available for review, leaving 49 patients. Mean age was 61.19 ± 14.54 years (M/F = 27/22). Six SBCE examinations were incomplete. Thirty three patients had evidence of portal hypertensive gastropathy (PHG) and 17 had evidence of oesophageal varices. In total, 29 patients had SCBE evidence of PHE (57%). 28/29 (96.5%) patients with PHE had also evidence of PHG. 13/17 (76.4%) patients with oesophageal varices had also evidence of PHE.
Conclusions. The prevalence of PHE in our study was 57%. SBCE is a useful tool in evaluating PHE in cirrhotic patients irrespective of aetiology.
Portal hypertension (PHT) is an inherent part of liver cirrhosis and the vast majority of cirrhotic patients will develop complications related to PHT. In the gastrointestinal (GI) tract specifically, there are many pathological changes attributed to PHT.1 The most well-recognised are oesophageal or gastric (OV/GV) and rectal varices, and portal hypertensive gastropathy (PHG). Small-bowel capsule endoscopy (SBCE) and double-balloon enteroscopy has enabled us to explore subtle findings in the small-bowel in great detail.2,3 Over the last few years, it has emerged that portal hypertensive enteropathy (PHE) is a common complication of PHT. First described by De Palma, et al.,4 it should now be considered well defined. Most studies looking at PHE have used SBCE, which is becoming the preferred tool to examine the small bowel mainly because of the accuracy of the results and the ease/non-invasive nature of the examination.
Clinically, PHE could be a source of bleeding in cirrhotic patients and the most common indication to perform SBCE in cirrhotics is obscure gastro-intestinal bleeding (OGIB) when prior bidirectional endoscopy is negative.3 To date, the abnormalities found in PHE have been grouped into inflammatory-like and vascular lesions.1 The prevalence of PHE varies widely between studies but as vascular lesions of PHE can cause bleeding and subsequent anaemia, it is important to be able to predict them. Thus far, studies have linked PHE to other clinical manifestations of cirrhosis. A recent study shows that PHT-related findings on other abdominal organs on computed tomography (CT) scan correlate well with the presence of PHE.5
In this study we aimed to determine the prevalence of PHE in our population of cirrhotic patients and correlate its presence with clinical and CT imaging findings.
Material and MethodsStudy design and patient populationThis was a retrospective study analyzing the records of the SCBE database of our unit at the Royal Infirmary of Edinburgh, south east of Scotland, UK. We searched through 1,477 patients who underwent SCBE between April 2005 and December 2013. Further manual search, through the electronic records of these patients, was conducted to identify patients with cirrhosis. The presence of cirrhosis was confirmed with a combination of physical and radiological evaluation, laboratory and endoscopic data. Cirrhosis severity was graded according to the MELD score. Patients with other underlying small-bowel disease, end stage cardiac or renal failure, evidence of sepsis or lack of sufficient data were excluded from analysis. Patients on non-steroidal anti-inflammatory drugs (NSAIDs) were also excluded. This study was conducted in accordance with UK research ethics guidelines. After review by the local ethics committee, further specific ethical review and approval were not required, as the study was considered an evaluation of previously collected data, obtained as part of regular clinical care.
Capsule endoscopyAll patients followed the Unit protocol for SBCE examination that was in place at the time. In our practice, a fasting time of 12 h and bowel preparation are used. Patients are allowed to have a snack 2 h post-capsule ingestion and a light meal 4 h into the test. Our standard protocol requires that the capsule is ingested with 100 mg of liquid simethicone. All SBCE studies had been re-reviewed by an experienced capsule endoscopist (KD). In case of any doubt/controversy, a SBCE expert (AK) provided adjudication. In our center, 2 different SBCE systems are currently in use since mid 2009 (PillCam®SB; Given®Imaging Ltd, Yoqneam, Israel and MiroCam®; IntroMedic Co. Ltd, Seoul, Korea).
The referral indications were divided into 3 categories:
- •
Obscure gastrointestinal bleeding (OGIB).
- •
Iron deficiency anaemia (IDA), and
- •
Other indications.
Furthermore, causes of incomplete SBCE examinations were recorded: critical luminal stricture, obstructing luminal mass, delayed gastric transit time, delayed small bowel transit time, and technical reasons/failures/ others (e.g., luminal bleeding, food residue).
