Obesity prevalence is rapidly increasing worldwide. It is associated with huge economic and health costs due to its clinical consequences, which includes increased incidence of type 2 diabetes, cardiovascular diseases, and development of different malignancies. In particular, obesity is an independent risk factor for the development of hepatocellular carcinoma (HCC). Indeed, obesity is highly prevalent in patients with non-alcoholic fatty liver disease (NAFLD) that is becoming one of the most frequent causes of liver disease worldwide. NAFLD-related HCC is the most rapidly growing indication for liver transplantation in many countries. The higher mortality rates found in obese HCC patients might be related not only to a worse outcome after HCC treatments, but also to a delayed diagnosis related to a low frequency and a poorer quality of abdominal ultrasonography surveillance that is the test universally used for HCC screening. Given its diffusion, obesity is frequently present in patients with chronic liver diseases related to different etiologies, and in these cases it may increase the HCC risk, acting as an additional co-factor. Indeed, growing evidence demonstrates that a healthy diet and regular physical activity may have an impact in reducing the overall HCC risk. Finally, an impact of obesity in the development of intrahepatic cholangiocarcinoma has been postulated, but more extensive studies are needed to definitively confirm this association.
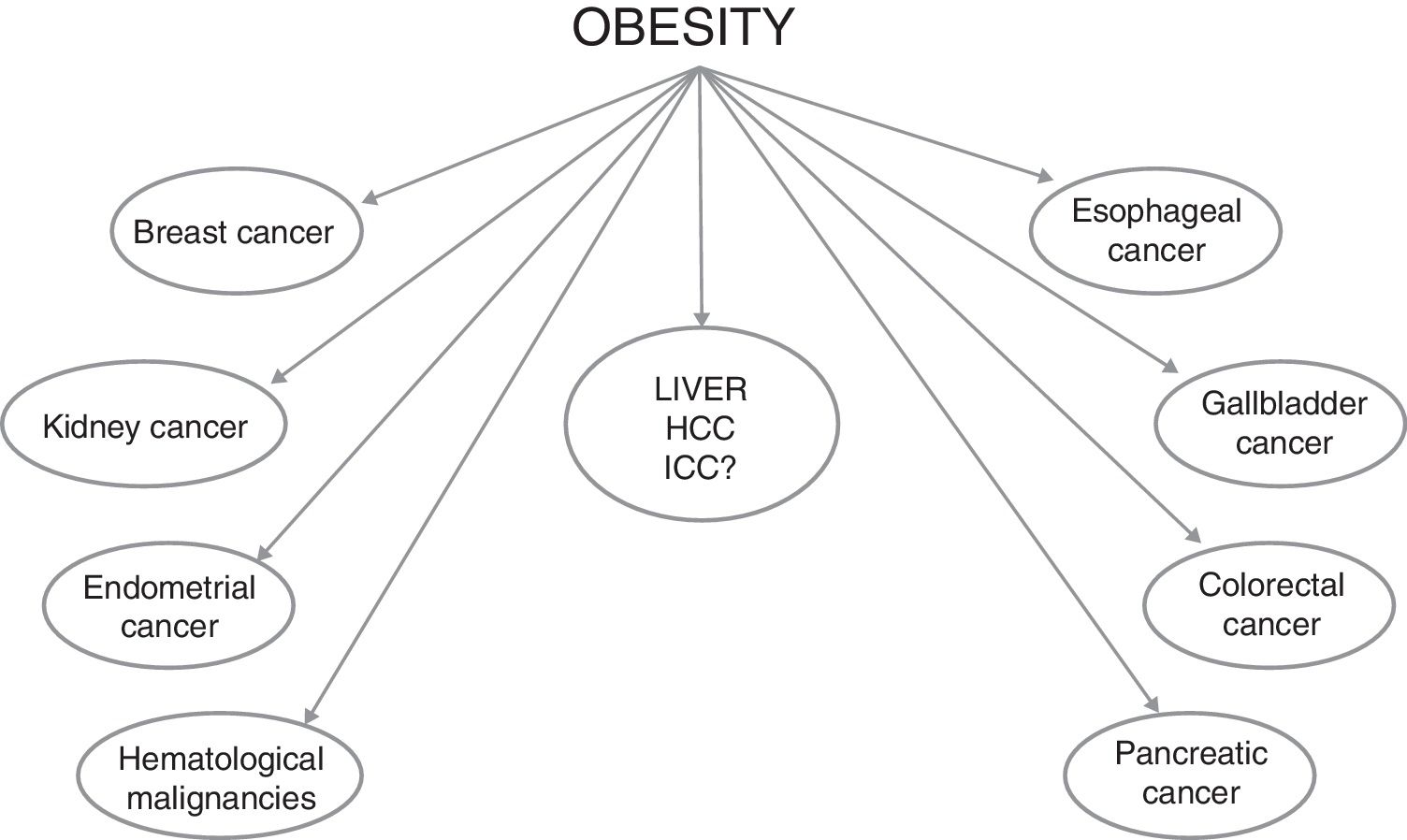
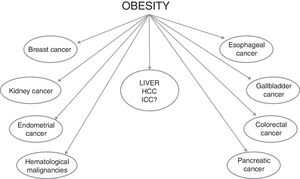
Obesity is recognized as a global pandemic, considering that figures have almost tripled worldwide since 1975. The World Health Organization estimated that obese people over 18 years of age were more than 650 million in 2016 (about 13% of the world's adult population [11% of men and 15% of women]) [1]. Furthermore, over 340 million children and adolescents aged 5–19 were estimated to be overweight or obese in 2016, and this implies that the number of obese subjects will increase in the near future, given that childhood obesity is linked to a much higher chance of adult obesity [2], with all the negative effects in terms of health care resources needed to deal with its consequences. Indeed, the development of cardiovascular diseases and of type 2 diabetes mellitus (T2DM) are the best known obesity-related complications. However, obesity is also an established risk factor for the development of several malignancies, such as breast, colorectal, endometrium, esophagus, gallbladder, kidney and pancreas cancers, as well as bone-marrow malignancies (Fig. 1) [3–6]. Overall, obesity increases mortality rates in all cancers, as showed in a study from the American Cancer Society in which subjects with a body mass index (BMI) greater than 40 had death rates higher than those in normal weight individuals (52% higher in men and 62% higher in women) [7]. On the basis of the relative risks and associations observed in this study, it was estimated that 14% of all deaths from cancer in men and 20% in women were attributable to being overweight or obese in U.S.A. [7].
