Background and aims. Percutaneous ethanol injection (PEI) is a well-established therapeutic option in patients with cirrhosis and hepatocellular carcinoma (HCC). The modified-Response Evaluation Criteria in Solid Tumors (m-RECIST) are an important tool for the assessment of HCC response to therapy. The aim was to evaluate whether HCC response according to the m-RECIST criteria could be an effective predictor of long-term survival in Barcelona Clinic Liver Cancer (BCLC) stage 0 and A HCC patients undergoing PEI.
Material and methods. 79 patients were followed-up for median time of 26.8 months. HCC diagnosis was based on the current guidelines of the American Association for Study of the Liver Diseases (AASLD) and European Association for Study of the Liver (EASL). Patient survival was calculated from the first PEI session to the end of the follow-up.
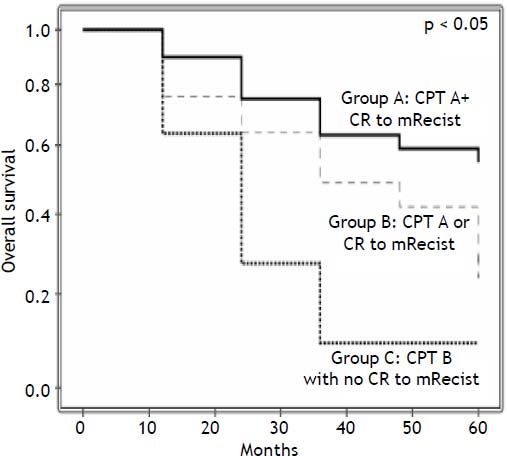
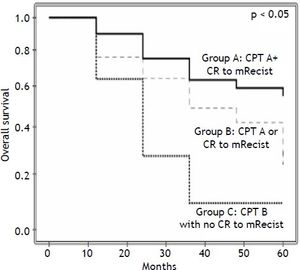
Results. The 1-, 3-, and 5-year overall survival rates were 79, 48 and 37%, respectively. In the multivariate analysis, Child-Pugh-Turcotte (CPT) (p = 0.022) and the response to m-RECIST criteria (p = 0.016) were associated with patient survival. CPT A patients who achieved Complete Response (CR) 1 month after PEI presented a 5-year survival rate of 55%. By contrast, the worst scenario, the group with CPT B but without CR had a 5-year survival rate of 9%, while the group with either CPT A or CR as a survival predictor had a 5-year survival rate of 31%. In conclusion, in BCLC stage 0 and A HCC-patients, m-RECIST at 1 month and Child A may predict survival rates after PEI.
Hepatocellular carcinoma (HCC) is a malignant tumor that has attracted attention in recent decades due to its rising worldwide incidence. This is mainly related to the burden of chronic hepatitis C infection and the subsequent development of cirrhosis.1–3 As a result of surveillance programs aimed at identifying HCC at early stages, many patients have been eligible for therapeutic interventions such as liver transplantation (LT), liver resection (LR) and percutaneous ablation.2 LT has been shown to be a feasible treatment option with survival rates of up to 75% after 4 years, provided that the Milan criteria are fulfilled (a single nodule up to 5 cm or up to 3 nodules no larger than 3 cm each with no macrovascular invasion or extra-hepatic spread).4 The current AASLD and EASL guidelines recommend LT as the preferred option in most patients within the Milan criteria.5,6 Child-Pugh-Turcotte (CPT) A and patients with a single tumor without significant portal hypertension (SPH) have been shown to be the best candidates for LR.7 Percutaneous ablation procedures are suggested for patients who are not suitable for surgical procedures and are considered alternative techniques to avoid tumor growth while patients are waiting transplantation.
Moreover, it is important to emphasize that curative therapeutic options should be largely offered to patients within the Barcelona Clinic Liver Cancer (BCLC) class 0 and A, provided that in addition to tumor morphological characteristics and liver function, a patient’s performance status is also considered.5,6,8
Percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) are the most commonly used methods of percutaneous ablation.9,10 Recent studies have demonstrated that RFA is more effective than PEI in patients who meet the Milan criteria with regards to the patient survival and tumor recurrence.11–14 Nevertheless, it is also accepted that both alternatives have similar outcomes for patients with a single liver nodule up to 2 cm in diameter.15 In addition, it should be noted that the higher cost of RFA in comparison with PEI may make PEI a more cost-effective treatment.