Study outcomeAll studies were evaluated for the presence of cirrhosis-related findings in the oesophagus (where feasible), stomach and small-bowel. OVs, GVs and PHG were classified as absent or present. In the small-bowel, inflammatory-like lesions (oedema, granularity, friability and erythema) and vascular lesions (varices, angiodysplasias, telangiectasias and cherry-red spots) in patients with PHE were recorded. Small-bowel varices were defined using the criteria presented by Canlas, et al.6
The relationships between PHE and patients’ clinical characteristics were evaluated. The latter includes: age, sex, aetiology of cirrhosis, and MELD score. We also identified four variables based on abdominal CT scans which are considered secondary to cirrhosis and PHT. These variables were: the presence of OVs or GVs, the presence of a transjugular intrahepatic portosystemic stent (TIPSS) shunt, the presence of splenomegaly and the presence of ascites. A composite score was calculated and compared between the two groups.
Statistical analysisWe used the OpenOffice Calc program to perform our statistical analysis. All continuous variables were compared using a Student’s t test. All categorical variables were compared using the Fischer’s exact test. Logistic regression analysis was used for multivariate analysis. A two-tailed P < 0.05 was considered statistically significant.
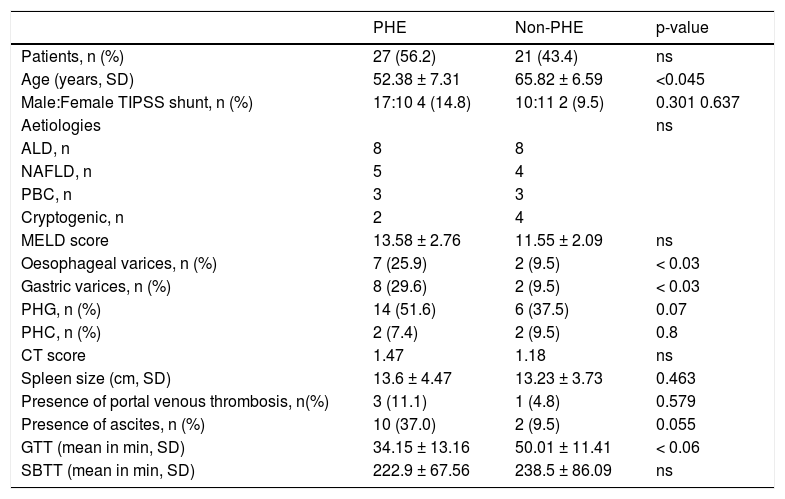
ResultsIn total, 53 patients with cirrhosis were identified; five patients were excluded due to lack of sufficient capsule endoscopy data. The remaining 48 patients were 27M/21F; mean age 61.19 ± 14.54 years. Thirty-two patients were examined using the PillCam® (SB1/SB2) system (Given®Imaging, Yokneam, Israel) and sixteen patients were examined using the MiroCam® (IntroMedic, Seoul, South Korea). The main reasons for referral in the 48 patients of our cohort were IDA (n = 30) and OGIB (n = 15). Three patients were referred for other indications. Aetiologies for liver disease were alcoholic liver disease (ALD; n = 16), non-alcoholic liver disease (NAFLD; n = 9), primary biliary cirrhosis (PBC; n = 6), cryptogenic cirrhosis (n = 6), hepatitis C (n = 5), autoimmune hepatitis (n = 4), chronic rejection (n = 4), portal vein thrombosis (n = 3), primary sclerosing cholangitis (n = 2) hepatitis B (n = 1), Wilson’s disease (n = 1) and Budd chiari syndrome (n = 1). Some patients had more than one aetiology for cirrhosis. Clinical characteristics of the two groups of PHE and non-PHE patients are shown in table 1.
