Acute cholangitis, which is characterized by biliary infection and acute liver injury, may impact cirrhosis prognosis. However, the prognosis itself remains unclear.
Materials and methodsThis multicenter retrospective cohort study compared the mortality and liver function change between patients with and without cirrhosis who underwent endoscopic treatment for acute cholangitis caused by choledocholithiasis between January 2004 and December 2019.
ResultsWe analyzed 699 patients, 44 of whom had cirrhosis. The cirrhotic group had a significantly higher 30-day mortality rate than the noncirrhotic group (14% vs. 1%; P < 0.001). The cirrhotic group also had significantly lower total bilirubin and albumin recovery. However, all patients with cirrhosis who survived achieved total-bilirubin recovery, and 91% achieved albumin recovery within 90 days. In multivariable Cox regression analysis, the independent risk factors for total-bilirubin recovery included cirrhosis (hazard ratio, 0.37; 95%CI, 0.24‒0.58; P < 0.001) and high total-bilirubin level (0.46; 95%CI, 0.34‒0.60; P < 0.001), whereas those for albumin recovery were cirrhosis (0.51; 95%CI, 0.33‒0.79; P = 0.002), high age (0.62; 95%CI, 0.47‒0.82; P < 0.001), organ dysfunction (0.62; 95%CI, 0.39‒0.96; P = 0.03), low albumin level (0.57; 95%CI, 0.36‒0.91; P = 0.02), and high C-reactive protein level (0.73; 95%CI, 0.56‒0.95; P = 0.02).
ConclusionsPatients with cirrhosis complicated with acute cholangitis had poor prognosis. Recovery of liver function after endoscopic treatment was slow; nevertheless, most patients who survived could recover within 90 days.
Cirrhosis is the late stage of liver fibrosis caused by many forms of liver diseases and conditions, resulting in decreased liver function and portal hypertension; in fact, it is one of the leading causes of death, with over 1 million deaths in 2010 [1]. From 1990 to 2013, cirrhosis-related mortality increased by 46% worldwide [1]. The most common cause of death in cirrhosis is developing a syndrome called acute-on-chronic liver failure (ACLF), which is characterized by acute decompensation of chronic liver disease associated with multiple organ failure and high short-term mortality [2–4]. Although approximately 50% of ACLF cases have no identifiable trigger, bacterial infection is the most commonly reported precipitating factor [2–5]. Bacterial infection-triggered ACLF has a higher mortality rate than non-infection-triggered ACLF [5].
Meanwhile, acute cholangitis is a clinical syndrome caused by stasis and biliary tract infection, resulting in acute liver injury [6]. With the increased biliary tract pressure in acute cholangitis, bacteria and endotoxins may migrate from the bile to the blood and lymphatic stream, causing severe and fatal infections, such as sepsis [6].
Cirrhosis complicated with acute cholangitis may likely have a high mortality rate because of bacterial infection and permanent liver dysfunction caused by acute liver injury and bile stasis. In a recent study, 11% of patients with cirrhosis developed ACLF after endoscopic retrograde cholangiopancreatography (ERCP) [7], but this study had a few number of patients with cholangitis before ERCP. Therefore, the impact of acute cholangitis on mortality and liver function in cirrhosis remain inadequately understood. Furthermore, the Tokyo Guidelines 2018 (TG18) [8–10], which are guidelines for acute cholangitis treatment, do not specifically address cirrhosis cases. If cirrhosis complicated with acute cholangitis has a high mortality rate or progresses to liver failure, healthcare professionals should be attentive to superior care in clinical practice. However, data regarding this issue are insufficient.
Hence, this study aimed to compare the changes in liver function and prognosis after undergoing endoscopic treatment for acute cholangitis between cirrhotic and noncirrhotic groups. To strictly evaluate liver function, we limited the study to acute cholangitis caused by choledocholithiasis, which involves inflammation of the whole liver, not regional cholangitis.
2Material and methods2.1Patient selectionThe research ethics committee approved this multicenter, retrospective cohort study, which was conducted in two tertiary care centers (Approval number: 3640 at Chiba University, 118 at Eastern Chiba Medical Center), and waived informed consent from the enrolled patients. Chiba University Hospital and Eastern Chiba Medical center are high-volume centers with more than 600 and 300 annual ERCPs, respectively. We reviewed the medical and procedural records of consecutive patients who underwent ERCP in the two centers between January 2004 and December 2019. These records were acquired from each center's database. These patients should be over 20 years of age and have undergone initial ERCP to treat acute cholangitis caused by choledocholithiasis within the abovementioned period. Patients were excluded if they had a negative or suspected diagnosis of acute cholangitis according to TG18 [8–10] and were subjected to percutaneous drainage before ERCP. All patients received antibiotics before ERCP and sedated with midazolam plus pentazocine and hydroxyzine.
2.2Clinical parameters and outcomesUsing the medical records, we retrieved the entire clinical course of the study population, collecting the following clinical parameters: baseline demographic characteristics, etiology of cirrhosis, symptoms and laboratory data at acute cholangitis diagnosis, ERCP procedural details, procedure-related adverse events, ACLF development, and mortality. Laboratory data and albumin–bilirubin grade (ALBI-grade [11]) before the cholangitis onset were also collected, if available. In addition, total bilirubin (T-BIL) and albumin (ALB) level trends within 3 months after ERCP were collected only in patients with laboratory data before the cholangitis onset.
We examined and compared the post-ERCP adverse events, 30-day mortality, ACLF development, T-BIL and ALB level cumulative recovery rate, and functional success rates between patients with and without cirrhosis. Specifically, the cumulative recovery rate of T-BIL and ALB levels and functional success rates were analyzed in patients who had data on T-BIL and ALB levels within 6 months before the cholangitis onset, post-ERCP data, and successful ERCP.
2.3Definitions2.3.1Cirrhosis, acute cholangitis, post-ERCP adverse events, and ACLFThe diagnosis of cirrhosis was based on clinical history, laboratory testing, liver biopsy (if available), and imaging studies [12]. In this study, acute cholangitis was defined as a definite diagnosis case according to TG18 [8–10]. The severity of acute cholangitis was also defined according to TG18 [8–10]. Post-ERCP adverse events were defined according to Cotton's criteria [13, 14], while ACLF was defined according to the European Association for the Study of the Liver–Chronic Liver Failure (EASL-CLIF) definition [3, 4, 15].
2.3.2Cumulative recovery rate of T-BIL and ALB levelsWe defined T-BIL recovery as a T-BIL level < 2.0 mg/dL or less than or equal to the level before the cholangitis onset, and ALB recovery as an ALB level > 3.5 g/dL or greater than or equal to the level before the cholangitis onset.
2.3.3Functional successAccording to Tokyo criteria 2014, functional success was defined as a 50% decrease in or normalization of the bilirubin level within 14 days of stent placement [16]. In this study, normalization of the bilirubin level was defined as a T-BIL level < 2 mg/dL or less than or equal to the pre-cholangitis level, as well as T-BIL recovery.