A large body of evidence shows a particularly strong association between obesity and hepatocellular carcinoma (HCC) [6,8–14], and this concise review will focus on the epidemiological and clinical aspects of the liver cancers in obese people.
2The burden of obesity-associated HCCHCC is the fifth most frequent cancer and the second leading cause of cancer-related mortality worldwide in men [15]. A constantly increasing trend of HCC incidence and mortality has been observed in U.S.A. and many European countries. In U.S.A., HCC shows an incidence increasing by 4.5% annually, and it is reported to be the most rapidly growing cause of cancer-related deaths [16]. Most cases of HCC arise in the context of liver cirrhosis, mainly due to chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV) infections and/or heavy alcohol drinking [17]. Chronic HBV infection is the leading cause of HCC worldwide and the main risk factor for HCC development in eastern Asia and sub-Saharan Africa, while chronic HCV infection remains an important risk factor in U.S.A. and Europe. Indeed, there is the hope that the cure of HCV by direct antiviral agents (DAA) and the efficacious treatments available against HBV will reduce the rates of HCC incidence and mortality. However, despite these very important advances in the treatment of viral hepatitis, liver cancer is still a globally recognized health care issue. Possibly, the impact on the global HCC incidence rates of the HCV cure by DAA will become more evident over time [18]. Nevertheless, there is clear evidence of a constant rise of HCC incidence that is commonly attributed to the parallel increase of non-alcoholic fatty liver disease (NAFLD), which has become the leading cause of liver disease in many areas of the world [19], with a prevalence reaching 30% of the general population [20,21]. In about 20% of NAFLD subjects, liver histology may show features of hepatitis – non-alcoholic steatohepatitis (NASH) – characterized by the presence of necro-inflammation and often of fibrosis, potentially evolving toward cirrhosis and HCC [22,23]. NASH-related HCC is the most rapidly growing indication for liver transplantation in U.S.A. [24].
NAFLD is strongly associated with features of metabolic syndrome (MS), and the probability of developing NASH increases with the number of risk factors involved (obesity, T2DM, hypertension and dyslipidaemia) [25,26]. In a meta-analysis study of global epidemiology, obesity prevalence among patients with NAFLD was estimated at 51.3%, whereas among patients with NASH it was calculated to be 81.8% [27]. Not surprisingly, giving the continuous rise of obesity prevalence, NAFLD has become the most common etiologic factor of liver disease worldwide [21]. The very strong association between HCC and MS has become more evident in the last two decades. Of note, obesity in itself has been shown to be an independent risk factor of HCC [8].