Clinical characteristics of our cohort.
| PHE | Non-PHE | p-value | |
|---|---|---|---|
| Patients, n (%) | 27 (56.2) | 21 (43.4) | ns |
| Age (years, SD) | 52.38 ± 7.31 | 65.82 ± 6.59 | <0.045 |
| Male:Female TIPSS shunt, n (%) | 17:10 4 (14.8) | 10:11 2 (9.5) | 0.301 0.637 |
| Aetiologies | ns | ||
| ALD, n | 8 | 8 | |
| NAFLD, n | 5 | 4 | |
| PBC, n | 3 | 3 | |
| Cryptogenic, n | 2 | 4 | |
| MELD score | 13.58 ± 2.76 | 11.55 ± 2.09 | ns |
| Oesophageal varices, n (%) | 7 (25.9) | 2 (9.5) | < 0.03 |
| Gastric varices, n (%) | 8 (29.6) | 2 (9.5) | < 0.03 |
| PHG, n (%) | 14 (51.6) | 6 (37.5) | 0.07 |
| PHC, n (%) | 2 (7.4) | 2 (9.5) | 0.8 |
| CT score | 1.47 | 1.18 | ns |
| Spleen size (cm, SD) | 13.6 ± 4.47 | 13.23 ± 3.73 | 0.463 |
| Presence of portal venous thrombosis, n(%) | 3 (11.1) | 1 (4.8) | 0.579 |
| Presence of ascites, n (%) | 10 (37.0) | 2 (9.5) | 0.055 |
| GTT (mean in min, SD) | 34.15 ± 13.16 | 50.01 ± 11.41 | < 0.06 |
| SBTT (mean in min, SD) | 222.9 ± 67.56 | 238.5 ± 86.09 | ns |
ALD: alcoholic liver disease. GTT: gastric transit time. MELD: score Model for End-stage Liver Disease. NAFLD: non-alcoholic fatty liver disease. ns: nonsignificant PBC: primary biliary cirrhosis. PHC: portal hypertensive colopathy. PHG: portal hypertensive gast-opathy. SBTT: small-bowel transit time.
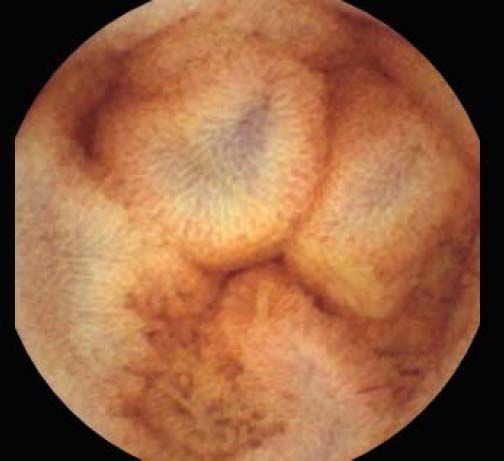
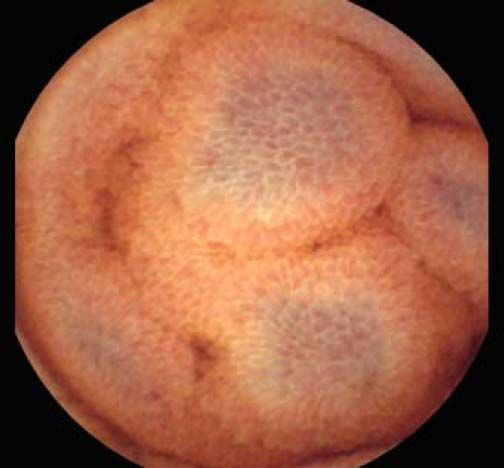
The overall prevalence of PHE was 57%. Patients with PHE on SBCE exhibited both vascular and inflammatory-like lesions. Oedema was present in 23/27(85.1%) and erythema in 24/27 (88.8%) patients. Cherry red spots were seen in 12/27 (44.4%) patients. Angiodysplasias were seen in 6/27 (22.2%) and telangiectasias in 3/27 (11.1%). Finally, small bowel varices were observed in 9/27 (33,33%) patients with PHE. Examples of such lesions are shown in figures 1–6. Thirty three patients had evidence of portal hypertensive gastropathy (PHG) and 17 had evidence of oesophageal varices. In total, 29 patients had SCBE evidence of PHE (57%). 28/29 (96.5%) patients with PHE had also evidence of PHG. 13/17 (76.4%) patients with oesophageal varices had also evidence of PHE.