2.4Statistical analysisContinuous variables are expressed as median and interquartile range, whereas categorical variables are expressed as numbers and percentages. For the statistical analysis, Mann–Whitney U test was employed for the continuous data, and Fisher's exact test for the categorical data. The factors for 30-day mortality were assessed by logistic regression analysis. The T-BIL and ALB recovery rates were estimated using Kaplan–Meier survival methods and compared using log‐rank tests. The hazard ratio (HR) and 95% confidence interval (CI) were estimated by Cox proportional regression analyses. All statistical data were analyzed using SPSS statistical software (version 25; SPSS-IBM, Chicago, IL, USA). Furthermore, P < 0.05 was considered statistically significant.
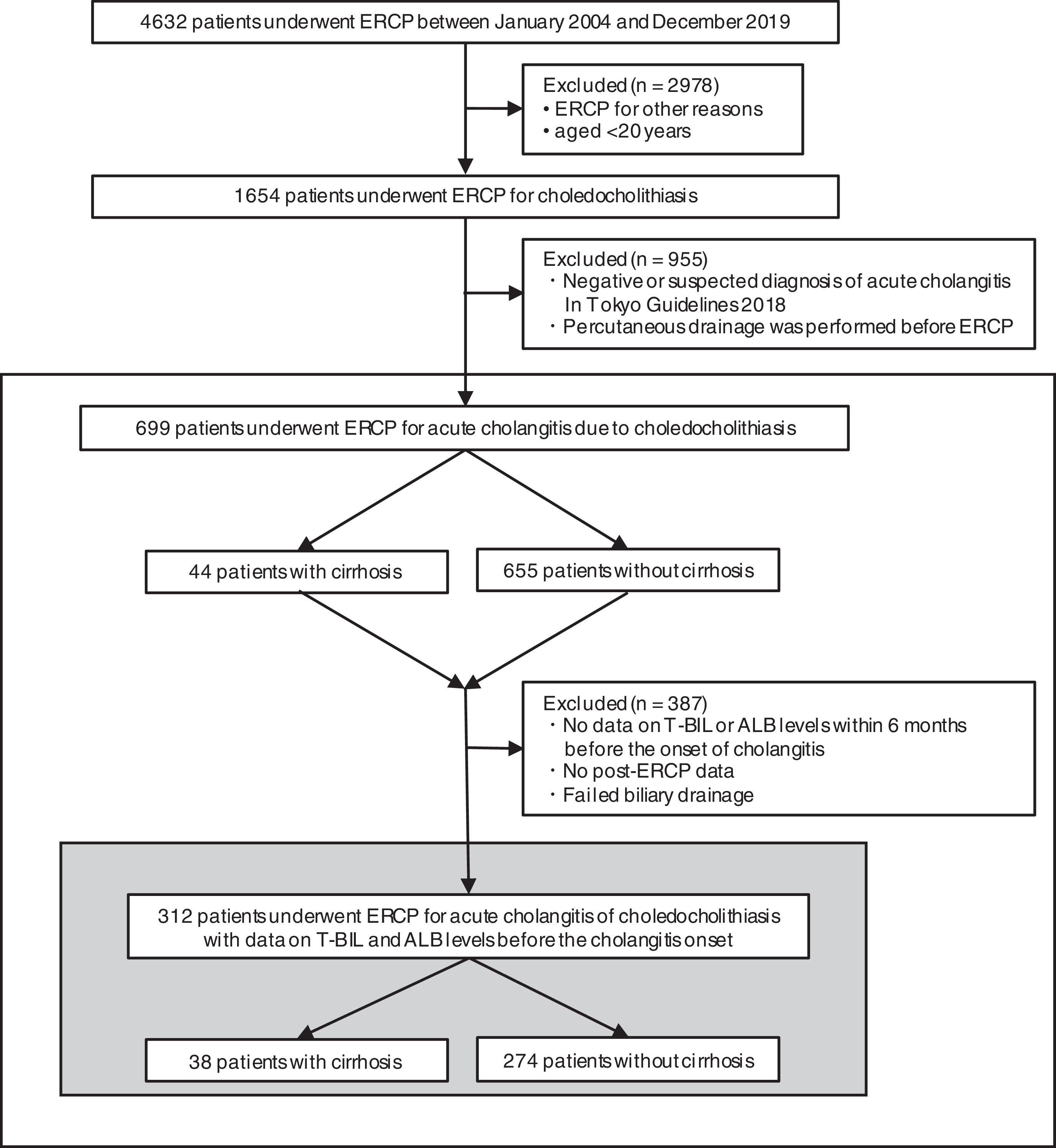
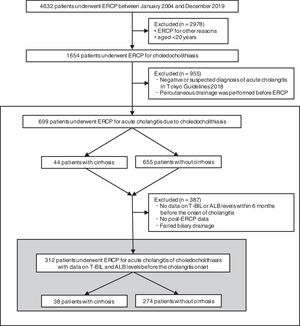
3Results3.1Study populationFig. 1 presents the flowchart of patient selection. We identified 4632 patients who underwent ERCP between January 2004 and December 2019. Among them, 1654 patients, who were over 20 years of age, had acute cholangitis caused by choledocholithiasis. However, we excluded 955 patients because of the negative or suspected diagnosis of acute cholangitis according to TG18 or percutaneous drainage before ERCP. Overall, we included 699 patients for the analysis, with 44 and 655 patients categorized as the cirrhotic and noncirrhotic groups, respectively.
Flowchart of patient selection. This study included 699 people who underwent ERCP for acute cholangitis caused by choledocholithiasis. The cumulative recovery rate of T-BIL and ALB levels and functional success rates were analyzed in 312 patients with data on T-BIL and ALB levels before the cholangitis onset. ERCP, endoscopic retrograde cholangiopancreatography; T-BIL, Total bilirubin; ALB, Albumin.
