Introduction. Hepatitis C virus (HCV) infection is an important risk factor for the development of liver fibrosis and progression to cirrhosis. Liver transplantation as terminal treatment option for liver disease requires life-long immunosuppression. However, immunomodulatory therapy may promote reinfection and renewed fibrogenesis. Immunosupressive agents may also affect the life cycle of hepatic stellate cells (HSC), the main source of extracellular matrix. We thus aimed to characterize the effects of three common immunosuppressive agents on HSC apoptosis with or without engulfment of HCV infected apoptotic bodies.
Material and methods. LX2 cells were incubated with three different immunosuppressants (rapamycine, mycophenolic acid or cyclosporine A) and co-incubated for 24 and 48 h with apoptotic bodies (AB), produced from Huh7 cells or from Con1 cells (Huh7-cells containing a subgenomic HCV replicon). The engulfment of AB was confirmed by immunofluorescence staining. HSC viability, apoptosis rate and expression of profibrogenic and proapoptotic genes were quantified.
Results. In LX2 cells that engulfed Conl AB, the treatment with mycophenolic acid induced HSC apoptosis and reduced collagen lalpha 1 expression compared to cylosporine A or rapamycine treatment. In conclusion mycophenolic acid is a potent inducer of HSC apoptosis and attenuates HSC activation and consecutively fibrogenesis in HCV infection. Translational studies will need to confirm these mono-culture results in vivo.
Among chronic liver diseases, hepatitis C virus (HCV) infection with a worldwide prevalence of approximately 170 million infected people, represents still the most common risk factor for development of liver cirrhosis.1 At present, orthotopic liver transplantation is the ultimate therapy of end-stage liver disease, including HCV associated liver cirrhosis. Besides the need of life-long immunosuppression, the increasing number of HCV-infected patients undergoing liver transplantation requires a targeted therapeutical option, which is capable of repressing transplant re-infection and consecutive progression to (re-) fibrosis and cirrhosis.2
A characteristic feature of the liver tissue structure is the balance between extracellular matrix (ECM) synthesis and secretion of matrix degrading enzymes, e.g. matrixmetalloproteinases (MMP). The activation of previously quiescent hepatic stellate cells (HSC) plays a pivotal role in fibrogenesis, potentially culminating in liver cirrhosis and organ dysfunction. HSC activation leads to altered gene expression, ECM production and secretion of proinflammatory cytokines.3 The HSC perpetuation phase is characterized by proliferation, contractility, chemotaxis, loss of retinoid acid storage capacity, and cytokine production resulting in fibrogenesis.4 Upon re-infection with HCV following transplantation for terminal liver disease, renewed HSC activation and fibrogenesis may occur. This comprises a significant health risk for transplanted patients, resulting in increased post-transplant mortality.
Immunosuppression after liver transplantation is mandatory to retain graft function by inhibiting possible rejection processes. In contrast, suppression of the immune system promotes the risk of re-infection with HCV post transplantation. Immunosuppressants might thus affect HSC activation and fibrogenesis indirectly by enhancing HCV-dependent hepatocyte apoptosis or by directly activating HSC. In the present project effects of immunosuppressants in combination with hepatocyte apoptosis on HSC activation and viability were assessed. For this purpose an established model of HSC activation in HCV was utilized, the profibrogenic engulfment of apoptotic bodies (AB) derived from a standard hepatocellular cell line (Huh7) and one cell line with a genotype 1B subgenomic HCV replicon (Con1) by HSC(5) has been characterized.
Material and MethodsCulture of Huh-7, Huh-7Con1+ (ConA) and LX-2 CellsHuman cell lines were cultured as described before at 37°C, 5%CO2 in humidified atmosphere.6 Huh7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 μg/l glucose, 1% glutamine, 10% heat-inactivated fetal bovine serum (FBS) and 100 U/mL penicillin/100 g/mL streptomycin (all antibiotics and media: PAA, Pasching, Austria). When cells reached about 80% confluence, cells were passaged by Trypsin / EDTA method. Huh-7Con1+ (Con1) cells were cultured in the same medium as Huh7 cells, whereby 1% Geneticin/ G418 was added for selection. LX2 cells, a human hepatic stellate cell line, was cultured under identical conditions, while culture medium contained 1% FBS only.