The cohort was then subdivided into those who exhibited PHE (n = 29) and those who did not have evidence of PHE by SCBE (n = 19). OVs and GVs were statistically more frequent in the PHE group (p < 0.033 and < 0.027 respectively). Furthermore, the non-PHE group was significantly older than the PHE group (p < 0.045). No other significant differences between the two groups were noted. The composite CT score was not statistically different between the two groups; however, as the numbers are small, no statistical comparison can be made. Four patients in the PHE group had a TIPSS shunt, whereas only two in the non-PHE group had a TIPSS.
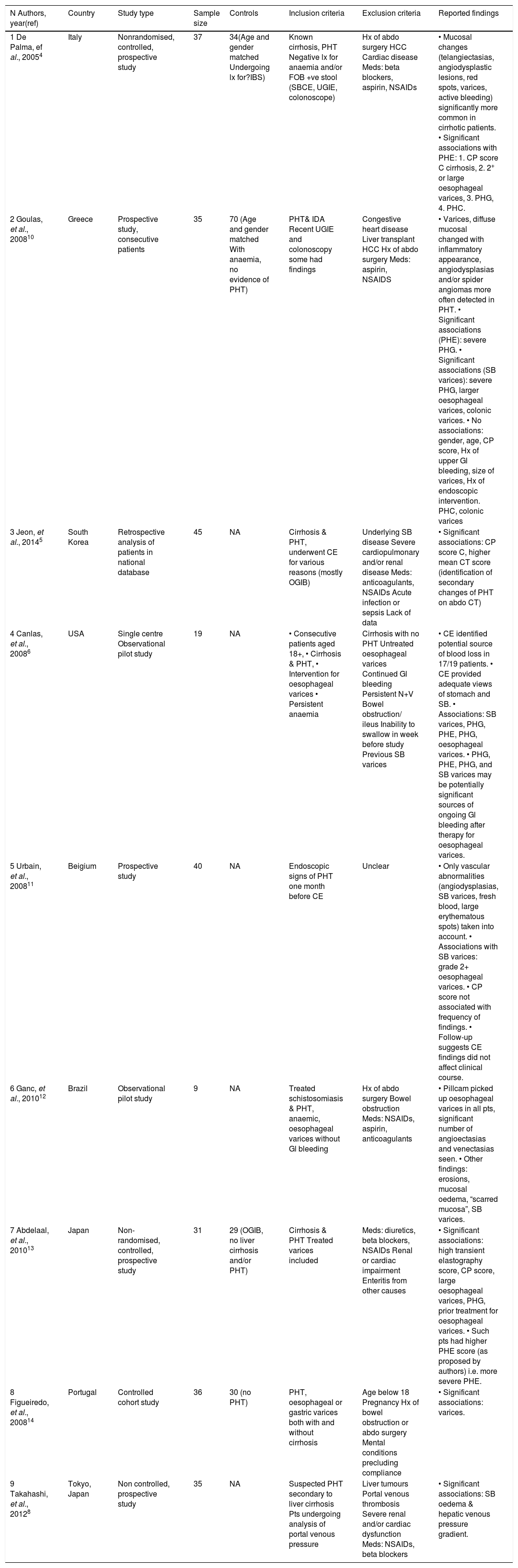
DiscussionPortal Hypertension can be a cause of life-threatening bleeding, mainly from OVs and/or GVs. PHG and colop-athy are also well-characterized manifestations of PH. The advent of SCBE a few years back enabled us to first describe and then characterise PHE. Further data on PHE have recently been produced but the true prevalence and associated clinical factors remain unclear (Table 2). The prevalence of PHE in our study was 57%, which is in accordance with many previous studies.7 Inflammatory-like lesions were very common in our cohort and we observed the presence of ectopic small-bowel varices in greater numbers than other studies reported so far.7 This is in accordance with our observation that GVs and OVs were more common in our PHE cohort. This is an indication that these patients had more prominent PHT. However we were unable to corroborate that, with enough data from Hepatic Venous Pressure Gradient (HVPG) measurements. It is important to stress that MELD, which is a good predictor of liver disease staging, was not significantly different between the PHE and the non-PHE group.