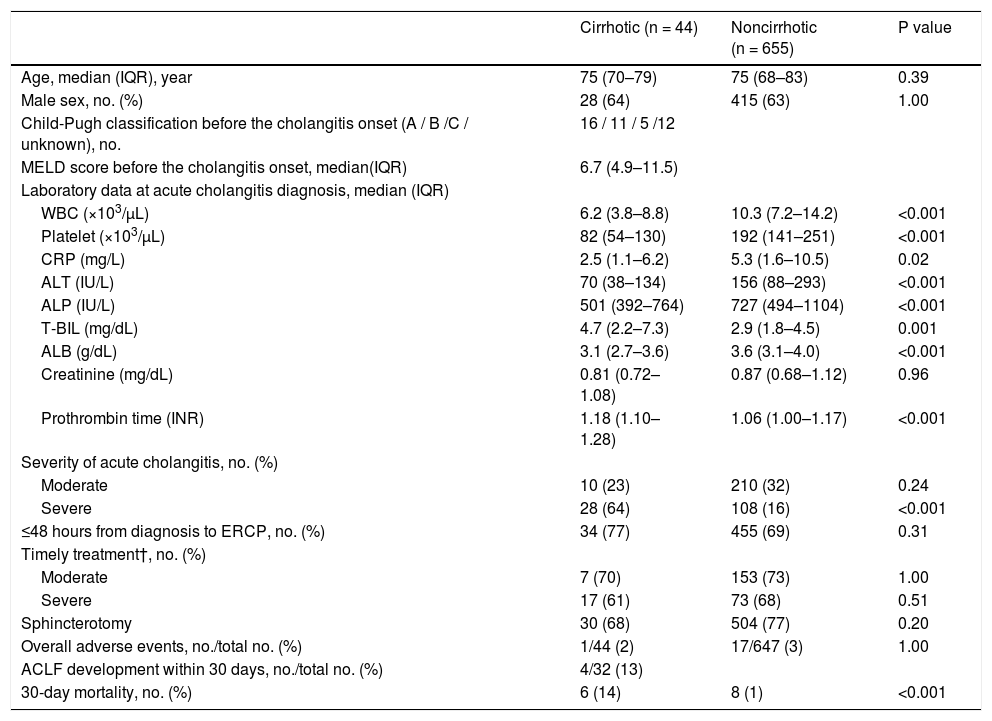
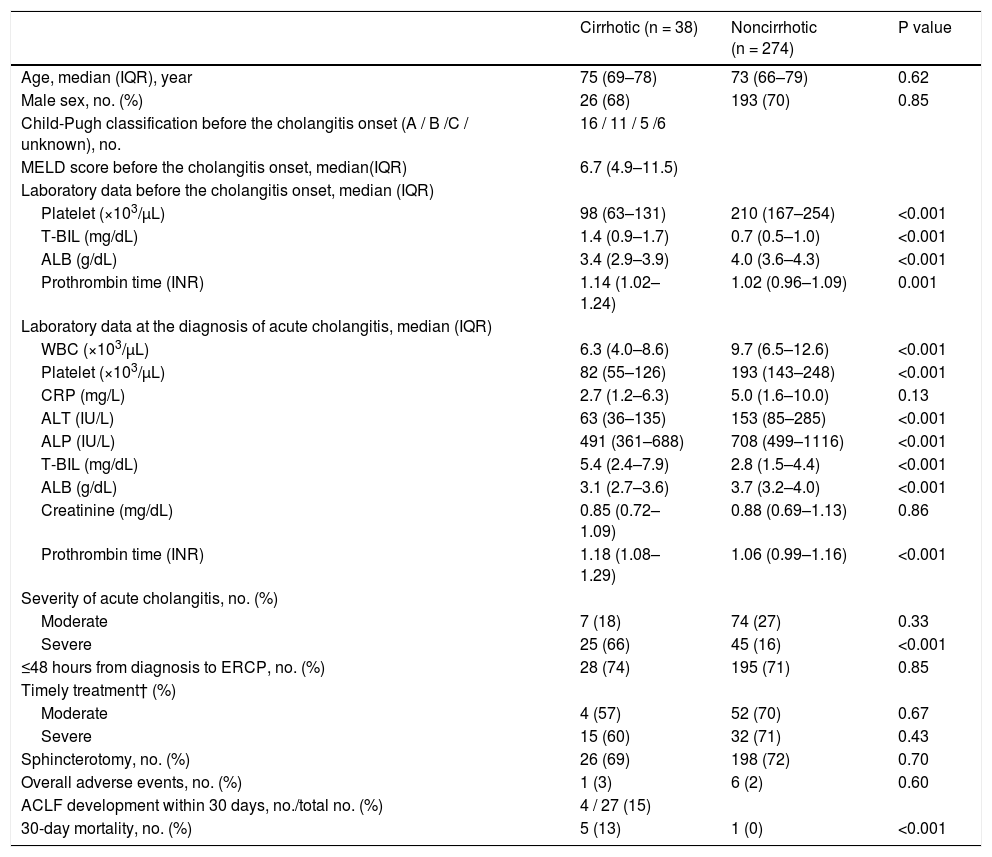
The median age was 75 years, and 443 of the study population (63%) were male (Table 1). The etiologies of cirrhosis were viral hepatitis (n = 22, 50%), alcohol abuse (n = 9, 21%), primary sclerosing cholangitis (n = 1, 2%), and others (n = 12, 27%). Median model for end-stage liver disease score was 6.7; 16 cases were Child–Pugh class A (36%), 11 were class B (25%), 5 were class C (11%), and 12 were unknown (27%).
Characteristics and outcome of 699 patients who underwent ERCP for acute cholangitis caused by choledocholithiasis.
IQR, interquartile range; MELD, model for end-stage liver disease; WBC, White blood cell; CRP, C-reactive protein; ALT, alanine aminotransferase; ALP, alkaline phosphatase; T-BIL, Total bilirubin; ALB, Albumin; INR, International normalized ratio; ERCP, endoscopic retrograde cholangiopancreatography; ACLF, acute-on-chronic liver failure.
† Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
During cholangitis diagnosis, the following symptoms were observed: abdominal pain in 496 (75%) of 658 patients (28 [65%] of 43 in the cirrhotic group, 468 [76%] of 615 in the noncirrhotic group); fever in 319 (48%) of 665 patients (22 [50%] of 44 in the cirrhotic group, 297 [48%] of 621 in the noncirrhotic group); neurological dysfunction in 32 (5%) of 676 patients (4 [9%] of 44 in the cirrhotic group, 28 [4%] of 632 in the noncirrhotic group); respiratory dysfunction in 51 (7%) of 683 patients (3 [7%] of 44 in the cirrhotic group, 48 [8%] of 639 in the noncirrhotic group); and cardiovascular dysfunction in 13 (2%) of 692 patients (2 [5%] of 44 in the cirrhotic group, 11 [2%] of 648 in the noncirrhotic group). No significant differences were found in patients with such symptoms of cholangitis.
Table 1 summarizes the laboratory results at the diagnosis of acute cholangitis. White blood cell, platelet, C-reactive protein (CRP), alanine aminotransferase, alkaline phosphatase, and ALB levels were significantly higher in the noncirrhotic group, but T-BIL and prothrombin time were significantly higher in the cirrhotic group. Creatinine results were no significantly different between the two groups.
On the basis of the TG18, cholangitis was mild in 323 patients (46%), moderate in 220 patients (31%), severe in 136 patients (19%), and unknown in 20 patients (3%). Severe condition was significantly more common in the cirrhotic group than in the noncirrhotic group (64% vs. 16%, P < 0.001) (Table 1).
Regarding the time from diagnosis of cholangitis to ERCP, 489 patients (70%) underwent ERCP within 48 hours of diagnosis. According to the TG18 [8–10], ERCP should be performed within 48 hours for moderate disease and 24 hours for severe disease. These recommendations were applied in 160 (73%) of 220 patients with moderate disease and 90 (66%) of 136 patients with severe disease; no significant difference was found between the cirrhotic and noncirrhotic groups in either case (Table 1).
Moreover, 614 (88%) patients had native papilla, and endoscopic biliary treatments were successful in 662 patients (95%). The rate of native papilla and the success of endoscopic treatments were not significantly different between the two groups. Meanwhile, endoscopic sphincterotomy was performed in 534 patients (77%). No significant differences were observed between the two groups (Table 1). There was no difference in the success rate of endoscopic treatments between the two centers (334 [93%] of 359 in Chiba University Hospital, 328 [96%] of 340 Eastern Chiba Medical Center).