The modified-Response Evaluation Criteria in Solid Tumors (m-RECIST) are a major advance in the evaluation of HCC radiological responsiveness.16 The revised version of the RECIST criteria establishes that the assessment of tumor response should consider only the area of viable tumor as defined by arterial enhancement, without the necrotic features. This has been incorporated into the AASLD and EASL practice guidelines.5,6
Little is known regarding the impact of the response to the m-RECIST criteria to PEI on longterm survival rates in HCC-patients.16 The aim of the present study is to evaluate whether or not the response to m-RECIST 1 month after PEI would identify a subset of BCLC 0 or A HCC-patients who would most benefit from this procedure.
Material and MethodsBetween September 1997 and December 2005, HCC was diagnosed in 431 patients with cirrhosis in the Department of Gastroenterology at the University of São Paulo School of Medicine. All radiological diagnostic assessments were reviewed following the current recommendation of the AASLD and EASL-HCC guidelines.5,6
Hepatic resection was the initial treatment in 34 cases, liver transplantation in 16, and transarterial chemoembolization in 74; 173 patients received palliative care. Forty-nine patients were lost of follow-up after diagnosis. PEI was performed in 85 cases. The criteria to select patients to undergo PEI were:
- •
A single nodule up to 5 cm or up to 3 nodules less than 3 cm in diameter, without macrovascular invasion or extra-hepatic spread.
- •
Nodules located at least 1 cm away from the hepatic hilum or the gallbladder.
- •
Prothrombin activity > 50%.
- •
- •
Ineligibility for hepatic resection or transplantation [except in cases in which PEI was performed as treatment for HCC while on a transplant waiting list, n = 54 (63%)].
Six patients were excluded due to the absence of reliable data available as a result of either technical problem during the test or an inability to administer it within the 30 day cutoff period. Seventy-nine patients were analyzed and comprise the population of the present study. All patients were classified as Eastern Cooperative Oncology Group performance status 0;17 thus all of the analyzed cases were BCLC class 0 or A.
PEI procedureEach session was performed under local anesthesia and conscious sedation with midazolan, using ultrasound real-time guidance of a 22-gauge needle. All sessions were performed by trained operators (FJC and DCPV). The intended total volume of absolute ethanol was calculated using the formula V = 4/3 π (r + 0.5)3.14 The actual injected volume of alcohol in each session varied depending on limiting factors such as patient tolerance of pain or leakage of ethanol outside the lesion. The number of PEI sections was determined based on the number and size of HCC lesions. Each treatment cycle consisted of up 4 or 5 sessions. One month after each cycle a four phase-computed tomography (CT) scan was performed. If viable residual tumor was detected, another treatment cycle was given, up to a maximum of 3 cycles.
Assessment of treatment responseShort-term treatment effectiveness was assessed according to the m-RECIST criteria16 1 month after the last PEI cycle. The response was independently assessed by 2 examiners. Disagreements were resolved by consensus.
Additional treatmentsDuring the follow-up period, 22 patients (28%) received transarterial embolization or chemoembolization as a rescue therapy for PEI failure, and 16 of the 54 patients on the LT waiting list (29.6%) received a transplant.
Follow-upPatients were follow-up from September 1997 until March 2013. Data were censored in case of death, the last visit or LT. In the first 5 years post-PEI, a CT or MRI was performed every 6 months to evaluate tumor status.
Statistical analysisCategorical variables were ordered and compared using a χ2 test (with Yates correction whenever appropriate). Continuous variables were expressed as the mean ± standard deviation and compared through Student’s t test. When a normal distribution could not be assumed, continuous variables were represented by the median value and ranges and were compared using the Mann-Whitney test. The probability curves of survival and recurrence were calculated using the Kaplan-Meier method and compared using the log-rank test. Variables with p < 0.1 were selected for multivariate analysis. A value of p < 0.05 was considered significant in the final analysis. The calculations were conducted using the SPSS for Windows 16.0 package.
