Introduction. Chronic liver disease (CLD) is becoming a major cause of mortality in patients who are positive with human immunodeficiency virus (HIV). Our aim was to assess the prevalence of CLD in HIV+ individuals.
Material and methods. We utilized the National Health and Nutrition Examination Survey (1999-2008) to assess the association of CLD with HIV infection. In eligible participants (18-49 years), HIV infection was defined as positive anti-HIV by enzyme immunoassay further confirmed by Western blot. The diagnosis of CLD included chronic hepatitis C (CH-C), alcohol-related liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD). Clinic-demographic and laboratory parameters were used to assess differences between those with and without HIV infection.
Results. 14,685 adults were included. Of those, 0.43 ± 0.08% were HIV-positive and 13.8% had evidence of CLD, including 26.3% in HIV-positive individuals and 13.7% in HIV-negative controls (p = 0.0341). In the U.S. population, independent predictors of CLD included HIV positivity [OR = 1.96 (1.02-3.77), p = 0.04], older age [OR = 1.03 (1.02-1.03), p < 0.0001], male gender [OR = 2.15 (1.89-2.44), p < 0.0001] and obesity [OR = 2.10 (1.82-2.43), p < 0.0001], while African American race/ethnicity was associated with lower risk for CLD [OR = 0.68 (0.58-0.80), p < 0.0001].
Conclusions. CLD is common in HIV positive individuals. With successful long term treatment of HIV, management of CLD will continue to remain very important in these patients.
According to the World Health Organization (WHO), as of 2011, 34 million people are infected with human immunodeficiency virus (HIV).1 From the time when the combination antiretroviral therapy (cART) was developed, there has been a significant decrease in deaths related to acquired immunodeficiency syndrome (AIDS).2 Although AIDS was the 8th cause of mortality prior to 1996, in 2012, it is no longer in the top 10 causes of death.3 In fact, recent studies have shown that HIV-positive individuals on cART have greater than 50% chance of dying for reasons not related to AIDS.4,5
The most common non-AIDS related cause of death is liver disease which accounts for approximately 14-18% of deaths.6 One study to provide evidence to support this shift in the cause of death collected data related to adverse events of Anti-HIV Drugs (D.A.D study). This study followed 23,441 HIV individuals over 5 years during which 1,235 deaths were registered.4 Although in the HIV+ cohort, AIDS remained the leading cause of death at 31.1%, liver disease was the second leading cause of death at 14.5%. Of the liver-related causes of death, 66% were due to hepatitis C (HCV), 16.9% were due to hepatitis B (HBV), and 7.1% were due to co-infection to both HCV and HBV. In addition to establishing liver disease as the second most common cause of death, this study also established a strong association between liver related deaths and the extent of advanced immunodeficiency.4
This and other studies continue to provide strong evidence that CLD is increasingly becoming an important cause of morbidity and mortality in HIV positive individuals.7–12 Individuals with HIV who become co-infected with HCV or HBV are less likely to clear the virus spontaneously, tend to have higher viral loads and are more likely to have progressive liver disease.9–10,12 HIV infected patients may also have a decreased response to viral hepatitis vaccines.7,8
In addition to chronic viral hepatitis, NAFLD is becoming an important liver disease related to metabolic syndrome.13–15 In the era of cART, HIV-infected patients are susceptible to metabolic disorders such as abdominal obesity and lipodystrophy.13 However, in addition to metabolic syndrome, in HIV infected individuals, NAFLD and hepatic fibrosis may be caused by anti-HIV treatment regimens, specifically, non-nucleoside reverse transcriptase inhibitors such as stavudine and didanosine.16,17 Additionally, some protease inhibitors can contribute to abdominal lipodystrophy and interrupt glucose and lipid homeostasis leading to the clinical manifestation of NAFLD.18
Finally, excessive alcohol use can lead to progressive liver disease, especially in HIV-HCV co-infected individuals.19,20 Additionally, excessive alcohol consumption may lead to HIV progression and poor viral suppression.