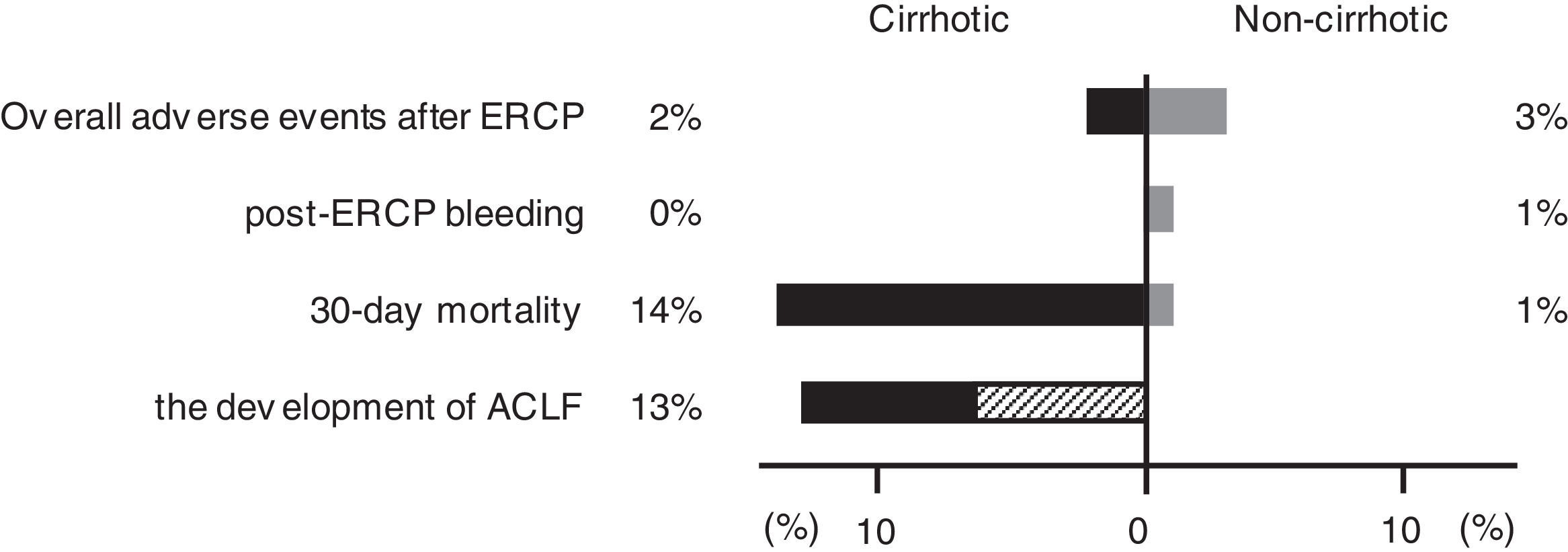
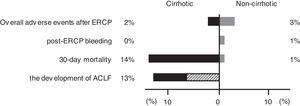
3.3Post-ERCP adverse eventsAdverse events occurred in 18 (3%) of 691 patients (1 [2%] of 44 in the cirrhotic group, 17 [3%] of 647 in the noncirrhotic group) (Fig. 2). These post-ERCP adverse events were as follows: pancreatitis in 9 (1%) patients (1 [2%] in the cirrhotic group, 8 [1%] in the noncirrhotic group); bleeding in 7 (1%) patients (0 [0%] in the cirrhotic group, 7 [1%] in the noncirrhotic group) (Fig. 2); perforation in 1 (0.1%) patient (0 [0%] in the cirrhotic group, 1 [0.2%] in the noncirrhotic group); aspiration pneumonia in 1 (0.1%) patient (0 [0%] in the cirrhotic group, 1 [0.2%] in the noncirrhotic group). No significant differences were found between the two groups (Table 1). And no significant differences were found between the two centers (12 [3%] of 351 in Chiba University Hospital, 6 [2%] of 340 Eastern Chiba Medical Center).
Difference in prognosis between the cirrhotic and noncirrhotic groups. The figure shows the proportion of adverse events, 30-day mortality, and the development of ACLF in the cirrhotic and noncirrhotic groups. The shaded area in the development of ACLF shows the proportion of death cases. ERCP, endoscopic retrograde cholangiopancreatography; ACLF, acute-on-chronic liver failure.
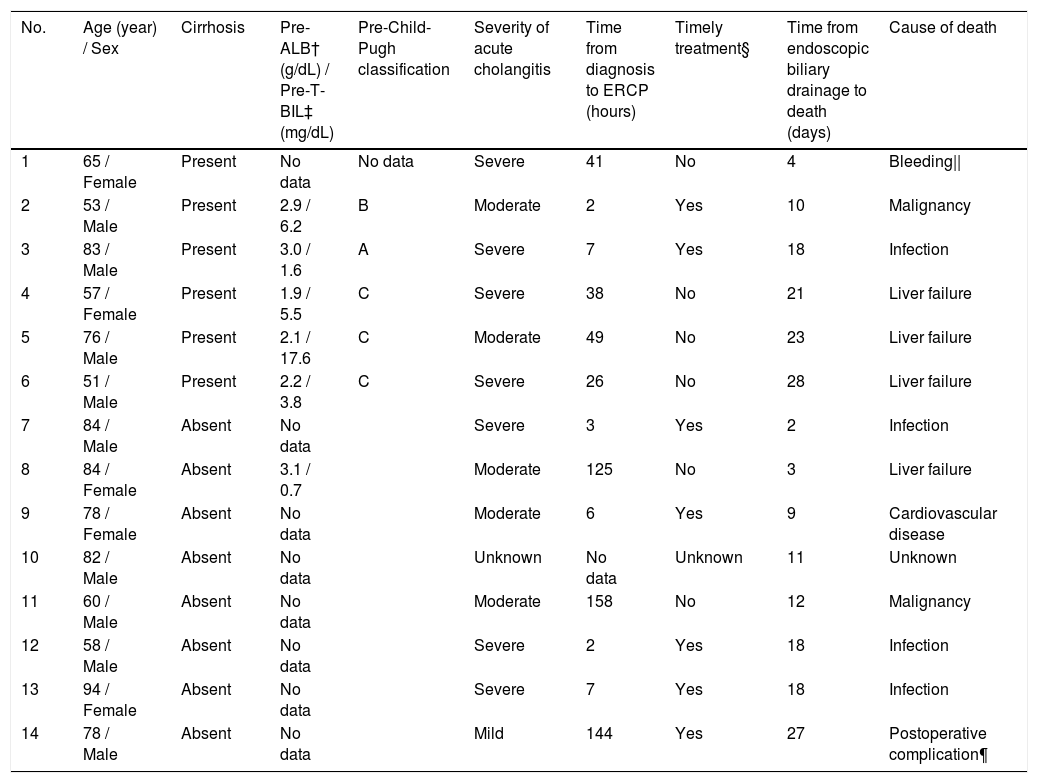
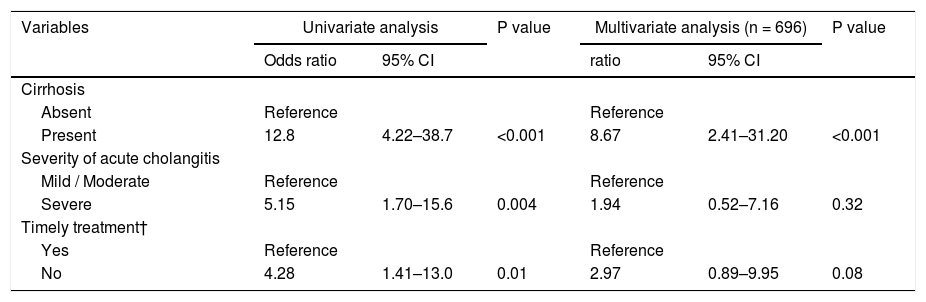
A total of 14 patients (2%) died within 30 days, 6 (14%) of whom were from the cirrhotic group and 8 (1%) from the noncirrhotic group (Fig. 2). The 30-day mortality rate was significantly higher in the cirrhotic group than in the noncirrhotic group (P < 0.001) (Table 1). No significant differences were found between the two centers (8 [2%] of 359 in Chiba University Hospital, 6 [2%] of 340 in Eastern Chiba Medical Center) Table 2. summarizes the details of the 14 deceased patients within 30 days. The severity of cholangitis at diagnosis was mostly moderate and above, and roughly half of the patients did not comply with the TG18 for ERCP timing. Four patients died because of liver failure, and another four patients died because of infection. Half of the cirrhotic group died of liver failure. Nonetheless, no one died because of post-ERCP adverse events. In multivariate logistic regression analysis, cirrhosis was an independent risk factor for 30-day mortality (OR, 8.67; 95% CI, 2.41‒31.20; P < 0.001) (Table 3).
