Introduction and aim. Interferon-free regimen has been reported to be highly efficient in treatment of HCV infection, including patients with compensated cirrhosis. We compared the efficacy of Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir and Ribavirin (OBT/PTV/r, with DSV and RBV) therapy in patients with chronic HCV genotype 1b infection and compensated cirrhosis with and without prior treatment experience with pegylated interferon and ribavirin (IFN/RBV).
Material and methods. A prospective two-center study was conducted in Mures County Hospital and Brasov County Hospital, Romania in period November 2015-July 2016. Both treatment naïve and PegIFN/RBV experienced patients with chronic HCV genotype 1b infection received 12 weeks of OBT/PTV/r, with DSV and RBV. Sustained virologic response 12 weeks after the treatment and eventual discontinuation of therapy due to adverse events were assessed in order to estimate safety and efficiency of therapeutic regimen.
Results. Fifty nine patients were included in study, 35 (59.3%) of them were previously treated with IFN/RBV. Forty four (74.5%) patients were previously diag-nosed with cirrhosis Child Pugh score 5, while 15 (25.4%) with Child Pugh score 6. All 59 patients achieved a SVR12 of 100% and one patient from treatment naïve cohort discontinued the therapy due to hyperbilirubinemia and encephalopathy. However viral load assessed at 12 weeks after discontinuation of therapy in this patient was undetectable.
Conclusion An all-oral regimen of co-for-mulated OBT/PTV/r with DSV and RBV results in high rate of sustained virologic response at post-treatment week 12 among HCV GT1b infected patients associated with compensated cirrhosis, regardless of previous treatment experience with PegIFN/RBV.
The prevalence of hepatitis C virus (HCV) infection is increasing worldwide with recent studies showing 3.1% of population to be infected in Eastern Europe.1 The primary goal of treatment in patients with HCV infection is to achieve sustained virologic response (SVR), which has been associated with improvement in liver histology and health related quality of life, as well as reduced risk of hepatocellular carcinoma (HCC) and liver related mortality.2-8 As HCV patients associated with liver cirrhosis are at greatest risk for HCC and liver related mortality, American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommend these patients to be prioritized for the treatment.8-10
For a long time the treatment of choice for patients with chronic HCV infection was the combination of pegylated interferon and ribavirin (PegIFN/RBV). However the efficacy and tolerability of this therapeutic regime was not ideal. Low response rates (33-77%) and the occurrence of significant toxicities leading to drug discontinuation, especially in patients with cirrhosis, required major improvements in HCV therapy and development of new antiviral drugs.11-13 Therapeutic regimes with addition of protease inhibitors such as telaprevir, boceprevir or simeprevir have brought improvement in SVR rates (ranging from 68 to 89% in naive patients with HCV genotype 1 infection), but were poorly tolerated due to interferon side effects.14-16 Nevertheless, the development of oral direct antiviral drugs (DAAs) has lead to rapid advance in HCV treatment.17 DAAs have been reported to be highly efficient, with SVR over 90% and better tolerable in compare with Interferon based therapy.17 However, achieving a SVR with DAAs has so far been more challenging in patients with cirrhosis.17,18 According to Zuckerman, et al., treatment with DAAs in patients with advanced fibrosis is highly effective, but caution should be used and closer monitoring may be needed compared to those without cirrhosis.19
Interferon free regimen containing Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir and Ribavirin has been approved for the treatment of HCV genotype 1 patients with Child Pugh class A cirrhosis in Romania. However no study in Romania so far compared the efficiency of such a therapy in treatment of naïve patients to those previously treated with other therapeutic regimes. We have conducted a study in order to compare the efficacy of Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir and Ribavirin therapy in patients with chronic HCV genotype 1b infection and compensated cirrhosis with and without prior treatment experience with PegIFN/RBV.
Material and MethodsWe have conducted a prospective two-center study in Mures County Hospital, Mures, Romania and Brasov County Hospital, Brasov, Romania in period November 2015 - July 2016. All the patients who referred to two dedicated hospital-based HCV treatment sites for initiating of novel DAA antiviral therapy were eligible for the inclusion. Both hospitals where patient recruitment took place are general hospitals admitting both patients with and without social insurance.