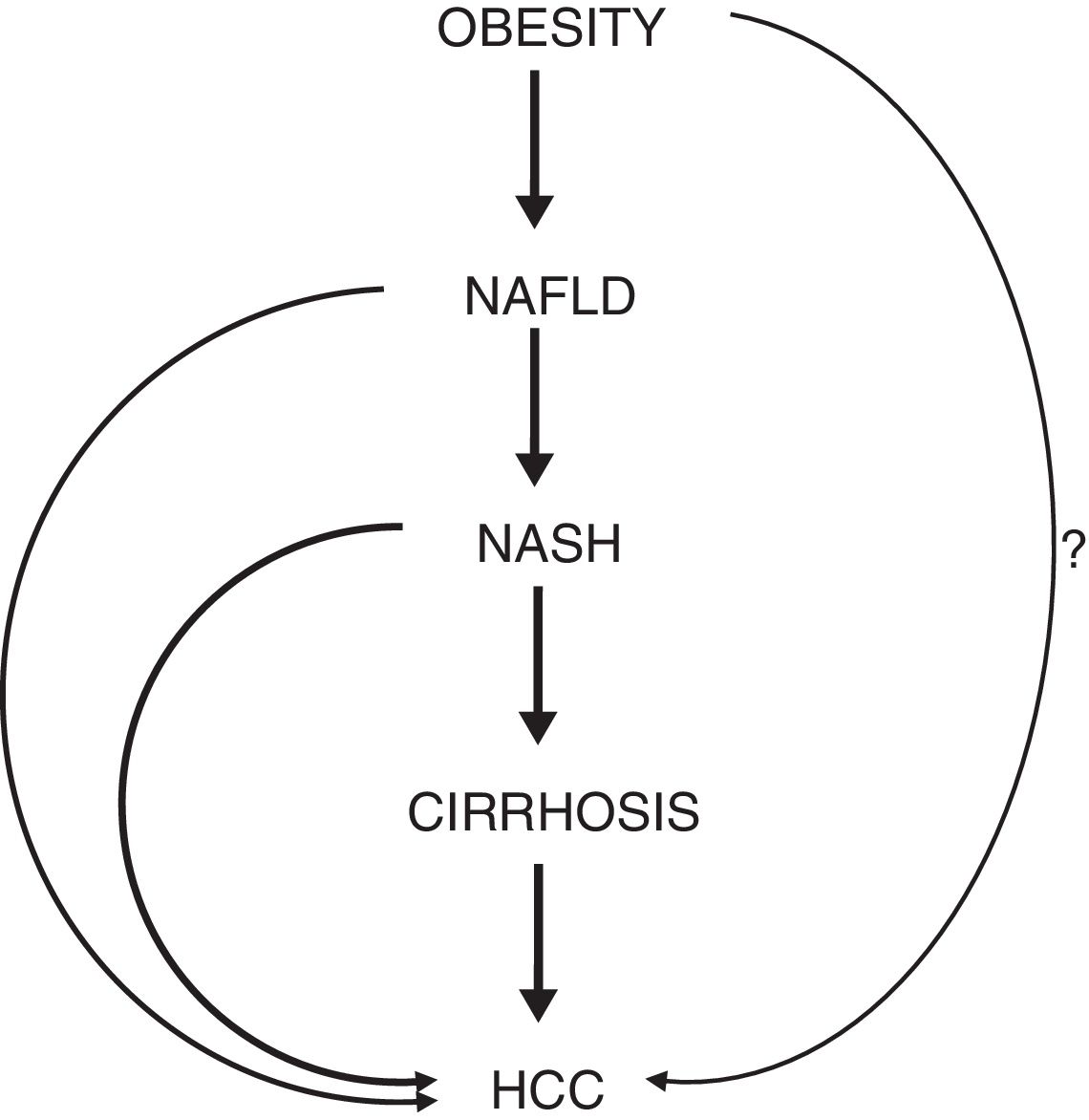
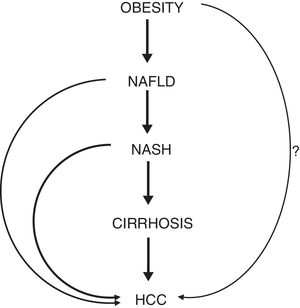
In a cohort of about 18,000 London-based government employees, followed up for a median of 28 years, the hazard ratio (HR) of HCC development in obese individuals was 3.76 [9]. A meta-analysis of cohort studies assessing the association between obesity and liver cancer showed that overweight or obese subjects had a 17% and 89% increased risk of HCC, respectively, compared to normal weight individuals [10]. Another meta-analysis, evaluating prospective observational studies assessing the strength of associations between BMI and different sites of cancer, showed that the risk of liver cancer increases by about 25% for each 5kg/m2 increase of BMI [11]. An additional meta-analysis evaluated 21 prospective studies, showing a relative risk of HCC of 1.39 for each 5kg/m2 increase in BMI, with the most pronounced risk increase among individuals with a BMI>32kg/m2[12]. A systematic review of 10 cohort studies conducted in 2010 showed a positive association between obesity and risk of HCC in the majority of the studies analyzed [13]. The necessity to take action in order to reduce the spread of childhood obesity is further highlighted by a Danish study, revealing that higher BMI in childhood increases the risk of primary liver cancer in adults, with a HR of HCC development of 1.36 per unit increase in BMI [14]. Despite the fact that some of these studies have limitations (particularly related to the absence of data on the presence of co-factors of liver damage [i.e., hepatitis virus infections and/or alcohol intake]), the link between obesity and HCC is very strong and unanimously considered unquestionable (Fig. 2).
Schematic representation of the phases connecting obesity to HCC. The question mark (?) highlights the hypothesis of a hepatocarcinogenic role of obesity independent of the non-alcoholic fatty liver disease. Abbreviations: NAFLD, non-alcoholic fatty liver disease. NASH, non-alcoholic steatohepatitis. HCC, hepatocellular carcinoma.