Variables for analysisThe following variables were analyzed:
- •
Demographic; age (continuous and ≤ 55/> 55 yo), gender, etiology of the liver disease (viral/ non-viral).
- •
Clinical features; significant portal hypertension [SPH-defined as a hepatic vein pressure gradient ≥ 10 mmHg or the presence of gastro-esophageal varices, splenomegaly (spleen length ≥ 12 cm) with platelet count ≤ 100 x 103/mm3, or need for diuretics to control ascites (yes / no)], CPT (A/ B).
- •
Radiological characteristics; size of tumor (continuous, ≤ 2/> 2 cm, BCLC class (0/A) (6), number of tumors (1/2 or 3 nodules), location in the liver (uni/bilobar).
- •
Serum laboratory analysis; bilirubin (continuous and ≤ 1/> 1 mg/dL), alfa feto-protein (continuous and ≤ 100/> 100.ng/mL and ≤ 200/> 200 ng/mL), platelet count (continuous and ≤100/> 100 x 103/mm3).
- •
Treatment parameters; total ethanol volume (continuous and ≤ 20/> 20 mL), response according to the m-RECIST criteria (CR/non-CR) and PEI used while on waiting list for LT (yes/no).
This study was approved by the Institutional Review Board fulfilling all requirements for studies in humans, following the guidelines of the Declaration of Helsinki.
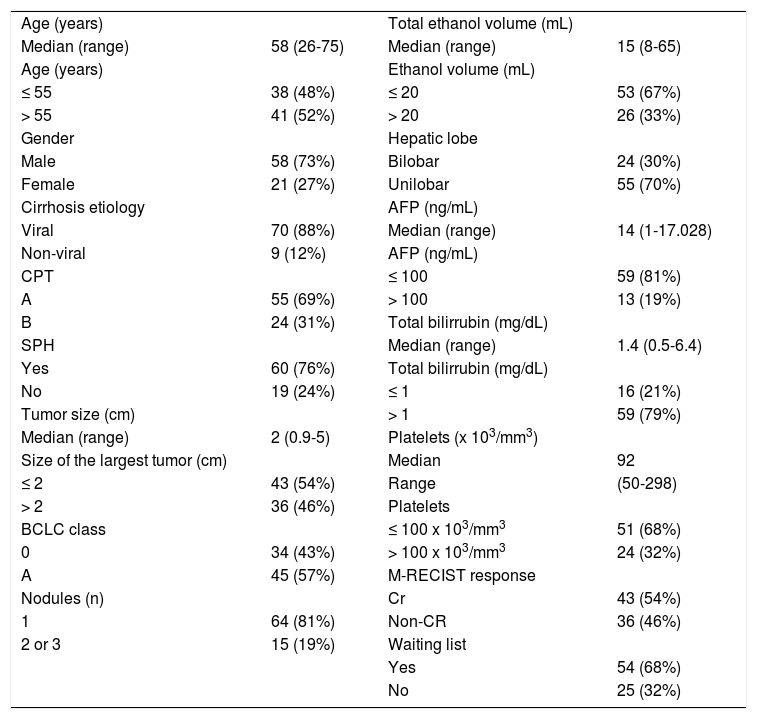
ResultsBaseline characteristics of the enrolled patients are presented in table 1. The median age was 58 (ranging from 26-75), and 58 (73%) of the patients were male. All patients had underlying cirrhosis, diagnosed by biopsy (n = 17, 22%) or clinical, radiological, and laboratorial features (n = 62, 78%). The etiology was hepatitis C, hepatitis B, alcohol, and miscellaneous in 67, 21, 8%, and 4% of patients, respectively. Fifty-five (69%) patients were CPT A. A single nodule was observed in 64 patients (81%), and 2 or 3 nodules were observed in 15 patients (19%). The median size of the primary tumor was 2 cm in diameter (ranging from 0.9-5 cm) at diagnosis. Forty-three patients (54%) had a primary tumor of 20 mm in diameter or less at time of diagnosis. The ultrasound pattern was hypoechoic in 54%, hyperechoic in 13%, isoechoic in 13% and mixed in 20% of patients. The median AFP level was 14 ng/mL (ranging from 1-17,028). AFP levels were higher than 100 ng/mL (19%) in 13 patients.