In the context of this growing appreciation of the importance of chronic liver disease in HIV + patients, our aim was to assess the prevalence and associations of chronic liver diseases in patients infected with HIV using a population-based data.
Material and MethodsStudy populationFor this study, we used five consecutive two-year cycles of the National Health and Nutrition Examination Survey (NHANES) collected by the National Center for Health Statistics between 1999 and 2008. NHANES is a nationwide survey conducted in the United States to collect information representing the health and nutritional status of the non-institutionalized civilian U.S. population. The survey includes an interview as well as standardized physical examination and data from blood samples collected at the exam centers. The description of these surveys has been reported in detail elsewhere.21 Participants were included in this study if they met the following criteria: at least 18 years of age, available demographic data together with the laboratory and clinical data necessary to rule in or rule out chronic liver disease (CLD). Furthermore, HIV data was available for participants aged 18-49 years who did not refuse HIV antibody test.
Study definitionsEligible participants were considered HIV+ if their serum tested positive for HIV antibody by a triple test that included two enzyme immunoassays followed by a Western blot. Furthermore, eligible individuals who refused phlebotomy or who did not have a sufficient blood sample for the serum HIV assay, but who did not refuse HIV testing had their urine tested for HIV type 1 antibody using the Calypte HIV-1 Urine EIA (except for the cycle of 2007-2008 when the urine test was not conducted.22 As a result, individuals tested positive were also considered HIV+. And, for the purpose of the study, all antibody-negative individuals of 18-49 years of age were considered HIV-controls.
In this study, we included 3 major categories of chronic liver disease: alcoholic liver disease, chronic hepatitis C, and non-alcoholic fatty liver disease. Alcohol related liver disease (ALD) was defined as excessive alcohol use (20+ g/day for men, 10+ g/day for women) in the year before enrollment for NHANES in the presence of elevated serum aminotransferases (ALT > 40 U/L or AST > 37 U/L in men, ALT or AST > 31 U/L in women). Serologic tests for hepatitis C antibody were available in eligible participants and, if positive, HCV RNA was tested. Participants with positive HCV RNA were considered to have chronic hepatitis C (CH-C). Finally, subjects were presumed to have non-alcoholic fatty liver disease (NAFLD) if they had elevated serum aminotransferases (defined above) in the absence of any other evidence of chronic liver disease such as excessive alcohol use or positive viral hepatitis serology. We excluded patients with diagnosis of other causes chronic liver diseases. All together, individuals with ALD, CH-C and NAFLD were considered to have chronic liver disease (CLD) while those with normal aminotransferases, negative viral hepatitis serology and no history of excessive alcohol use were presumed to have no chronic liver disease.
Statistical analysisThe demographic and clinical parameters were compared between HIV+ individuals and HIV-controls using stratum-specific chi-square and t-test for a contrasted mean. To study independent predictors of CLD, we used multiple logistic regression analysis where demographic and clinical parameters were tested for independent association with CLD in HIV+ and HIV-individuals separately. P-values of 0.05 or less were considered potentially significant unless stated otherwise. Statistical analyses were conducted using SUDAAN 10.1 (Research Triangle Institute, Research Triangle Park, NC). Sampling weights and stratum/sampling units that accounted for the complex survey design were used as recommended, and adjustment coefficients were applied when merging the five analytic cycles into one dataset according to the NHANES Analytic and Reporting Guidelines.21
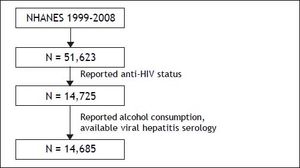
ResultsThe initial NHANES cohort (1999-2008) included 51,623 participants. Of these, 14,685 were considered eligible for the study and were considered the study cohort (Figure 1). After weighing on the basis of race/ethnicity, gender, and age, the cohort included 66.77 ± 1.84% non-Hispanic Whites, 12.22 ± 1.06% non-Hispanic Blacks and 10.22 ± 1.05% Hispanic Americans. Of the study cohort, 79 individuals (0.43 ± 0.08%) were HIV+.