Generation of apoptotic bodies (AB)To mimic conditions under chronic HCV infection, human hepatic stellate cells were incubated with AB, obtained from Con1 cells. AB generated from untransfected Huh7 cell line served as controls (condition in non-infected patients). For generation of AB 3.5 × 106 cells were seeded in 15cm petridishes and incubated in DMEM with above described additives but without 1% Geneticin/ G418 to avoid toxic side effects on LX2 cells. When confluence of more than 80% was reached, apoptosis was induced by irradiation with 100 mJ/cm2 UV light (λ = 254 nm) in a UV cross-linker (SpectroLinker XL1000, Spectronics Corporation, West-bury, NY, USA). After 24h formation of AB was visualized by inverted phase contrast microscopy. For selective harvesting of AB, cell culture supernatant was centrifuged at 300 g for 10 min at room temperature twice. The AB pellet was suspended in LX2 medium. Concentration of AB was determined by measurement of OD600 (absorbance λ = 595 nm).
Quantification of AB uptake by LX-2 cellsThe qualitative engulfment of ABs into cultured LX2 cells was detected by fluorescence microscopy. LX2 cells were labeled with Green Fluorescent Cell Linker Kit for General Cell Membrane Labeling (Sigma) for 2 min according to manufacturers instructions. Cells were washed three times at 400 g and RT for 10 min. Labeled 1 × 105 LX2 cells were seeded on cover slips and let rest for 24 h to become adherent. AB harvested from either Huh7 or Con1 cells were labelled for 2 min with the Red Fluorescent Cell Linker Kit for General Cell Membrane Labeling (Sigma) according to manufacturers instructions. After a washing step AB were resuspended in LX2 medium and added to adherent LX2 cells for 24 h. Non-engulfed AB were removed by washing with PBS twice. Slides were embedded with Antifade Gold medium with DAPI (Invitrogen, Paisley, Great Britain) and sealed with cover slips. Samples were evaluated by inverted fluorescence microscopy (Axiovert, Zeiss, Oberkochen, Germany) with a mercury vapor lamp and a multiple-band filter cube for simultaneous detection of green (λ = 515-530 nm) and red ( λ = 580-630 nm) fluorescence. Digital imaging was performed via the confocal LSM 510 LASER scanning module combined with Axiovision 4 software (both Zeiss).
Immunosuppressive treatment of LX2 cellsLX2 cells were seeded in tissue culture grade 6-well plates (Greiner, Frickenhausen, Germany) with a starting concentration of 1×105 cells per well and incubated for 24 h. Cells were pre-incubated with AB derived from Huh7 or Con1 cells for 1h. Vehicle, cyclosporine A (100 nM), rapamycin (25 nM), or mycophenolic acid (5 μg/mL) were added for 24 and 48 h. Cells were harvested to perform MTT, immunoblotting or rt-PCR.
MTT-assayLiving cells can reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) (pale yellow color) to a dark blue formazan product, that can be detected at a wavelength of λ = 550 nm. The resulting product concentration corresponds directly to the number of living cells. To asses viability of LX2 cells supernatant was removed and MTT solution (0.7 mg/mL) (Sigma-Aldrich, Munich, Germany) was added for 2 h. The produced formazan was solved from cells using MTT extraction solution (95% isopropanolol, 5% acetic acid) and measured at λ = 550 nm in a multiplate reader (ELISA Reader LAMBDA E, MWG-Biotech, Ebersberg, Germany).