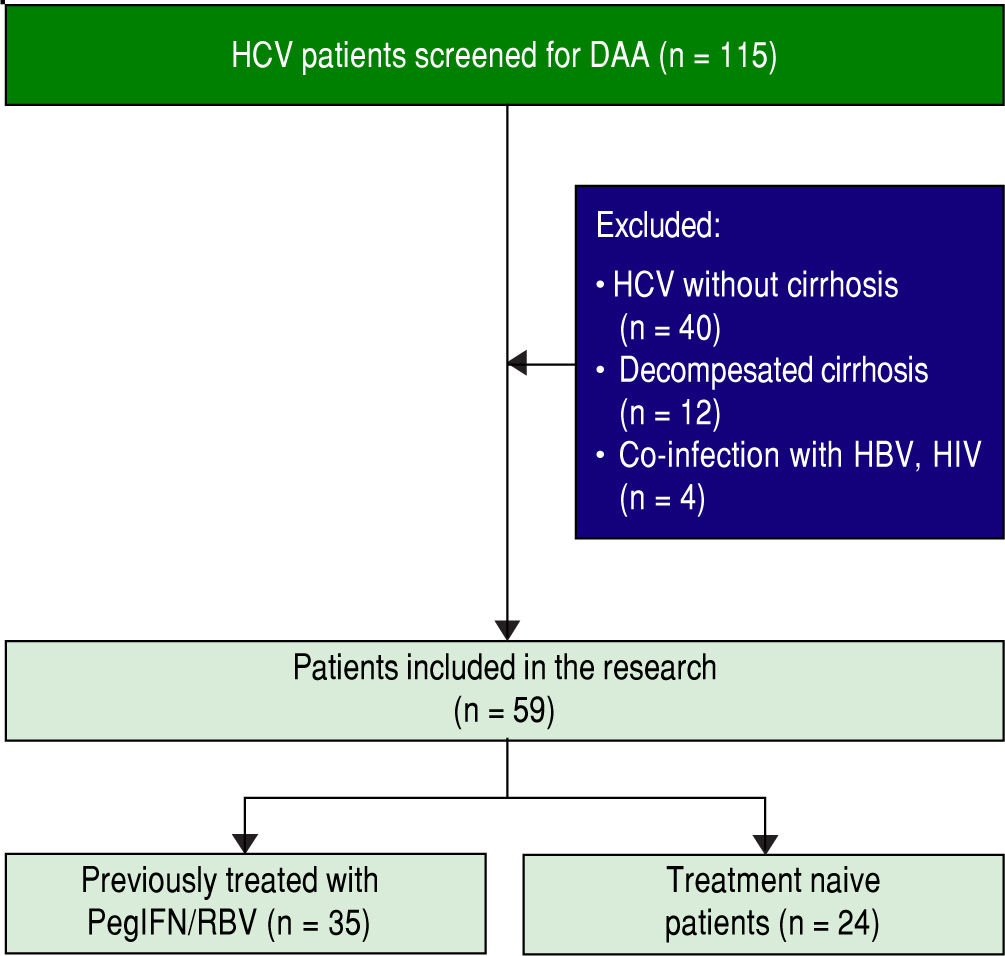
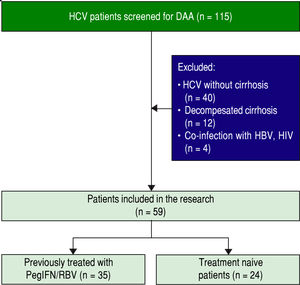
Figure 1 illustrates the process of patient enrollment. Inclusion criteria implied age of 18 years or older, pres- ence of HCV genotype 1b infection for at least 6 months prior to inclusion and documented liver cirrhosis with a Child Pugh score of 5 or 6. Patients were included regard- less to previously treatment with PegInterferon/Ribavirin. Exclusion criteria implied presence of HCC, co-infection with hepatitis B virus or human immunodeficiency virus, any other cause of clinically significant liver disease, de- compensated liver cirrhosis (class Child Pugh B and C), uncontrolled diabetes mellitus, severe psychiatric disor- der, and active substance abuse. Study was approved by in- stitutional review board. Written informed consent was obtained from each patient before enrollment.