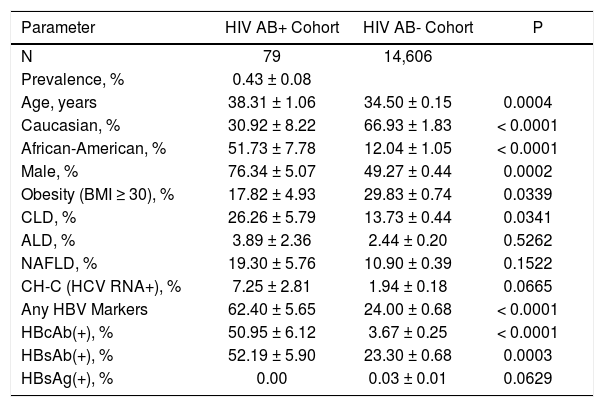
Pairwise comparison of HIV+ individuals to HIV-controls is summarized in table 1. Specifically, HIV+ individuals were predominantly male (76.34 ± 5.07% in HIV+ vs. 49.27 ± 0.44% in HIV-, p = 0.0002) and African American/Black (51.73 ± 7.78% vs. 12.04 ± 1.05%, respectively, p < 0.0001). HIV+ individuals were less likely to be obese (BMI > 30: 17.82 ± 4.93% in HIV+ vs. 29.83 ± 0.74% in HIV-, p = 0.034) and older (age: 38.31 ± 1.06 years in HIV+ vs. 34.50 ± 0.15 years in HIV-, p = 0.0004).
Clinical and demographic characteristics of the HIV+ individuals.
| Parameter | HIV AB+ Cohort | HIV AB- Cohort | P |
|---|---|---|---|
| N | 79 | 14,606 | |
| Prevalence, % | 0.43 ± 0.08 | ||
| Age, years | 38.31 ± 1.06 | 34.50 ± 0.15 | 0.0004 |
| Caucasian, % | 30.92 ± 8.22 | 66.93 ± 1.83 | < 0.0001 |
| African-American, % | 51.73 ± 7.78 | 12.04 ± 1.05 | < 0.0001 |
| Male, % | 76.34 ± 5.07 | 49.27 ± 0.44 | 0.0002 |
| Obesity (BMI ≥ 30), % | 17.82 ± 4.93 | 29.83 ± 0.74 | 0.0339 |
| CLD, % | 26.26 ± 5.79 | 13.73 ± 0.44 | 0.0341 |
| ALD, % | 3.89 ± 2.36 | 2.44 ± 0.20 | 0.5262 |
| NAFLD, % | 19.30 ± 5.76 | 10.90 ± 0.39 | 0.1522 |
| CH-C (HCV RNA+), % | 7.25 ± 2.81 | 1.94 ± 0.18 | 0.0665 |
| Any HBV Markers | 62.40 ± 5.65 | 24.00 ± 0.68 | < 0.0001 |
| HBcAb(+), % | 50.95 ± 6.12 | 3.67 ± 0.25 | < 0.0001 |
| HBsAb(+), % | 52.19 ± 5.90 | 23.30 ± 0.68 | 0.0003 |
| HBsAg(+), % | 0.00 | 0.03 ± 0.01 | 0.0629 |
Chronic liver disease of any etiology was detected for 26.26 ± 5.79% of HIV+ individuals, which was significantly higher than in HIV- controls (13.73 ± 0.44%, p = 0.03). Considering different etiologies of CLD separately, a close to significant difference was noted only for CH-C: 7.25 ± 2.81% vs. 1.94 ± 0.18%, p = 0.06. However, other liver diseases including NAFLD and ALD were not significantly different between HIV+ and HIV- individuals (Table 1). Although HBsAntigen+ was not significantly different between the two groups, other markers of HBV infection (presence of HBsAntibody and HB-cAntibody) were highly more prevalent in HIV+ individuals.