Details of patients who died within 30 days after endoscopic biliary drainage.
† Pre-ALB means albumin level before the onset of cholangitis.
‡ Pre-T-BIL means total bilirubin level before the onset of cholangitis.
§ Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
|| Upper gastrointestinal bleeding
¶ Death from post-operative intra-abdominal hemorrhage of intrahepatic bile duct cancer
Logistic regression analysis on 30-day mortality.
ERCP, endoscopic retrograde cholangiopancreatography; CI, confidence interval
† Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
According to EASL-CLIF definition, we included 32 patients but excluded 12 patients (7 with hepatocellular carcinoma outside the Milan criteria, 2 with cholangitis immediately after radiofrequency ablation or transcatheter arterial chemoembolization treatment, 2 with renal failure, and 1 who already had multiple organ failure before the cholangitis onset). Of the 32 included patients, 4 (13%) developed ACLF within 30 days (Fig. 2), and 2 (50%) of them soon died. Of the 28 patients who did not develop ACLF, 1 (4%) died. Overall, 3 (9%) of the 32 patients died within 30 days.
3.5Cumulative recovery rate of T-BIL and ALB levelsOf the total of 699 patients, 312 had data on T-BIL and ALB levels within 6 months before the cholangitis onset, post-ERCP data, and successful ERCP (Fig. 1) Table 4. describes the cohort, consisting of 38 and 274 patients with and without cirrhosis, respectively. Furthermore, Table 4 presents the laboratory results before the cholangitis onset. Regarding ALBI grades before the cholangitis onset, 9 (24%) had grade 1, 21 (55%) had grade 2, and 8 (9%) had grade 3. The results of the patient characteristics, ERCP procedure, and outcomes were similar to the analysis results of 699 patients (Table 4).
Characteristics and outcome of 312 patients who underwent ERCP for acute cholangitis caused by choledocholithiasis, with data on T-BIL and ALB levels before the cholangitis onset.
IQR, interquartile range; MELD, model for end-stage liver disease; T-BIL, Total bilirubin; ALB, Albumin; WBC, White blood cell; INR, International normalized ratio; CRP, C-reactive protein; ALT, alanine aminotransferase; ALP, alkaline phosphatase; ERCP, endoscopic retrograde cholangiopancreatography; ACLF, acute-on-chronic liver failure. † Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