At enrollment, data were collected on patients’ medical history (time since cirrhosis diagnosis, viral load, fibrosis grade, previous interferon-based antiviral therapy). Patients were submitted to physical examination, abdominal ultrasonography, esophagogastroduodenoscopy and routine blood labs (aspartate aminotransferase-AST, alanine aminotransferase-ALT, total bilirubin-TBIL, gamma glutamyl transpeptidase-GGT, alkaline phosphatase-ALK, international normalized ratio-INR, albumin, white blood cell count-WBC, haemoglobin-Hb, platelet count-Plt, creatinine, urea). Data were collected on demographics and anthropometric measures (body mass index-BMI). Severity of cirrhosis was assessed accordingly to Child Pugh classification. Liver fibrosis was evaluated by Fibro-Test™ (GGT, TBIL, alpha-2-macroglobulin, apolipoprotein A1, haptoglobin-weighted depending on the patient’s age and gender) according to BioPredictive, Paris, France Manufacturer.20
All patients enrolled in the present study received 12 weeks treatment with Ombitasvir (OBV), Paritaprevir (PTV), Ritonavir (r), Dasabuvir (DSV) and Ribavirin (RBV). Co-formulated OBV/PTV/R was administered once daily (25/150/100 mg) and DSV twice daily 250 mg. RBV was administrated three times daily with the dose determined according to body weight (1,000 mg daily in patients with a body weight of < 75 kg, and 1,200 mg daily in patients with a body weight ≥ 75 kg). Viral load was assessed at the moment of screening, at the end of the treatment (EOT) and 12 weeks after the end of treatment (SVR12). HCV RNA levels were quantitatively processed using semi-automated reverse transcriptase-PCR assay. Lower limit of quantification (LLOQ) was considered 25 IU/mL according to Abbott Diagnostic, Chicago, Illinois Manufacturer.21 Efficacy of therapeutic regime was assessed by the percentage of patients with SVR12, defined as a HCV RNA below the LLOQ at 12 weeks after last dose of study drug.
Among the patients included in study, two cohorts were defined: cohort A-treatment experienced patients previously treated with PegIFN/RBV and cohort B-treatment naive patients. Treatment experienced patients had previously documented treatment failure, partial response or they discontinued the therapy due to adverse events. Comparison was made between the cohorts in relation to SVR12 rates and the percentage of patients with virologic failure during treatment or relapse after treatment. Furthermore baseline factors (clinically and laboratory ones) that could predict lower rates of response or the development of adverse events were assessed.
Statistical analysisData were considered as nominal or quantitative variables. Nominal variables were characterized using frequencies. Quantitative variables were tested for normality of distribution using Kolmogorov-Smirnov test and were characterized by median and percentiles (25-75%) or by mean and standard deviation (SD), when appropriate. A γ2 test was used in order to compare the frequencies of nominal variables. Quantitative variables were compared using t test, Mann Whitney test. The correlation between quantitative variables was assessed using Spearman’s rho, when appropriate. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 20, Chicago, IL, USA).
ResultsA total of 59 patients with HCV-associated liver cirrhosis were enrolled in the study. Tables 1 and 2 reports demographics, clinical, biological and virological characteristics of the patients included.
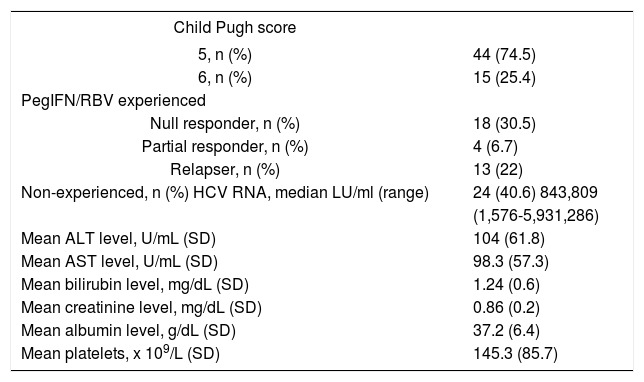
Clinical characteristics of 59 patients before treatment with OBV/PTV/r+DSV+RBV.
| Child Pugh score | |
|---|---|
| 5, n (%) | 44 (74.5) |
| 6, n (%) | 15 (25.4) |
| PegIFN/RBV experienced | |
| Null responder, n (%) | 18 (30.5) |
| Partial responder, n (%) | 4 (6.7) |
| Relapser, n (%) | 13 (22) |
| Non-experienced, n (%) HCV RNA, median LU/ml (range) | 24 (40.6) 843,809 |
| (1,576-5,931,286) | |
| Mean ALT level, U/mL (SD) | 104 (61.8) |
| Mean AST level, U/mL (SD) | 98.3 (57.3) |
| Mean bilirubin level, mg/dL (SD) | 1.24 (0.6) |
| Mean creatinine level, mg/dL (SD) | 0.86 (0.2) |
| Mean albumin level, g/dL (SD) | 37.2 (6.4) |
| Mean platelets, x 109/L (SD) | 145.3 (85.7) |
Median age of included patients was 60 and 52.5% of the patients were females. Forty four (74.5%) were previously diagnosed with liver cirrhosis Child Pugh score 5, while 15 (25.4%) were diagnosed with Child Pugh score 6. All patients were in F4 fibrosis stage. All patients were infected with genotype 1b HCV infection and more than half of them had prior treatment experience with PegIFN/RBV (35 of 59 patients, 59.3%). Null responders to previous PegIFN/RBV treatment comprised 51.4%, partial responders 11.4% and relapsers 37.2% of the study population (Table 2).