Baseline characteristics of the 79 enrolled patients.
| Age (years) | Total ethanol volume (mL) | ||
| Median (range) | 58 (26-75) | Median (range) | 15 (8-65) |
| Age (years) | Ethanol volume (mL) | ||
| ≤ 55 | 38 (48%) | ≤ 20 | 53 (67%) |
| > 55 | 41 (52%) | > 20 | 26 (33%) |
| Gender | Hepatic lobe | ||
| Male | 58 (73%) | Bilobar | 24 (30%) |
| Female | 21 (27%) | Unilobar | 55 (70%) |
| Cirrhosis etiology | AFP (ng/mL) | ||
| Viral | 70 (88%) | Median (range) | 14 (1-17.028) |
| Non-viral | 9 (12%) | AFP (ng/mL) | |
| CPT | ≤ 100 | 59 (81%) | |
| A | 55 (69%) | > 100 | 13 (19%) |
| B | 24 (31%) | Total bilirrubin (mg/dL) | |
| SPH | Median (range) | 1.4 (0.5-6.4) | |
| Yes | 60 (76%) | Total bilirrubin (mg/dL) | |
| No | 19 (24%) | ≤ 1 | 16 (21%) |
| Tumor size (cm) | > 1 | 59 (79%) | |
| Median (range) | 2 (0.9-5) | Platelets (x 103/mm3) | |
| Size of the largest tumor (cm) | Median | 92 | |
| ≤ 2 | 43 (54%) | Range | (50-298) |
| > 2 | 36 (46%) | Platelets | |
| BCLC class | ≤ 100 x 103/mm3 | 51 (68%) | |
| 0 | 34 (43%) | > 100 x 103/mm3 | 24 (32%) |
| A | 45 (57%) | M-RECIST response | |
| Nodules (n) | Cr | 43 (54%) | |
| 1 | 64 (81%) | Non-CR | 36 (46%) |
| 2 or 3 | 15 (19%) | Waiting list | |
| Yes | 54 (68%) | ||
| No | 25 (32%) |
CPT: Child-Pugh-Turcotte. SPH: significant portal hypertension. BCLC: Barcelona Clinic Liver Cancer. AFP: alpha-fetoprotein. CR: complete response.
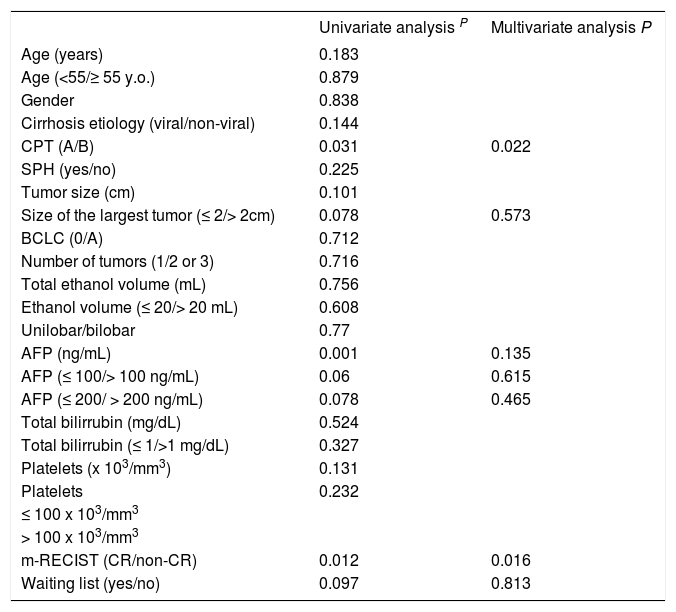
The median time to follow-up was 26.8 months (ranging from 0.7-136). The overall survival rates at 1, 3, and 5 years were 79%, 48%, and 37%, respectively. The variables tested as predictors of survival are listed in table 2. In the univariate analysis CPT (p = 0.031), the response according to the m-RECIST p = 0.012), size of the largest tumor (p = 0.078), AFP levels (continuous, p = 0.001, and ≤/>100 ng/mL, p = 0.060, AFP ≤/> 200 ng/mL, p = 0.078) and PEI performed while on a waiting list for LT (p = 0.097) were the factors associated with patient survival. In the multivariate analysis, only CPT (HR 0.466; 95% CI, 0.243-0.896; p = 0.022) and the response according to the m-RECIST (HR 0.441; 95% CI, 0.227-0.856; p = 0.016) predicted the survival probability (Table 2). CPT A patients who achieved CR 1 month after PEI presented a 5-year survival rate of 55%. By contrast, the worst scenario, the group with CPT B but without CR had a 5-year survival rate of 9%, while the intermediate group (with just 1 survival predictor) had a 5-year survival rate of 31%, p < 0.05 (Figure 1). Forty-three patients died between treatment and follow-up. Liver decompensation (n = 22) and HCC progression (n = 11) were the most frequent causes of death.
Prognostic factors associated with patient survival
| Univariate analysis P | Multivariate analysis P | |
|---|---|---|