A milestone epidemiology paper published by Calle et al. in 2003, which evaluated more than 900,000 U.S. adults, showed that the relative risk of liver cancer-related mortality in subjects with a BMI between 30 and 34.9kg/m2 and in those with a BMI>35kg/m2 was 1.9 and 4.5 times higher, respectively, than that of normal weight individuals [7]. Similarly, the previously reported paper analyzing a large English population, showed that obese individuals had a liver cancer-related mortality 4 times higher than that of normal weight subjects [9]. Recently, a meta-analysis including 9 observational studies for a total number of more than 1,500,000 individuals showed that obese subjects had a two-fold increase of HCC-related mortality. Such association was more evident in men and Western populations [28]. A study conducted in UK showed that, between 2000 and 2010, mortality for liver cancer rose 1.8-fold, with a 10-fold increase in HCC associated with NAFLD and with obesity and other metabolic risk factors being present in 66% of the cases, independently of the underlying etiology of the liver disease [29]. Obesity may also have an impact on the outcome after any treatment of the cancer nodules. In a study analyzing a cohort of 159 HCC patients treated with orthotopic liver transplantation (OLT), an increased incidence of life-threatening complications was found in overweight and obese patients compared to normal weight subjects, as well as a doubled incidence of HCC recurrence after OLT (15% vs. 7%) [30]. In a U.S. retrospective cohort of 342 HCC patients who underwent OLT between 1999 and 2010, a BMI higher than 30 was a predictor of HCC recurrence, microvascular invasion and of a poor overall survival (OS), doubling the mortality risk after transplantation [31]. Another study performed in Japan showed lower survival rates in overweight or obese patients undergoing hepatectomy for recurrent HCC compared to individuals with normal BMI [32]. These studies might lead to hypothesize that obesity negatively influences the outcome after HCC treatments, and this may be due to a higher risk of postoperative complications [33]. However, in this context it is worthy to be mentioned that several studies showed that HCC patients with higher BMI have a better outcome after hepatectomy than those with lower BMI [34–36], whereas other studies did not find any difference in outcomes after surgery in patients with different BMI [37–39].