Quantitative real-time polymerase chain reaction (qrt-PCR)Total RNA was isolated after 24 or 48 h from lysed LX2 cells with the RNeasy mini kit (Qiagen, Hilden, Germany) according to manufacturer’s specifications. RNA purities and concentration were measured photometrically (Biophotometer, Eppendorf, Hamburg, Germany) at λ = 260 nm and λ = 280 nm. Reverse transcription of 1 μg RNA was performed with the QuantiTect RT kit (Qiagen, Hilden, Germany) following the protocol. Quantitative qrt-PCR of the cDNAs were performed using QuantiTect SybrGreen system (Qiagen, Hilden, Germany), the iCycler iQ thermal cycler (Bio-Rad, California, USA) with real time detection system software 3.0a and Genex software (Bio-Rad). Each 30 μl reaction contained 15 μl QuantiTect SybrGreen master mix (Qiagen), 2 μl cDNA, 1 μl forward primer, 1 μl reverse primer (at 10 pmol/μl each), and 11 μl aqua dest. Enzyme activation was achieved by a single step for 15 min at 95 °C, followed by the actual PCR in 40 cycles of 30 s at 95 °C, 30 s at 55 °C, and 30 s at 72 °C. A melting curve was collected 95 °C to 55 °C, at -0.5 °C steps for 10 s each. Relative gene expressions were calculated from the threshold cycles in relation to the reference gene HPRT 1 (hypoxanthine-phosphoribosyltransferase 1). PCR primers were generated as based on information by OMIM, ENTREZ, GeneBank, and the BLAST algorithm (all of which: National Center for Biotechnology Information, Bethesda, MD, USA) as well as Primer3 (http://frodo.wi.mit.edu) and all obtained from Eurofins (MWG, Operon, Ebersberg, Germany). Oligonucleotide sequences are given in supplemental.
Detection of apoptosis by M30-ElisaA surrogate marker for apoptosis (M30) was assessed in the supernatant of LX2 cells using the M30 Apoptosense-Kit (Peviva, Bromma, Sweden) following the manufacturers’ instructions. M30 represents a cytokeratin-18 (CK-18) neo-epitope only exposed upon apoptotic cleavage by activated caspase-3.
StatisticsStatistical analysis was performed with Microsoft Excel 2010TM (Microsoft GmbH, Frankfurt/M, Germany) and Prism 5 (GraphPad Software, La Jolla, CA, USA). Data is presented as mean ± SEM of 3 to 5 independent experiments. Only differences with p < 0.05 were considered significant. Statistic significant levels were detected by two-way ANOVA and Bonferroni post-test for all groups. For immunoblotting data one-way ANOVA with Bonferroni post-test was used.
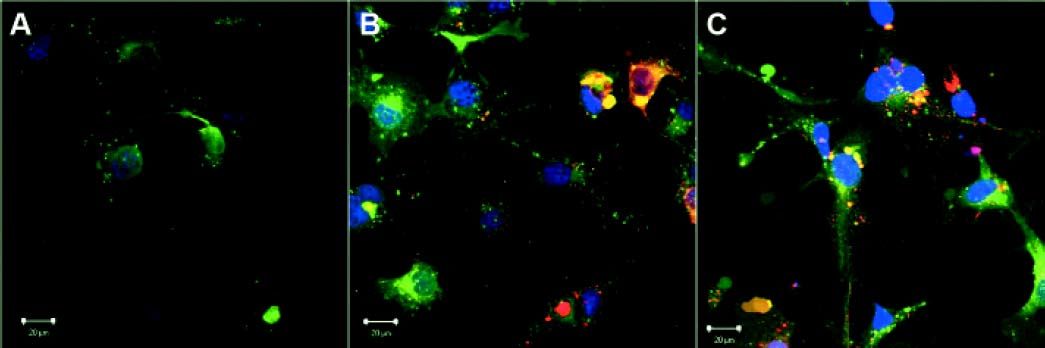
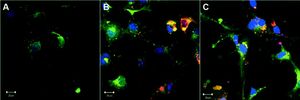
ResultsEngulfment of apoptotic bodies by HSCEngulfment of Huh7 and Con1 AB by LX2 cells under in vitro conditions mimics activation of quiescent HSC, as observed during chronic HCV infection in humans. Engulfment of AB by LX2 cells was visualized by fluorescence microscopy (Figure 1). AB incorporation was not affected by treatment with immunosuppressants and was independent of the surrogate viral load (for comparison please refer to reference 7).
Visualization of apoptotic body engulfment by LX2 cells by fluorescence microscopy. LX2 cells were labeled with a General membrane labeling (green) and embedded in antifade cover medium with DAPI (blue). Apoptotic bodies (AB) were labeled with general membrane labeling in red. Depicted are control cells receiving no AB (A), cells receiving AB from Huh7 cells (B), and cells receiving AB from Con1 cells (Huh7 containing the Con1 replicon) (C). No difference in uptake of AB from Huh7 cells or from Con1 cells was visible.