Summary of results.
| N Authors, year(ref) | Country | Study type | Sample size | Controls | Inclusion criteria | Exclusion criteria | Reported findings |
|---|---|---|---|---|---|---|---|
| 1 De Palma, ef al., 20054 | Italy | Nonrandomised, controlled, prospective study | 37 | 34(Age and gender matched Undergoing lx for?IBS) | Known cirrhosis, PHT Negative lx for anaemia and/or FOB +ve stool (SBCE, UGIE, colonoscope) | Hx of abdo surgery HCC Cardiac disease Meds: beta blockers, aspirin, NSAIDs | • Mucosal changes (telangiectasias, angiodysplastic lesions, red spots, varices, active bleeding) significantly more common in cirrhotic patients. • Significant associations with PHE: 1. CP score C cirrhosis, 2. 2+ or large oesophageal varices, 3. PHG, 4. PHC. |
| 2 Goulas, et al., 200810 | Greece | Prospective study, consecutive patients | 35 | 70 (Age and gender matched With anaemia, no evidence of PHT) | PHT& IDA Recent UGIE and colonoscopy some had findings | Congestive heart disease Liver transplant HCC Hx of abdo surgery Meds: aspirin, NSAIDS | • Varices, diffuse mucosal changed with inflammatory appearance, angiodysplasias and/or spider angiomas more often detected in PHT. • Significant associations (PHE): severe PHG. • Significant associations (SB varices): severe PHG, larger oesophageal varices, colonic varices. • No associations: gender, age, CP score, Hx of upper Gl bleeding, size of varices, Hx of endoscopic intervention. PHC, colonic varices |
| 3 Jeon, et al., 20145 | South Korea | Retrospective analysis of patients in national database | 45 | NA | Cirrhosis & PHT, underwent CE for various reasons (mostly OGIB) | Underlying SB disease Severe cardiopulmonary and/or renal disease Meds: anticoagulants, NSAIDs Acute infection or sepsis Lack of data | • Significant associations: CP score C, higher mean CT score (identification of secondary changes of PHT on abdo CT) |
| 4 Canlas, et al., 20086 | USA | Single centre Observational pilot study | 19 | NA | • Consecutive patients aged 18+, • Cirrhosis & PHT, • Intervention for oesophageal varices • Persistent anaemia | Cirrhosis with no PHT Untreated oesophageal varices Continued Gl bleeding Persistent N+V Bowel obstruction/ ileus Inability to swallow in week before study Previous SB varices | • CE identified potential source of blood loss in 17/19 patients. • CE provided adequate views of stomach and SB. • Associations: SB varices, PHG, PHE, PHG, oesophageal varices. • PHG, PHE, PHG, and SB varices may be potentially significant sources of ongoing Gl bleeding after therapy for oesophageal varices. |
| 5 Urbain, et al., 200811 | Beigium | Prospective study | 40 | NA | Endoscopic signs of PHT one month before CE | Unclear | • Only vascular abnormalities (angiodysplasias, SB varices, fresh blood, large erythematous spots) taken into account. • Associations with SB varices: grade 2+ oesophageal varices. • CP score not associated with frequency of findings. • Follow-up suggests CE findings did not affect clinical course. |
| 6 Ganc, et al., 201012 | Brazil | Observational pilot study | 9 | NA | Treated schistosomiasis & PHT, anaemic, oesophageal varices without Gl bleeding | Hx of abdo surgery Bowel obstruction Meds: NSAIDs, aspirin, anticoagulants | • Pillcam picked up oesophageal varices in all pts, significant number of angioectasias and venectasias seen. • Other findings: erosions, mucosal oedema, “scarred mucosa”, SB varices. |
| 7 Abdelaal, et al., 201013 | Japan | Non-randomised, controlled, prospective study | 31 | 29 (OGIB, no liver cirrhosis and/or PHT) | Cirrhosis & PHT Treated varices included | Meds: diuretics, beta blockers, NSAIDs Renal or cardiac impairment Enteritis from other causes | • Significant associations: high transient elastography score, CP score, large oesophageal varices, PHG, prior treatment for oesophageal varices. • Such pts had higher PHE score (as proposed by authors) i.e. more severe PHE. |
| 8 Figueiredo, et al., 200814 | Portugal | Controlled cohort study | 36 | 30 (no PHT) | PHT, oesophageal or gastric varices both with and without cirrhosis | Age below 18 Pregnancy Hx of bowel obstruction or abdo surgery Mental conditions precluding compliance | • Significant associations: varices. |
| 9 Takahashi, et al., 20128 | Tokyo, Japan | Non controlled, prospective study | 35 | NA | Suspected PHT secondary to liver cirrhosis Pts undergoing analysis of portal venous pressure | Liver tumours Portal venous thrombosis Severe renal and/or cardiac dysfunction Meds: NSAIDs, beta blockers | • Significant associations: SB oedema & hepatic venous pressure gradient. |
CE: capsule endoscopy. CP score: Child-Pugh score. FOB: faecal occult blood. HCC: hepatocellular carcinoma. Hx: history. IBS: Irritable bowel syndrome. IDA: Iron deficiency anaemia, lx: Investigations. N+V: nausea and vomiting. NSAIDs: Non-Steroidal Anti-Inflammatory Drugs. OGIB: occult Gl bleeding. PHC: portal hypertensive colopathy. PHE: portal hypertensive enteropathy. PHG: portal hypertensive gastropathy. PHT: portal hypertension. SBCE: small-bowel capsule endoscopy. UGIE: upper gastrointestinal endoscopy.