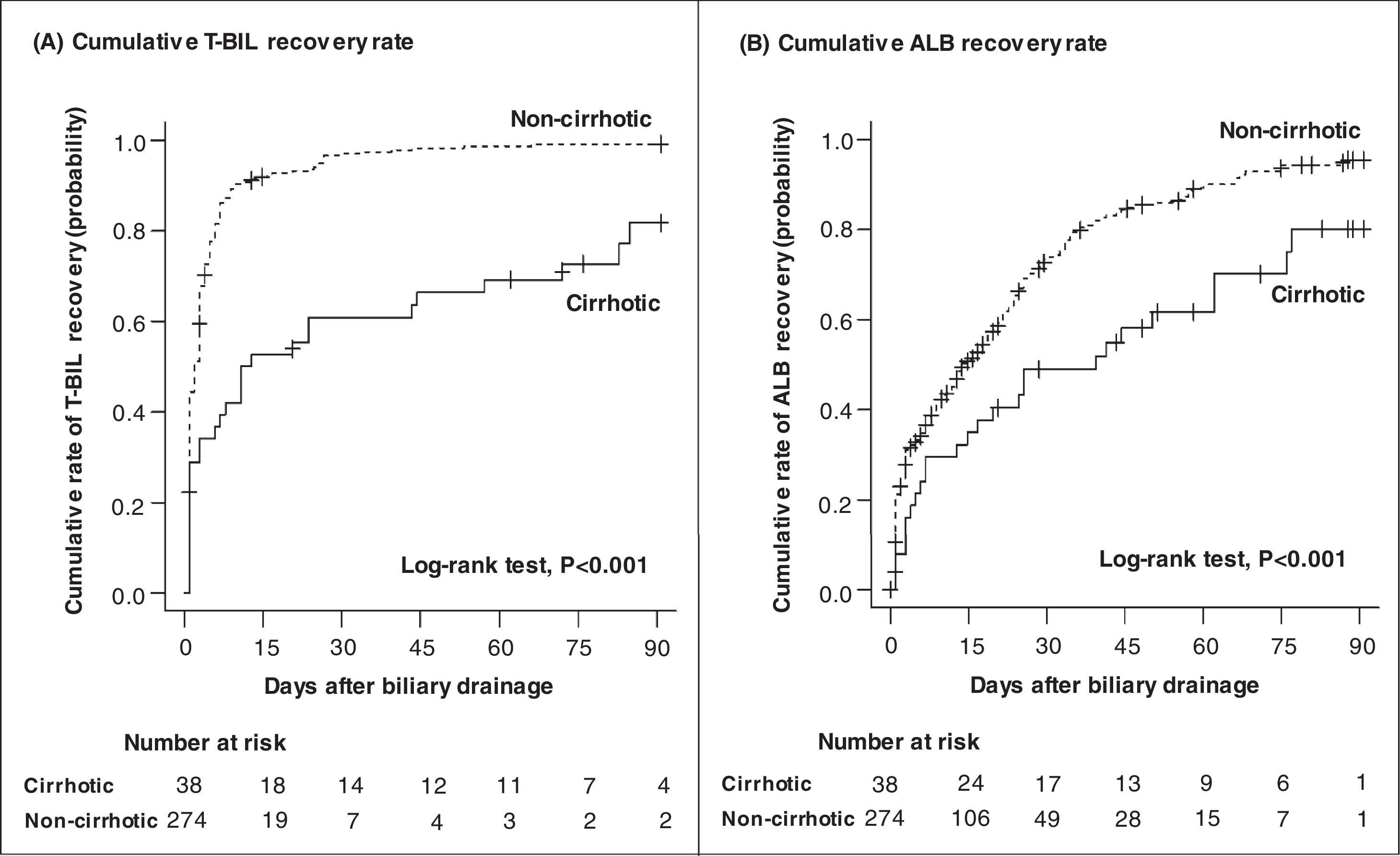
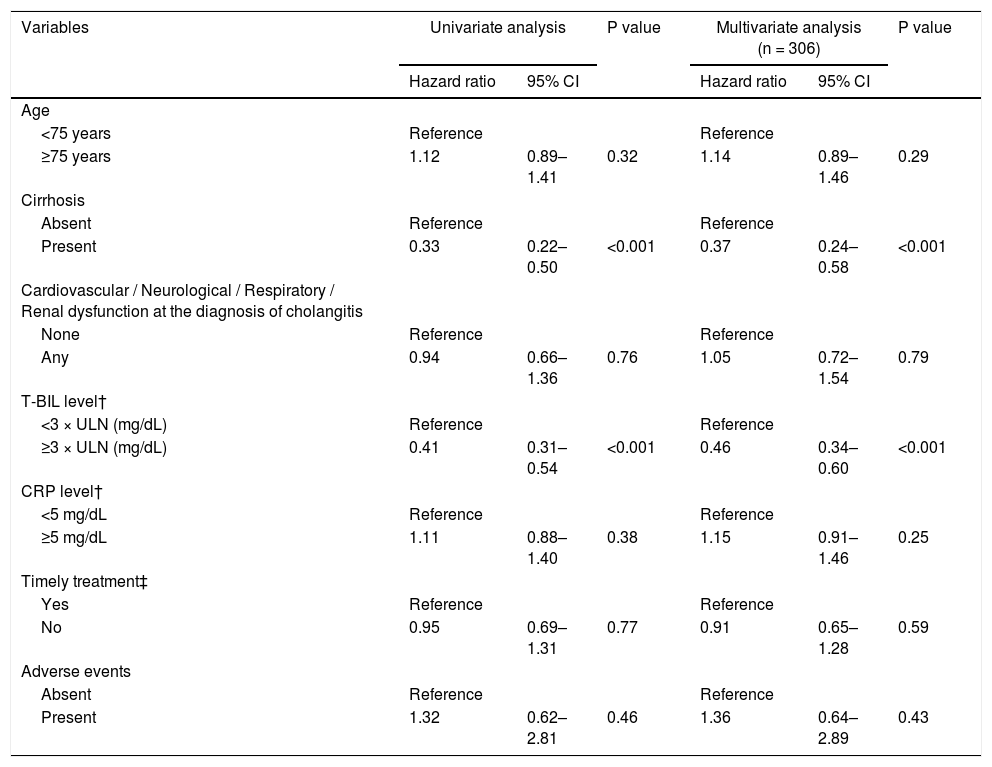
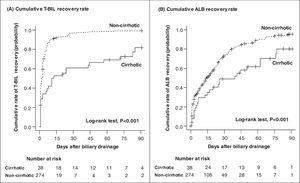
Fig. 3 shows the cumulative recovery rate of T-BIL and ALB levels. We could collect data for the day after ERCP, but subsequent data were sparse depending on the case, such as days or weeks later. The cirrhotic group had a significantly lower T-BIL recovery rate than the noncirrhotic group (HR = 0.33, 95% CI = 0.22‒0.50, P < 0.001) (Table 5). In the noncirrhotic group, half of the patients achieved T-BIL recovery within only 2 days compared with 12 days in the cirrhotic group. However, even in the cirrhotic group, approximately 80% of the patients achieved T-BIL recovery within 90 days. Multivariable Cox regression analysis identified that cirrhosis (HR, 0.37; 95% CI, 0.24‒0.58; P < 0.001) and high T-BIL level at the diagnosis of cholangitis (> 3 × upper limit of normal) (HR, 0.46; 95% CI, 0.34‒0.60; P < 0.001) are independent risk factors for T-BIL recovery (Table 5).
Cumulative recovery rates of T-BIL (A) and ALB (B) levels. The cumulative recovery rate of the T-BIL level was significantly lower in the cirrhotic group than in the noncirrhotic group (A). In the noncirrhotic group, half of the patients achieved T-BIL recovery within only 2 days compared with 12 days in the cirrhotic group. The cumulative recovery rate of the ALB level was also significantly lower in the cirrhotic group (B). In the noncirrhotic group, half of the patients achieved ALB recovery within 14 days compared with 40 days in the cirrhotic group. Censored data represent patients whose follow-up was lost before recovery. T-BIL, Total bilirubin; ALB, Albumin.
Multivariable Cox regression analysis on the independent predictors of T-BIL recovery.
T-BIL, total bilirubin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CRP, C-reactive protein; CI, confidence interval; ERCP, endoscopic retrograde cholangiopancreatography.
†Each level was at the diagnosis of acute cholangitis.