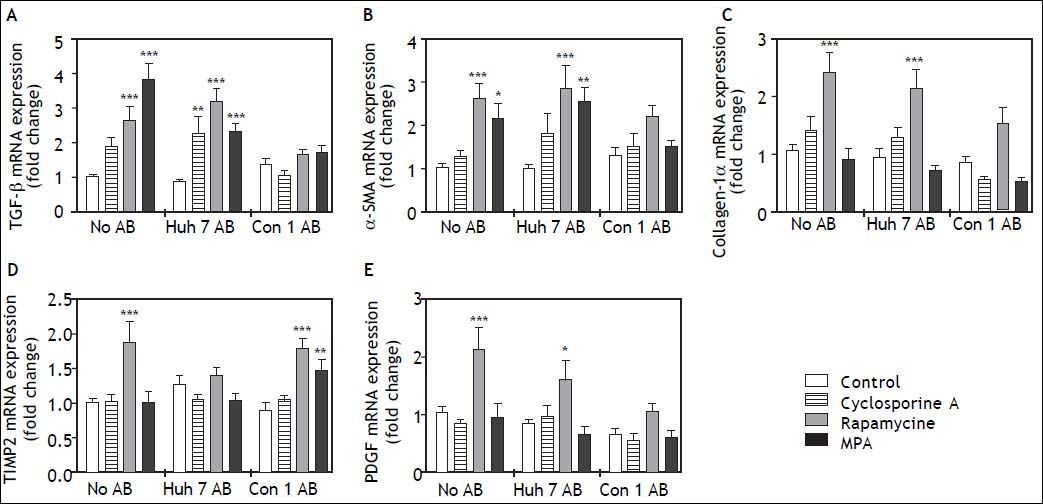
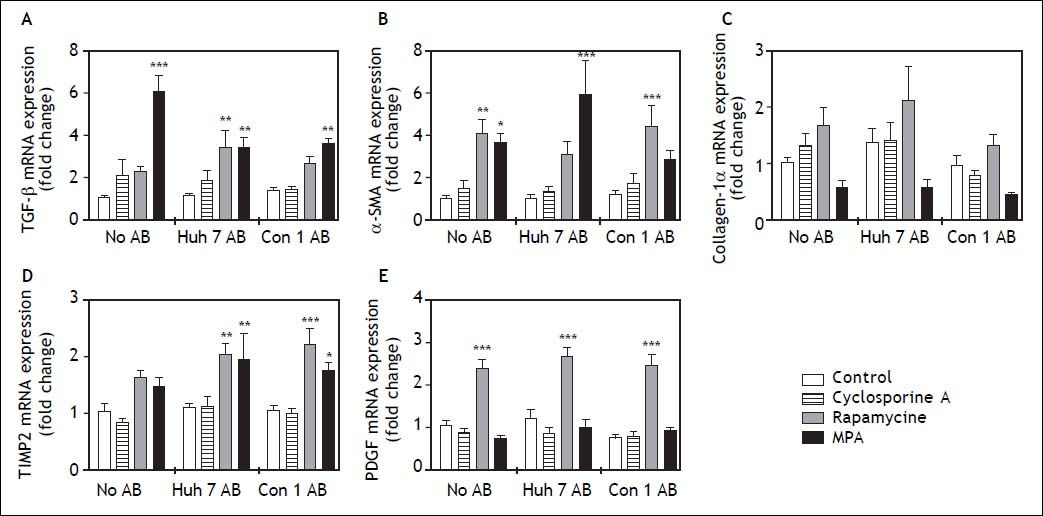
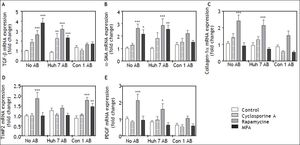
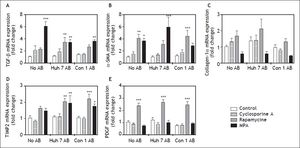
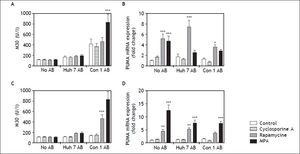
In order to determine the effects of immunosuppressive agents on HSC activation, mRNA expressions of TGFb, a-SMA, Col1a1, TIMP2, and PDGF in cell lysate were analyzed. Rapamycine induced expression of TGF-beta, alpha-SMA Collagen-1-alpha, TIMP2, and PDGF at 24 h after stimulation (Figure 2). Interestingly co-treatment with Con1 AB, but not Huh7 AB ameliorated this effect, except for TIMP2. Mycophenolic acid increased the mRNA expression of TGF-beta and alpha-SMA. Again a reduction of this effect was observed in co-stimulation with Con1 AB. Increased TIMP2 mRNA expression occurred under co-treatment with Con1AB and mycophenolic acid only. Cyclosporine A, Huh7 AB or Conl AB alone exhibited no effect on the analyzed mRNA expression levels at 24h. Rapamycine induced mRNA expression of PDGF independent of AB treatment after 48h (Figure 3). TIMP2 mRNA was increased upon co-treatment with Huh7 or Con1AB only, TGF-beta solely in combination with Huh7 AB. Rapamycine induced mRNA expression of alpha-SMA was observable without AB or Con1 AB. Again no effects of AB or cyclosporine A alone were detected.