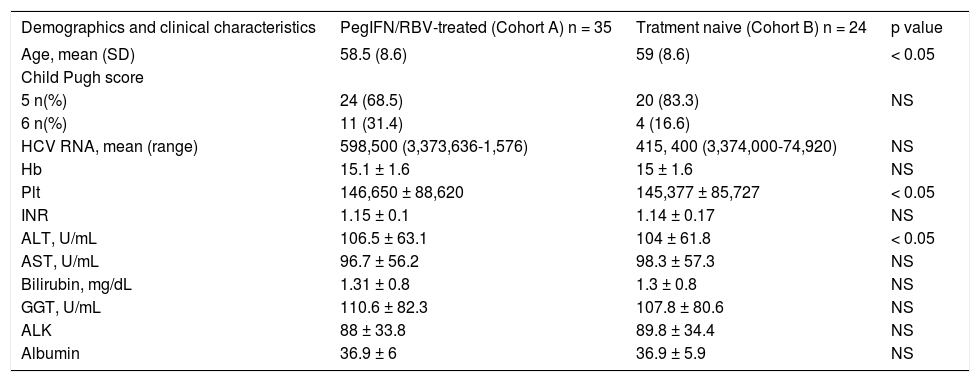
Table 3 reports the initial distribution of demographics, clinical, biological and virological characteristic of patients included in relation to previous treatment with PegIFN/RBV. Treatment naïve patients (Cohort B) were significantly older than patients previously treated with PegIFN/RBV (Cohort A) (p = 0.013) (Table 3). Mean levels of AST, ALT and GGT were above the normal range in both cohorts (Table 3). Patients from Cohort A had significantly higher mean level of ALT than patients in Cohort B (p = 0.049), while number of platelets was significantly lower among patients from cohort B (p = 0.031). There was no difference in HCV RNA levels or Child-Pugh score between the groups.
Distribution of patients demographics and clinical characteristics at the time of initiation of OBV/PTV/r+DSV+RBV therapy in relation to previous PegIFN/RBV-treated.
| Demographics and clinical characteristics | PegIFN/RBV-treated (Cohort A) n = 35 | Tratment naive (Cohort B) n = 24 | p value |