| Age (years) | 0.183 | |
| Age (<55/≥ 55 y.o.) | 0.879 | |
| Gender | 0.838 | |
| Cirrhosis etiology (viral/non-viral) | 0.144 | |
| CPT (A/B) | 0.031 | 0.022 |
| SPH (yes/no) | 0.225 | |
| Tumor size (cm) | 0.101 | |
| Size of the largest tumor (≤ 2/> 2cm) | 0.078 | 0.573 |
| BCLC (0/A) | 0.712 | |
| Number of tumors (1/2 or 3) | 0.716 | |
| Total ethanol volume (mL) | 0.756 | |
| Ethanol volume (≤ 20/> 20 mL) | 0.608 | |
| Unilobar/bilobar | 0.77 | |
| AFP (ng/mL) | 0.001 | 0.135 |
| AFP (≤ 100/> 100 ng/mL) | 0.06 | 0.615 |
| AFP (≤ 200/ > 200 ng/mL) | 0.078 | 0.465 |
| Total bilirrubin (mg/dL) | 0.524 | |
| Total bilirrubin (≤ 1/>1 mg/dL) | 0.327 | |
| Platelets (x 103/mm3) | 0.131 | |
| Platelets | 0.232 | |
| ≤ 100 x 103/mm3 | ||
| > 100 x 103/mm3 | ||
| m-RECIST (CR/non-CR) | 0.012 | 0.016 |
| Waiting list (yes/no) | 0.097 | 0.813 |
y.o.: years old. CPT: Child-Pugh-Turcotte. SPH: significant portal hypertension. BCLC: Barcelona Clinic Liver Cancer. AFP: alpha-fetoprotein. CR: complete response.
Thirteen patients developed complications related to the treatment, which included ascites (n = 6), pleural effusion (n = 2), ascites and pleural effusion (n = 1) and hepatic encephalopathy (n = 1). Three patients had major complications, two patients died because acute cholecystitis (one of them, only 1 month after PEI) and one had tumor seeding in the needle track.
DiscussionThe factors involved in the response to therapy and survival rates in HCC are not fully understood. It has been suggested that early HCC patients would have a 5-year survival rate of approximately 20% if no treatment were administered.18 No prospective randomized trials have been performed comparing treatment alternatives with the best supportive care, given the assumption that treatment improves life expectancy. In addition to the discussion regarding the most appropriate first-line definitive treatment for patients with early-HCC, it is well accepted that at least a 50% 5-year survival rate would be expected for resection, transplantation and percutaneous ablation.19 The results achieved with surgical procedures are generally better understood, however the benefits of percutaneous ablation techniques have not been clearly demonstrated in the subset of patients with early-HCC.11–14,20–27
The present study demonstrated that PEI appears to be useful in a subset of BCLC 0 and A patients who are classified as with a CPT score A and who attained CR according to the m-RECIST criteria 1 month after PEI, as evidenced by a 5-year survival rate higher than 50%. It is important to note that BCLC A patients comply with CPT A and B, with Milan criteria and with Performance Status 0.5,6 Therefore, the identification of the CPT score provides an important tool for the differentiation of BCLC A patients who could benefit from PEI.