Profibrogenic gene expression in LX2 cells treated with immunosuppressants and apoptotic bodies (AB) after 24h. AB from Huh7 cells (Huh7 AB) or from Con1 cells (Huh7 containnig the Con1 HCV replicon; Con1 AB) were added to LX2 cells. After 1 h incubation immunosuppressing agents were added and mRNA expressions of profibrogenic genes were determined after 24 h. Rapamycine treatment led to a strongly profibrogenic expression profile of LX2 cells, irrespective of added AB. *,**,*** p < 0.05, 0.01, 0.0001 compared to control/no AB (2-way ANOVA with Bonferroni correction). MPA: mycophenolic acid.
Profibrogenic gene expression in LX2 cells treated with immunosuppressants and apoptotic bodies (AB) after 48 h. AB from Huh7 cells (Huh7 AB) or from Con1 cells (Huh7 containnig the Con1 HCV replicon; Con1 AB) were added to LX2 cells. After 1 h incubation immunosuppressing agents were added and mRNA expressions of profibrogenic genes were determined after 48 h. Rapamycine treatment led to a strong induction of PDGF a pro-proliferative signal for hepatic stellate cells, irrespective of added AB. *,**,*** p < 0.05, 0.01, 0.0001 compared to control/no AB (2-way ANOVA with Bonferroni correction). MPA: mycophenolic acid.
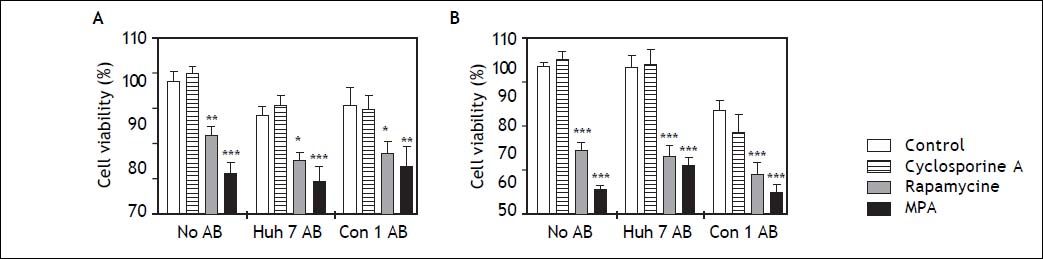
LX2 cell viability assessed by MTT was significantly reduced by rapamycine and mycophenolic acid after 24 and 48 h incubation (Figure 4). Both substances diminished LX2 cell viability independent of AB treatment. Cell viability was not affected by cyclosporine A or AB treatment alone.
Survival of LX2 cells after treatment with apoptotic bodies (AB) and/or immunosuppressants. MTT assays were performed to assess viability of LX2 cells after 24 h (A) and 48h (B) of treatment with AB and/or immunosuppressants. While AB treatment alone did not reduce viability of LX2 cells, mycophenolic acid (MPA) significantly diminished cell viability at 24 and 48 h. Rapamycine had a similar effect, which was slightly weaker at 24 h. *,**,*** p < 0.05, 0.01, 0.0001 compared to control/no AB (2-way ANOVA with Bonferroni correction).