‡ Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
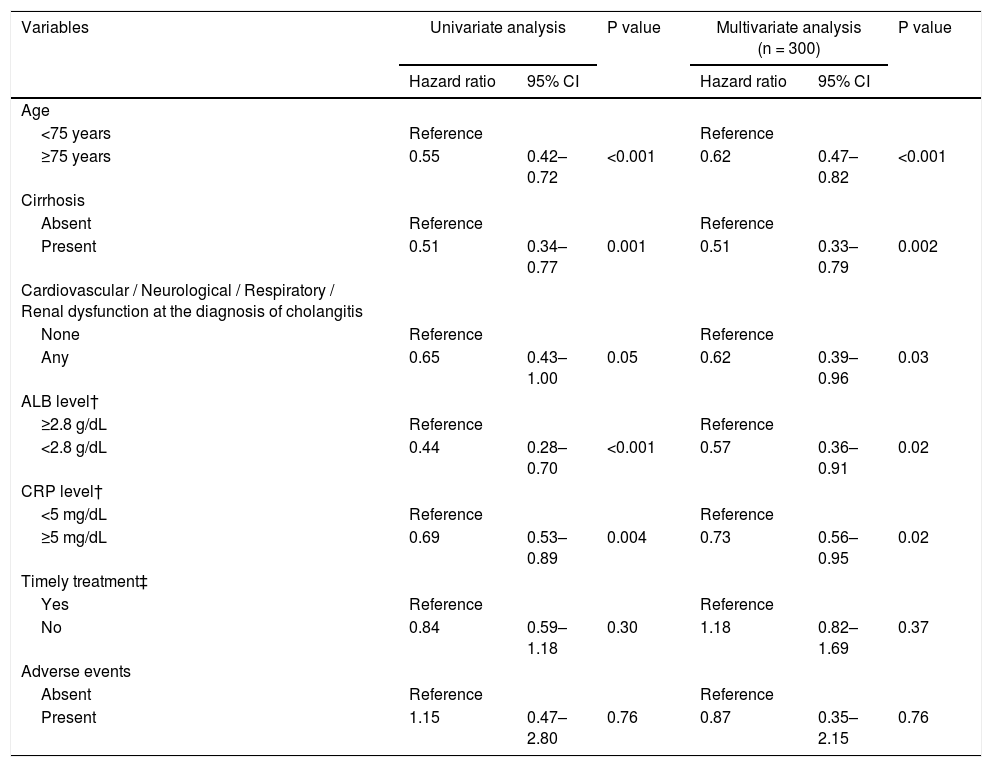
Likewise, ALB recovery rate was significantly lower in the cirrhotic group than in the noncirrhotic group (HR = 0.51, 95% CI = 0.34‒0.77, P = 0.001; Table 6). Half of those in the noncirrhotic group achieved ALB recovery within 14 days compared with 40 days in the cirrhotic group. However, as with T-BIL recovery, approximately 80% of the patients achieved ALB recovery within 90 days. In multivariable Cox regression analysis, the independent risk factors for ALB recovery were age ≥ 75 years (HR, 0.62; 95% CI, 0.47‒0.82; P < 0.001), cirrhosis (HR, 0.51; 95% CI, 0.33‒0.79; P = 0.002), cardiovascular / neurological / respiratory / renal dysfunction at the diagnosis of cholangitis (HR, 0.62; 95% CI, 0.39‒0.96; P = 0.03), low ALB level at cholangitis diagnosis (<2.8 g/dL) (HR, 0.57; 95% CI, 0.36‒0.91; P = 0.02), and high CRP level at cholangitis diagnosis (≥5 mg/dL) (HR, 0.73; 95% CI, 0.56‒0.95; P = 0.02) (Table 6).
Multivariable Cox regression analysis on the independent predictors of ALB recovery.
ALB, albumin; T-BIL, total bilirubin; CRP, C-reactive protein; CI: confidence interval; ERCP, endoscopic retrograde cholangiopancreatography.
†Each level was at the diagnosis of acute cholangitis
‡ Timely treatment is the treatment performed within 48 hours in moderate patients and within 24 hours in severe patients.
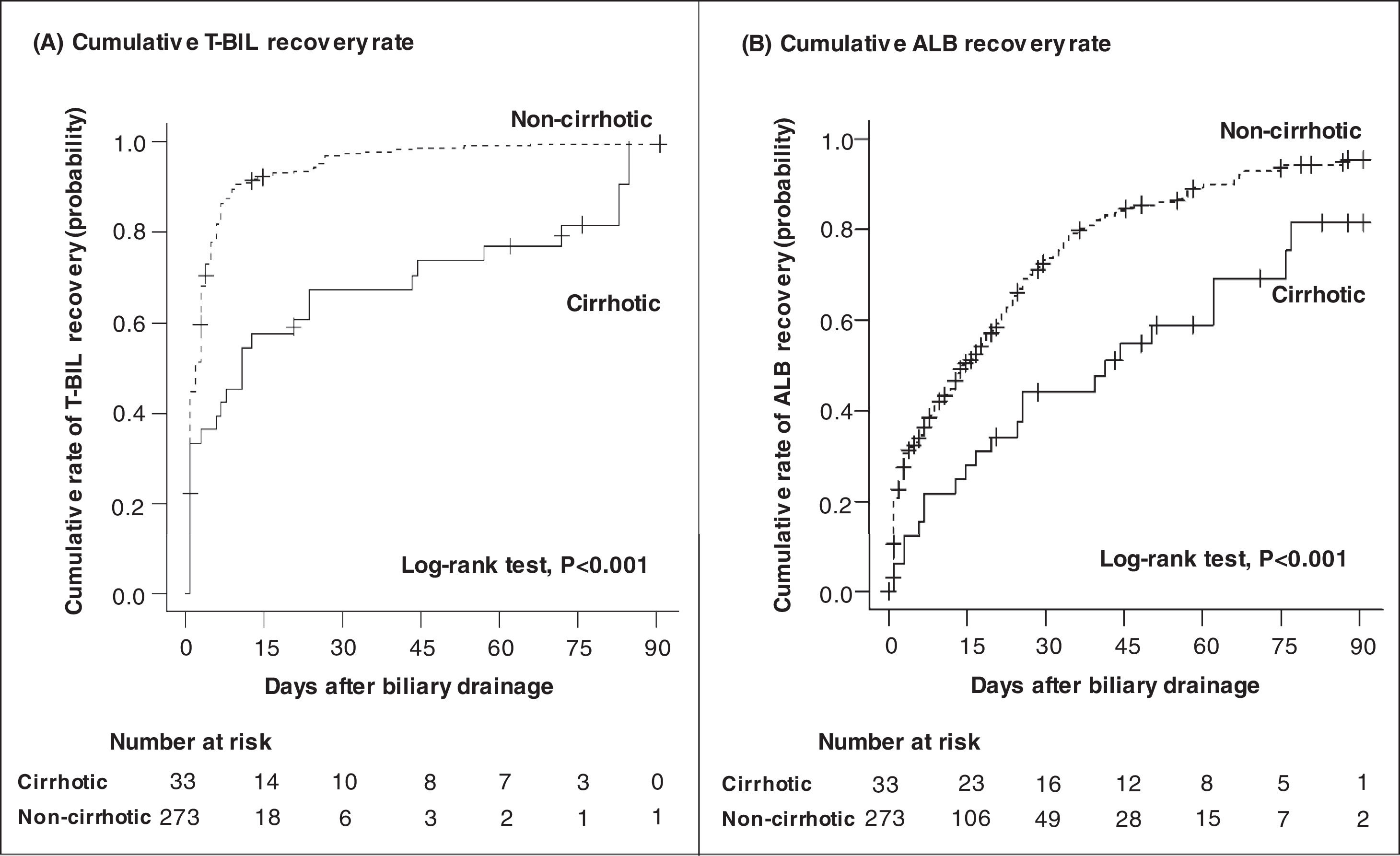
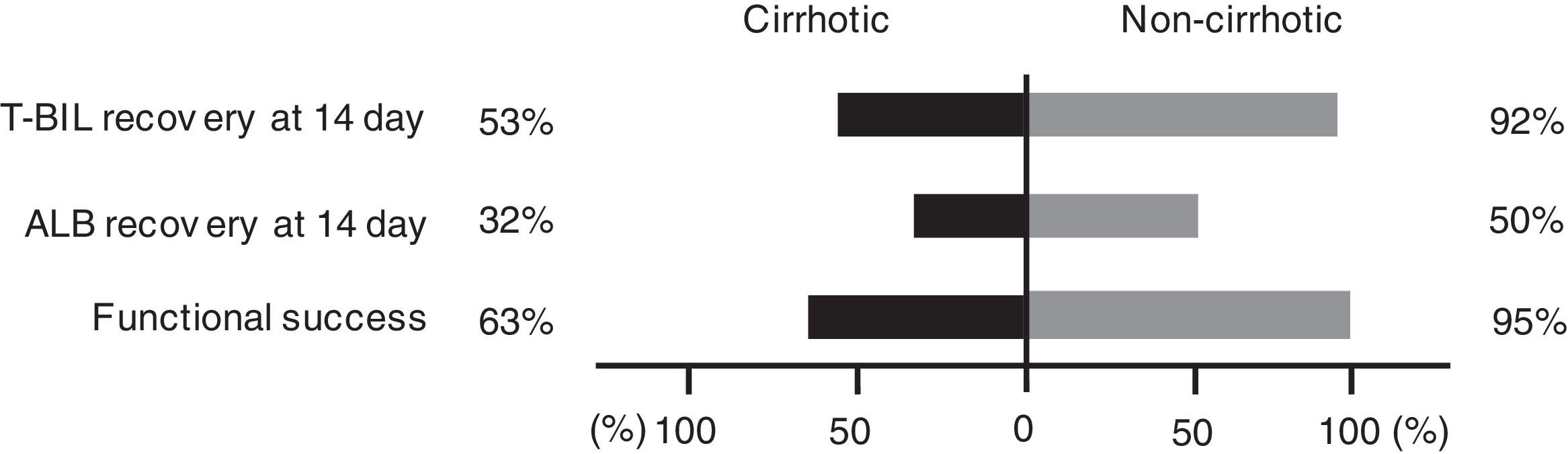
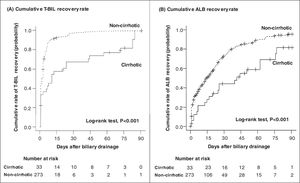
Fig. 4 depicts the rate of T-BIL and ALB recoveries among patients who survived. Both T-BIL and ALB recovery rates were significantly prolonged in the cirrhotic group. Nevertheless, all patients achieved T-BIL recovery, and 91% of the patients achieved ALB recovery in 90 days.
Cumulative recovery rates of T-BIL (A) and ALB (B) levels limited to patients who survived. Both T-BIL and ALB cumulative recovery rates were significantly prolonged in the cirrhotic group (A) (B). T-BIL recovery was achieved in all patients, and ALB recovery was achieved in 91% of patients in 90 days. T-BIL, Total bilirubin; ALB, Albumin.
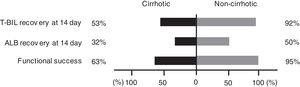
Functional success rates defined by Tokyo criteria were significantly lower in the cirrhotic group than in the noncirrhotic group (63% vs. 95%, P < 0.001) (Fig. 5).
4DISCUSSIONThis retrospective study shows two critical findings. First, patients with cirrhosis complicated with acute cholangitis could develop ACLF and had a higher 30-day mortality than those without cirrhosis; some patients died of liver failure even after the infection had cured. Second, liver function recovery after endoscopic treatment was slower in patients with cirrhosis than in those without cirrhosis; however, most cirrhosis survivors recovered within 90 days. Based on these findings, cirrhosis complicated with acute cholangitis should be considered as a severe disease with high mortality, and we should monitor decompensated symptoms for several weeks until liver function recovery. We should also formulate strategies to avoid developing cholangitis as much as possible in patients with cirrhosis. These patients should undergo endoscopic lithotomy for asymptomatic choledocholithiasis to avoid developing cholangitis, and prophylactic cholecystectomy should also be considered, taking into account the mortality rate of cholangitis versus that of surgery if gallstones exist.