The m-RECIST criteria are an important advancement in the treatment of HCC-patients. However, data describing the impact of the m-RECIST criteria response on long-term outcomes are scarce (Table 3). In 2008, the Panel of Experts on HCC suggested that radiological responsiveness could be used as a surrogate parameter to evaluate treatment alternatives in HCC-patients.28 Given the scarcity of data, it was necessary to verify the role of radiological responsiveness post-PEI. To our knowledge, the present study is the first to demonstrate that the m-RECIST criteria are a valid surrogate parameter to evaluate prognosis of patients submitted to PEI.
Tumor size was not associated with long-term survival rates in the present analysis. Germani, et al. demonstrated that patients with a single nodule up to 2 cm in diameter would benefit similarly from either PEI or RFA. In patients with larger nodules but still in the early stages, RFA is associated with higher long-term survival rates.15 It could be argued that our results are not in agreement with this observation. Nevertheless, the superiority of RFA over PEI is believed to be associated with an inherent limitation of PEI in larger tumors. In contrast to RFA, the injected ethanol does not always accomplish complete tumor necrosis because of its lack of homogeneous distribution within the lesion, especially in the presence of intra-tumoral septa, and the limited effect on extra-capsular cancerous spread. At our Institution, this was recently demonstrated through a total of 38 RFA sessions performed on 34 patients.29 All patients had early stage tumors. The initial rate of RFA-in-duced complete tumor necrosis was 90%. CPT class and the model for end-stage liver disease score were identified as predictors of survival by simple Cox regression, but only CPT class showed a statistically significant association to survival in multiple Cox regression analysis (HR = 15; 95%CI: 3-76 months; p = 0.001). Although the present study did not compare PEI and RFA directly, these findings are in agreement with the current understanding of the use of ablative techniques in early HCC.
The principal limitation of this study is the retrospective design. Thus, our findings should ideally be validated by a prospective cohort of patients undergoing PEI. However, it should be highlighted that some solutions might be considered appropriate when the standard recommendations do not fulfill the patient’s needs. For instance, the current AASLD and EASL HCC-guidelines recommend liver resection for the subset of CPT A patients with a single HCC up to 5 cm without SPH.5,6 This recommendation is largely based on the landmark study published in 1999 by the Barcelona group.7 Following a retrospective evaluation of 77 cases, a multivariate analysis demonstrated that only the patients who benefit from LR would be those cases without SPH (n = 35) because the estimated 5-year survival rate was higher than 70%. Despite similar methodological limitations to the present study, those recommendations remain a valid and well-accepted reference in the treatment of HCC-patients in early-stages.
ConclusionThe present study showed that BCLC HCC-patients 0 and A who benefit from PEI comprise the subgroup of CPT A cases with complete response 1 month after PEI following the fulfillment of the m-RECIST criteria. The remaining cases achieved a 5-year survival rate of less than 50%. However further prospective studies are still needed to confirm such results.
Abbreviations- •
AASLD: American Association for Study of the Liver Diseases.
- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CPT: Child-Pugh-Turcotte.
- •
CR: complete response.
- •
CT: computed tomography.
- •
EASL: European Association for the Study of the Liver Diseases.
- •
HCC: hepatocellular carcinoma.
- •
LR: liver resection.
- •
LT: liver transplantation.
- •
m-RECIST: modified-Response Evaluation Criteria in Solid Tumors.
- •
MRI: magnetic resonance imaging.
- •
PEI: percutaneous ethanol injection.
- •
RFA: radio-frequency ablation.
- •
SPH: significant portal hypertension.
The study was supported in part by Alves de Queiroz Family Fund for Research.