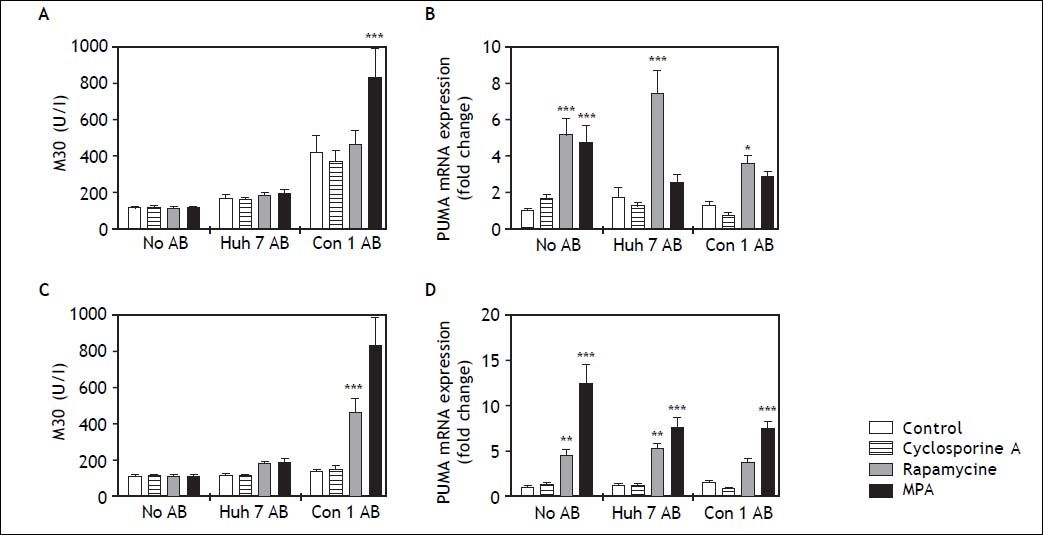
Since mycophenolic acid and rapamycine reduced HSC viability and affected activity, we analyzed HSC apoptosis. Apoptosis of HSC is an important mechanism in HSC inactivation and resolution of fibrosis.8 Caspase cleaved CK18 filaments (M30) were quantified as surrogate marker for apoptosis in the cell supernatant. While treatment with Con1 AB increased M30 concentrations in cell supernatants, this effect did not reach significance except for co-treatment with mycophenolic acid at 24h (Figure 5A). After 48 h incubation, co-stimulation with Con1 AB and rapamycin or mycophenolic acid increased detectable M30 concentrations (Figure 5C). PUMA is an important inducer of p53-dependent and independent apoptosis and PUMA mRNA levels comprise an early marker of apoptosis.9 PUMA mRNA expression was induced by rapamycine at 24h independent of AB co-treatment (Figure 5B). Mycophenolic acid increased PUMA mRNA only without AB co-stimulation at 24 h. In contrast, mycophenolic acid induced PUMA mRNA expression independent of AB treatment after 48 h incubation (Figure 5D).
Cell death in LX2 cells after treatment with apoptotic bodies (AB) and/or immunosuppressants. To determine the amount of apoptosis induced by immunosuppressant dosage, M30 (A, C) and mRNA expression of PUMA in LX2 cells were measured. Mycophenolic acid (MPA) treatment significantly enhanced the amount of M30 in cell culture supernatant 24 h (A) and 48 h (C) after stimulation, though this effect only occurred in the presence of Con1 AB. mRNA expression of PUMA was increased by rapamycine treatment after 24 h (B) and to some extent also at 48 h (D) after treatment. MPA increased PUMA mRNA only in absence of AB at 24 h, but independent of AB treatment after 48 h. p < 0.05, 0.01, 0.0001 compared to control/no AB (2-way ANOVA with Bonferroni correction).