4HCC surveillance in obese patientsBy definition, the aim of a surveillance program is to achieve a reduction in the mortality rates of the target disease through an early diagnosis, and it should be cost-efficient. The usefulness and the applicability of the diagnostic tests used for surveillance depend on many factors, such as the incidence of the disease in the target population and the availability of the test itself. Patients at high risk of developing HCC should be included in a surveillance program that essentially consists of an upper abdominal ultrasonography performed every six months [40,41]. Patients with cirrhosis are at the highest risk of HCC (in fact, more than 80% of HCC arise in the contest of advanced liver disease) [42]. However, gray areas exist, and there is the possibility of the development of HCC in non-cirrhotic livers, in particular in cases with HBV infection and in cases with NAFLD. However, the real risk of HCC development in non-cirrhotic NAFLD patients is still unknown. It is estimated that about 50% of NASH-associated HCC arise in the context of a non-cirrhotic liver [43,44], but such incidence of liver cancer is considered not sufficient to promote an active ultrasonographic surveillance, considering the very large prevalence of NAFLD in the general population. Thus, timely diagnosis of HCC arising in a NAFLD context is a true challenge for the hepatologists, and obesity – very frequently present in NAFLD patients – makes it more difficult, because of a higher chance of a poor-quality ultrasound examination in obese subjects.
In a U.S. study examining “Surveillance, Epidemiology and End Results” (SEER) registries between 2004 and 2009, and including almost 5000 HCC patients, those with NAFLD-associated liver cancer were older, had shorter OS and higher tumor-related mortality than patients with HCC related to other etiologies [45]. This is mainly due to a delay in the diagnosis due to uncertainty in the surveillance benefit. The issue related to the lack of surveillance has been assessed in a study on U.S. veterans, showing that a significantly higher proportion of patients with NAFLD-related HCC (56.7%) did not undergo surveillance in the 3 years preceding HCC diagnosis, compared with HCC patients with alcohol- (40.2%) or HCV-related disease (13.3%) [46]. Consequently, a lower number of patients with NAFLD-related HCC had the chance to receive tumor-specific treatments [46]. A retrospective cohort study conducted in the U.S. and including 941 patients undergoing ultrasound surveillance for cirrhosis showed that obese patients had 3–8-fold higher risk of having an inadequate examination, with increasing BMI leading to an increased risk of failure. In fact, one-third of cirrhotics with a BMI>35 had a qualitatively inadequate ultrasound [47]. For these reasons, the possibility of a surveillance with computed tomography or magnetic resonance imaging has been considered for these patients, although its cost-effectiveness would be impractical. Indeed, there is no consensus on what is the best surveillance strategy – if any – in non-cirrhotic obese patients with NAFLD, considering that the individual risk of HCC development is low, particularly if compared to the risk in cirrhotic subjects [48,49]. Although given the actual incidence rates surveillance cannot be recommended in this setting, it is undoubted that an efficacious HCC risk stratification in non-cirrhotic obese subjects with NAFLD is an unmet need at present.
5Obesity as a risk co-factor of HCC development in chronic liver diseasesBecause of its diffusion, obesity is frequently present in patients with chronic hepatitis B or C or with alcoholic liver disease, and it is considered an additional HCC risk factor in these subjects. A Taiwanese population-based study, conducted in about 24,000 subjects, revealed that obesity was associated with a 4-fold risk of HCC in anti-HCV positive subjects, a 1.36-fold risk in HBV-infected, and a 2-fold risk in subjects without viral infections [50]. Furthermore, when obesity and diabetes were present together, such association caused a more than 100-fold increased risk of HCC in both HBV and HCV infected subjects, suggesting a possible synergistic effect of metabolic factors and viral hepatitis [50]. A Japanese study which enrolled about 1500 patients with chronic hepatitis C showed that overweight and obesity were independent risk factors of HCC, with a HR of 1.86 and 3.1, respectively [51]. A retrospective study analyzing liver biopsies from HBV infected individuals, showed that histologically detected liver steatosis was an independent risk factor for HCC (HR 7.3) [52]. Similarly, the presence of radiologically-assessed NAFLD was shown to be a risk factor for HCC development in chronic hepatitis B patients in whom HBV was suppressed by means of antiviral therapy [53]. A synergistic effect of obesity and alcohol intake has been identified in a study prospectively evaluating a Taiwanese population with chronic hepatitis B, where the risk of incident HCC increased in both overweight (HR 2.4) and obese (HR 2.9) alcohol abusers [54]. A large prospective study from UK highlighted the role of obesity in patients with HCC arising in the context of liver diseases caused by other etiologies, with metabolic risk factors being present in up to two-thirds of patients with HCC [29]. A retrospective analysis conducted in the U.S. on about 20,000 explanted livers showed that obesity was an independent predictor of HCC in patients with alcoholic cirrhosis [55]. Similarly, a French retrospective study analyzing 110 patients with alcoholic cirrhosis who underwent OLT found that a previous history of being overweight or obese increased the risk of HCC (odds ratio [OR] 6.2), and that the contemporary presence of T2DM increased the risk with an additional effect (OR 9.1) [56]. In a cohort of French patients with well compensated alcoholic or HCV related cirrhosis, the contemporary presence of obesity and T2DM significantly increased the risk of HCC development (HR 6) [57]. Altogether, these data confirm that obesity is an important additional player in the development of HCC in patients with liver disease due to different causes.