Patients with cirrhosis complicated with acute cholangitis have a higher incidence of ACLF and 30-day mortality than those without cirrhosis. Acute cholangitis is often life-saving after successful biliary drainage, with a mortality rate of only 2.7% [17, 18]. In the present study, the cirrhotic group had a higher mortality rate (14%) than the noncirrhotic group within 30 days of successful drainage, and half of the patients with cirrhosis died of liver failure. Notably, the 30-day mortality rate was still as high as 10% in patients with cirrhosis without imminent fatal disease, such as hepatocellular carcinoma outside the Milan criteria, according to the ACLF criteria of EASL-CLIF definition. Therefore, acute cholangitis strongly impacts the mortality of patients with cirrhosis. High mortality rate in cirrhosis may be owing to the immunocompromised state in which bacterial infections can be fatal, or liver damage caused by bile stasis, which leads to progressive liver failure even if the infection is controlled.
The liver function recovery after endoscopic treatment in patients with cirrhosis was slower than in patients without cirrhosis; however, most of those who survived could recover within 90 days. One reason for the slower T-BIL recovery in the cirrhotic group was the higher T-BIL level than that in the noncirrhotic group before the treatment. However, the lower rate of functional success, which represents the rapidity of jaundice improvement, in the cirrhosis group suggests another mechanism of prolonged T-BIL recovery independent of the T-BIL level before the treatment. Acute cholangitis causes jaundice, which results from extrahepatic obstruction and intrahepatic cholestasis. Intrahepatic cholestasis is caused by hepatocellular disorders, leading to bilirubin excretion into the bile ducts [19–21]. In both cirrhotic and noncirrhotic groups, extrahepatic bile duct obstruction can be relieved by successful endoscopic treatment. Thus, the difference in intrahepatic cholestasis may lead to prolonged jaundice in the cirrhotic group, in which bilirubin-clearance capacity is reduced. Meanwhile, ALB recovered more slowly than T-BIL in both groups. Recovery of decreased ALB consumed by acute cholangitis depends on the capacity of the liver to produce ALB. A lower ALB level before the treatment and reduced albumin production capacity caused by hepatocellular damage may explain the slower recovery in the cirrhotic group.
Our study's strength is that we designed this study to evaluate liver function changes in patients with cirrhosis after endoscopic drainage by selecting consecutive patients with acute cholangitis caused by choledocholithiasis.
However, our study also has some limitations. First, this study is a retrospective analysis of two tertiary centers, with a small sample size. The prothrombin time or ascites before the cholangitis onset in our patients with cirrhosis was too small to analyze the prognosis by Child–Pugh classification. On the other hand, it was not investigated whether the liver function of non-cirrhotic patients was normal, which may have compromised the results of the trial because there was no significant difference in the distribution of liver function between the two groups. Second, in some cases, blood and bile cultures were not collected, and antimicrobial administration was not standardized, so we cannot confirm whether effective antimicrobials were administered. Thirdly, the timing of the post-ERCP T-BIL and ALB values varied from case to case, which may have influenced the results. Finally, some patients with cirrhosis who developed cholangitis did not undergo endoscopic treatment because they were considered too risky to do so.
5ConclusionsThe prognosis of patients with cirrhosis complicated with acute cholangitis was poor. Furthermore, the recovery of liver function after endoscopic treatment was slow, but most patients who survived could recover within 90 days.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
These authors would like to thank Enago (www. enago.jp) for the English language review.