|---|---|---|---|
| Age, mean (SD) | 58.5 (8.6) | 59 (8.6) | < 0.05 |
| Child Pugh score | |||
| 5 n(%) | 24 (68.5) | 20 (83.3) | NS |
| 6 n(%) | 11 (31.4) | 4 (16.6) | |
| HCV RNA, mean (range) | 598,500 (3,373,636-1,576) | 415, 400 (3,374,000-74,920) | NS |
| Hb | 15.1 ± 1.6 | 15 ± 1.6 | NS |
| Plt | 146,650 ± 88,620 | 145,377 ± 85,727 | < 0.05 |
| INR | 1.15 ± 0.1 | 1.14 ± 0.17 | NS |
| ALT, U/mL | 106.5 ± 63.1 | 104 ± 61.8 | < 0.05 |
| AST, U/mL | 96.7 ± 56.2 | 98.3 ± 57.3 | NS |
| Bilirubin, mg/dL | 1.31 ± 0.8 | 1.3 ± 0.8 | NS |
| GGT, U/mL | 110.6 ± 82.3 | 107.8 ± 80.6 | NS |
| ALK | 88 ± 33.8 | 89.8 ± 34.4 | NS |
| Albumin | 36.9 ± 6 | 36.9 ± 5.9 | NS |
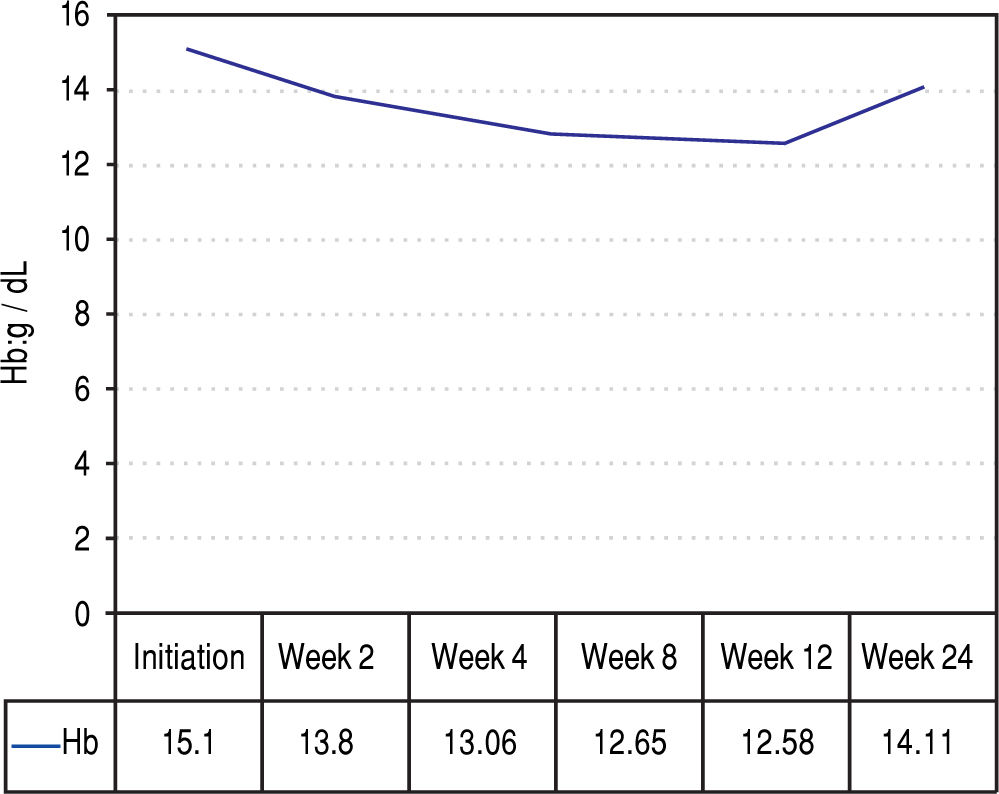
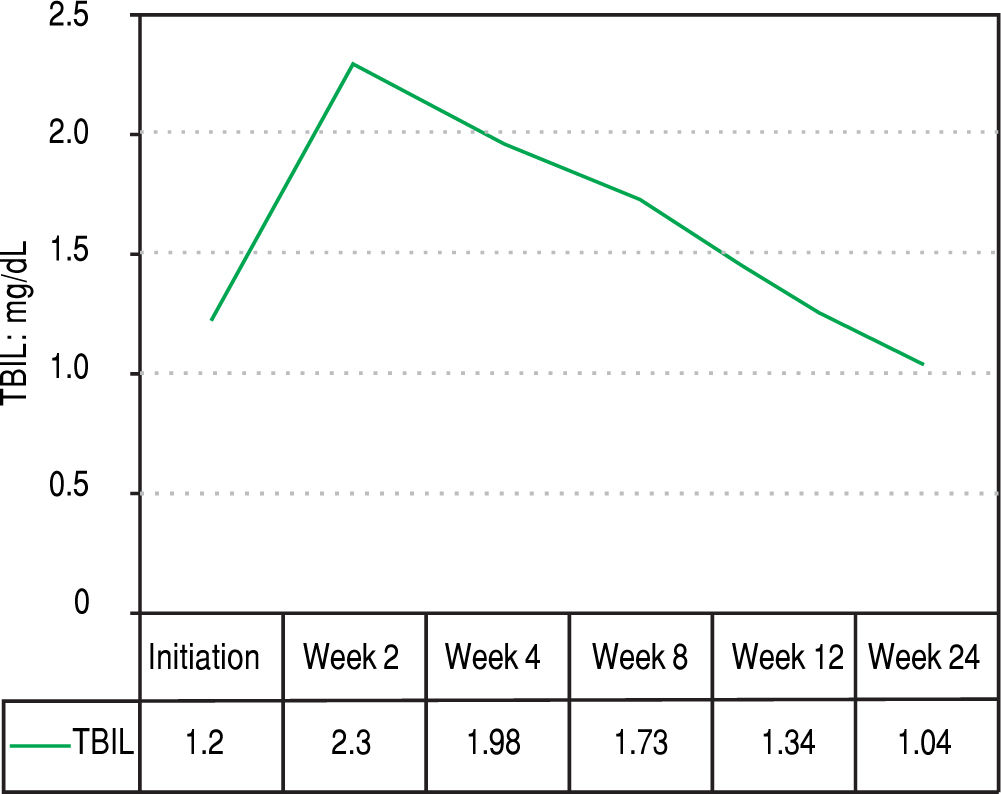
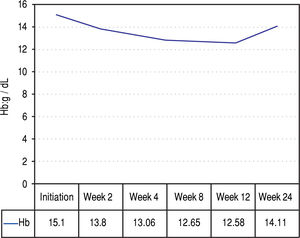
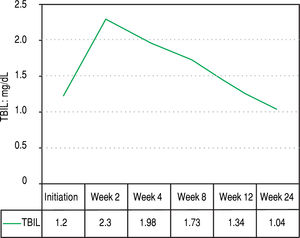
Figures 2 and 3 reports the changes in patients Hb and TBIL values under the treatment. Mean TBIL value increased after 2 weeks of treatment (Figure 3) while mean Hb levels decreased after 2-4 weeks (Figure 2).