We would argue then that PHE is commoner in patients with more pronounced PHT as manifested by the presence of OV, GV and PHG and is in accordance with observations by Takahashi, et al.,8 and Aoyama, et al.,9 who showed a correlation between PHE and HPVG. We were unable to correlate the presence of PHE with CT findings related to portal hypertension. As this is a retrospective study we had CT data on 16 patients with PHE and 11 patients in the non-PHE group. It is possible that the numbers were too small to draw any meaningful conclusions. Our centre is at the forefront of research and clinical applications of TIPSS. As part of clinical management patients with PHT end up having a TIPSS Shunt inserted either to control variceal bleeding or as prophylaxis against subsequent re-bleeding. As such the fact that more PHE patients had a TIPSS inserted reflects the more severe PHT of this cohort.
There are potential limitations for this study. This is a retrospective analysis of a large database and it can only reflect the data included in the data base or mined out of patients’ casenotes without a prospective perspective. Also no control group was included and we are unsure whether typical manifestations of PHE could exist in non-cirrhotic patients as reported by other studies albeit in very small numbers. Lastly no significant follow up data were acquired. We believe though that this study, which has one of the biggest patient populations from a single centre, presents accurate findings on PHE and its associations with PHT.
ConclusionIn conclusion this retrospective study shows a prevalence of PHE of 57%. PHE was more prevalent in patients with more severe PHT. As PHE is sometimes an obscure cause of bleeding in cirrhotic patients despite adequate treatment of varices, the diagnosis of PHE is important as it could guide management by suggesting patients who should be offered TIPSS. Our study corroborates data that SBCE is a valuable tool for the diagnosis of PHE. A large prospective multi-centre study could further evaluate the relationship between direct measurements of HVPG and degree of liver fibrosis as measured by Fibroscan or Hyaluronic acid and PHE.
Conflict of InterestNone.
Disclosure(S)Dr. A Koulaouzidis has received a research grant from GivenImaging Ltd and material support for research from SynMed UK. He has also received lecture honoraria from Dr. Falk UK, and travel support from Dr. Falk UK, Abbott, Almirall, Ferring UK, and MSD.
Abbreviations- •
ALD: alcoholic liver disease.
- •
CT: computed tomography.
- •
GI: gastrointestinal.
- •
GV: gastric varices.
- •
HVPG: hepatic venous pressure gradient.
- •
IDA: iron deficiency anaemia.
- •
MELD: Model for End-stage Liver Disease.
- •
NAFLD: Non-alcoholic Fatty Liver Disease.
- •
NSAIDs: Non-steroidal Anti-inflammatory Drugs.
- •
OGIB: obscure gastrointestinal bleeding.
- •
OV: oesophageal varices.
- •
PHE: portal hypertensive enteropathy.
- •
PHG: portal hypertensive gastropathy.
- •
PHT: portal hypertension.
- •
SBCE: small bowel capsule endoscopy.
- •
TIPSS: transjugular intrahepatic portosystemic shunt.