The activation of quiescent vitamin A-rich HSC into proliferative, contractile, and fibrogenic myofibroblasts plays a pivotal role in progression of HCV to fibrosis, finally culminating in cirrhosis. Since hepatitis progresses to end stage liver disease in a significant number of patients with HCV, many HCV patients face the need for liver transplantation. The selection of the immunosuppressive regimen after reception of a transplant liver, plays a pivotal role in HCV reinfection with rapid progression to re-cirrhosis. In the present study we assessed the possible impact of immunosuppressive agents on human HSC biology, in a model of HCV infection. The central findings are that rapamycine and mycophenolic acid (i) induce mRNA expression profiles associated with HSC activation, (ii) reduce HSC viability, and (iii) induce cell death of HSC. In contrast cyclosporine A did not affect HSC biology in vitro.
We observed that mycophenolic acid reduced HCV viability, which is supported by previous studies of Greuping, et al.10 Viability of HSC was substantially reduced with mycophenolic acid and rapamycine treatment independent of presence of AB. However, apoptosis rate, assessed by quantification of caspase cleaved collagen filaments was increased in mycophenolic acid and Con1 co-treated LX2 cells only, suggesting a cell specific pro-apoptotic effect by immunosupression and virus derived substances. Apoptosis is a central mechanism in HSC inactivation and regression of fibrosis. This is of clinical relevance, since hepatocyte apoptosis promotes HSC activation.7
Success of HCV treatment in patients with reinfection following liver transplantation is limited. HCV reinfection in HCV patients occurs in virtually all patients, in which viral clearance was not achieved before transplantation. Reinfection occurs rapidly and is accompanied by an accelerated fibrosis progression compared to patients without transplantation, which is most likely augmented by immunosuppressant regimen. Traditional dual HCV therapy, based on interferon and ribavirin was often associated with adverse events, high prevalence of thrombocytopenia and drug interactions, as well as a relatively low rate of sustained viral response (SVR).11,12 Recently, novel therapy regimens have been implied in the post-transplant setting.13 Still, triple therapy requires the application of pegylated interferon for up to 48 weeks and the experience in post-transplant patients is limited. Recently introduced interferon free regimens might improve SVR rate, quality of life and survival in HCV reinfection, but data on post-transplant patients is scarce at present.14–16
A possible supportive strategy to ameliorate new fibrogenesis in HCV positive transplant patients might be modification of the immunosuppressive regimen. Utilization of antifibrogenic drugs might improve survival and reduce the incidence of lethal complications like end-stage liver disease and concomitant hepatocellular cancer.17 In kidney disease, mycophenolic acid has previously been proven to have antifibrotic properties.18 Here, we were able to demonstrate a strong effect of mycophenolic acid on HSC apoptosis, viability and activation in a model of HCV induced HSC activation. Mycophenolic acid was the most potent inducer of HSC apoptosis compared to cyclosporine A and rapamycine in this study. However, rapamycine has previously been attributed to antifibrotic properties in a mouse model.19 In our study, rapamycine also induced HSC apoptosis and reduced HSC viability, tough to a lesser extent. In a clinical study, Firpi, et al. observed only a modest effect of cyclosporine A on fibrogenesis in HCV reinfection, but a potential positive effect on SVR.20 Here, cyclosporine A did not alter HSC viability or apoptosis, and seemed to have no effect on mRNA expression of genes involved in HSC activation.
In summary, mycophenolic acid had an overall strong effect on HSC apoptosis, viability and activation. If mycophenolic acid has potential to reduce renewed fibrosis in a post-transplant HCV setting still is open to debate, since strong HSC activation and HSC apoptosis might mutually diminish pro- and anti-fibrotic effects of this substance. In contrast, cyclosporine A seemed to have virtually no effect on HSC biology in vitro and might be an interesting immunosuppressant in clinical settings, were any interference with fibrogenic processes is unwanted. A conversion to a mycophenolic acid containing immunosuppressant regimen might be considered in patients with HCV recurrence to prevent rapid progression to cirrhosis. Further co-culture and in vivo studies will need to confirm these results.
Author Contributions- •
Study design: AZ, AC, LPB.
- •
Data collection: JB, AZ, AB, SS.
- •
Data analysis: JB, AZ, CF, JPS, OA.
- •
Material or technical support: SS, CF, VC, GG.
- •
Drafting of the manuscript: AZ, AB.
- •
Revision of the manuscript for important intellectual content: JB, JPS, AC, LPB.
- •
Study supervision: VC, GG, AC, LPB.