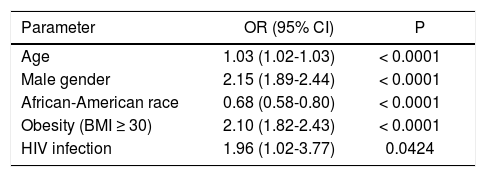
Using multivariate analysis, after controlling for major confounders, HIV infection was independently associated with having chronic liver disease (OR (95% CI) =1.96 (1.02-3.77), p = 0.04) (Table 2).
Independent predictors of CLD in the U.S. population of 18-49 years of age.
| Parameter | OR (95% CI) | P |
|---|---|---|
| Age | 1.03 (1.02-1.03) | < 0.0001 |
| Male gender | 2.15 (1.89-2.44) | < 0.0001 |
| African-American race | 0.68 (0.58-0.80) | < 0.0001 |
| Obesity (BMI ≥ 30) | 2.10 (1.82-2.43) | < 0.0001 |
| HIV infection | 1.96 (1.02-3.77) | 0.0424 |
We also assessed the prevalence of HIV in patients with CLD. In fact, 0.83 ± 0.21% of individuals with CLD were HIV+. This included 0.69 ± 0.40% of those with ALD, 1.58 ± 0.65% of those with CH-C and 0.76 ± 0.25% of those with NAFLD. In controls without liver disease, the prevalence of HIV infection was 0.37 ± 0.07% which is significantly lower as compared to those with CLD (p = 0.03) and those with CH-C (p = 0.05), and not significantly different from that in ALD and NAFLD (both p > 0.1).
DiscussionChronic liver diseases have become major causes of morbidity and mortality in HIV patients. In this population-based study, HIV infected individuals were more likely to have chronic liver disease as compared to non-infected controls. These findings are important since HIV infected individuals with CLD, especially those co-infected with HCV, are at significant risk for progressive liver disease and liver-related mortality. In fact, liver disease has become the most common non-AIDS related cause of death in the HIV population. Our data provides evidence of independent association between CLD and HIV at the population level. Additionally, both HCV and markers of HBV were both more common in HIV+ individuals, providing additional validity of our data.
Given these associations, it is imperative to screen individuals with HIV for CLD and reduce the risk of liver disease by vaccinating against HAV and HBV as well as by counseling against excessive alcohol use and modifying risk factors for NAFLD.
Once liver disease is identified in HIV-infected patients, steps can be taken not only to treat the underlying liver disease but also to closely monitor for its progression to cirrhosis or end-stage liver disease. Although anti-viral treatment for HCV in the HCV-HIV co-infected individuals may have lower response rates, the newer regimens are expected to provide better efficacy and improved treatment strategies.23,24 It is also important to note that access to treatment is also a major issue for HCV infected patients. This issue may be even more important for HCV-HIV co-infected patients and appropriate strategies are needed to help remove this barrier.
Our study does have some limitations. First, NHANES is a study looking at only civilian, non-institutionalized population in the United States which may have underestimated the prevalence of HIV and other forms of liver disease by not accounting for incarcerated individuals, homeless persons, nursing home residents, and active military duty. In addition, NHANES screens for HIV in only for individuals between 18 to 49 years old. Also, some individuals with NAFLD may have been categorized as controls without liver disease if, at the time of examination, their liver enzymes were normal. Although other causes of chronic liver diseases were excluded by historical data, complete serologic/diagnostic laboratory tests were not available to fully exclude autoimmune hepatitis or hemochromatosis. However, the relatively low prevalence of these types of CLD in general population (as compared to HCV, NAFLD and ALD), their impact should be minimum. Also, limited sample size was also potentially responsible for some of the nonsignificant findings due to type II error. Nevertheless, the size of the initial study population and the in-depth nature of the available data make the study quite unique.
ConclusionThe HIV-infected individuals are at risk of morbidity and mortality due to chronic liver diseases. The increasing burden of CLD in HIV infected individuals has made liver-realted mortality as the most common non-AIDS related cause of death in HIV positive individuals. It is important for health care providers involved in the care of HIV infected individuals to provide screening, early diagnosis and treatment of chronic liver diseases.
Conflict of InterestNo conflict of interest for any authors. Internal funding only.