6Possible interventions for reducing HCC risk in obese patients with NAFLDGiven the strong link between obesity and HCC, every intervention aimed at reducing the BMI at individual level should reduce the risk of HCC development. The impact of dietary factors and physical activity on HCC have been recently reviewed [58]. Growing evidence demonstrates that a healthy diet may have an impact in reducing the risk of HCC development. It has been shown that a fruit-rich diet reduces HCC risk, while a low vegetable consumption increases the possibilities of developing primary liver cancer [59,60]. Indeed, a good adherence to a Mediterranean diet appears to be associated with a 50% reduction of HCC incidence [61]. Moreover, epidemiologic studies have demonstrated that physical activity is able to reduce the risk of different cancers [62–66]. In a large prospectively-followed Taiwanese cohort, a correlation between a reduced risk of HCC and the degree of physical activity was observed [67]. An NIH study on about 500,000 individuals provided similar results, showing a significant decreased risk of HCC (relative risk 0.56) comparing the highest with the lowest level of physical activity [68]. Concerning pharmacologic interventions, metformin as well as statins have been associated with a significantly reduced risk of HCC in diabetics and dysplipidaemic patients with NAFLD [69–74]. However, studies specifically focused on the use of the above drugs in obese individuals are lacking. Bariatric surgery is a well recognized treatment of morbid obesity, and it has been shown to induce disappearance of NASH in about 85% of patients after one year of follow up post-surgery [75]. Thus, one may speculate that – in the long-term – this surgical approach could be beneficial in reducing the risk of HCC development in morbidly obese patients. However, the lack of long-term follow up investigations do not allow – at present – to confirm this hypothesis.
7Is there a link between obesity and cholangiocarcinoma?Cholangiocarcinoma (CC) is a malignant tumor of the biliary tract, the second most common primary liver cancer after HCC [76]. CC is classified based on anatomic locations as intra- (ICC) or extrahepatic (ECC), which are considered two distinct phenotypes, differing in their presentation, natural history, management, and probably also in their pathogenesis. ICC is similar to HCC in its presentation, and both are often classified as primary liver cancers in epidemiologic studies.
At present, contrasting data are available on a possible link between obesity and CC [77–80]. However, when the studies were limited to the ICC, the results showing an association with obesity appear to be more uniform. Indeed, the start of the obesity pandemic in the U.S.A. preceded by about 10 years the rapid increase of ICC incidence observed in the 1980s in that country [81]. In addition, data from the SEER program showed that MS was significantly more frequent in patients developing ICC, and in these patients obesity was identified as an independent risk factor of ICC [8]. Also a meta-analysis confirmed that obesity is an ICC risk factor (OR 1.6) [82]. A recently published paper from the National Cancer Institute linked early adulthood adiposity to ICC. In particular, it showed that higher BMI at age 18 was associated with a 34% higher risk of successive ICC development [83]. The most recently published meta-analysis, analyzing prospective cohorts and nested case-control studies, revealed that obesity was associated with a 49% increased ICC risk [84].
8ConclusionsMany epidemiologic data have identified obesity as an important risk factor for HCC development. Moreover, obesity is associated with reduced survival in HCC patients. This might be related to a less efficacious surveillance strategy and a consequent delay in diagnosis with more limited possibility of therapeutic interventions, although the possibility of a worse outcome after curative treatments in these patients cannot be ruled out. With the growing epidemic of obesity, a parallel increase of the prevalence of NAFLD is foreseen, making it the candidate to be the worldwide most important risk factor for HCC development in the near future.AbbreviationsBMI body mass index cholangiocarcinoma direct antiviral agents extrahepatic cholangiocarcinoma hazard ratio hepatitis B virus hepatitis C virus hepatocellular carcinoma intrahepatic cholangiocarcinoma metabolic syndrome non-alcoholic fatty liver disease non-alcoholic steatohepatitis odds ratio orthotopic liver transplantation overall survival surveillance epidemiology and end results type 2 diabetes mellitus
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.