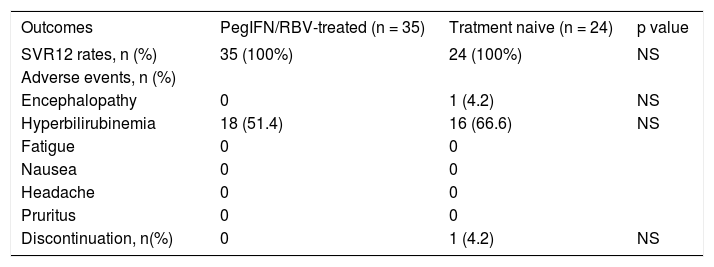
Tables 4 and 5 reports treatment outcomes in both cohorts. After 12 weeks of treatment with OBV/ PTVr + DSV+RBV, 100% of patients in each cohort achieved SVR12. One patient from treatment naive cohort presented herself with hepatic encephalopathy and hyperbilirubinemia (Bi: 6.79 mg/dL) after 6 weeks of treatment, and discontinued the therapy. Symptoms were ceased after two weeks of specific treatment with laxatives, rifaximin and hepatoprotectives. Her viral load determined at 12 weeks after discontinuation was undetectable. No patients from either cohort experienced on-treatment virologic failure or post-treatment relapse. Adverse events like hyperbilirubinemia were present for two weeks maximum.
Distribution of outcomes OBV/PTV/r+DSV+RBV therapy in relation to previous treatment.
| Outcomes | PegIFN/RBV-treated (n = 35) | Tratment naive (n = 24) | p value |
|---|---|---|---|
| SVR12 rates, n (%) | 35 (100%) | 24 (100%) | NS |
| Adverse events, n (%) | |||
| Encephalopathy | 0 | 1 (4.2) | NS |
| Hyperbilirubinemia | 18 (51.4) | 16 (66.6) | NS |
| Fatigue | 0 | 0 | |
| Nausea | 0 | 0 | |
| Headache | 0 | 0 | |
| Pruritus | 0 | 0 | |
| Discontinuation, n(%) | 0 | 1 (4.2) | NS |
Distribution of patients biochemical characteristics after the therapy with OBV/PTV/r+DSV+RBV in relation to previous treatment.
| Biochemical characteristics | PegIFN/RBV-treated (n = 35) | Tratment naive (n = 24) | p value |
|---|---|---|---|
| Hb, mean ± SD | 14.1 ± 1.9 | 14 ± 1.8 | < 0.05 |
| INR, mean ± SD | 1.26 ± 0.2 | 1.16 ± 0.1 | NS |
| ALT, U/mL | 21.2 ± 9.8 | 20.6 ± 9.6 | NS |
| AST, U/mL | 29.3 ± 12.1 | 28.5 ± 12.4 | < 0.05 |
| Bilirubin, mg/dL | 1.04 ± 0.55 | 0.98 ± 0.52 | NS |
| GGT, U/mL | 34.4 ± 19.5 | 34.9 ± 18.8 | NS |
After 12 weeks of treatment patients from cohort A had significantly higher mean levels of hemoglobin (p = 0.03). Mean values of AST were also significantly higher (p = 0.043) among patients previously treated with PegIFN/RBV (Cohort A) (Table 5).
DiscussionWe report a 12-week treatment with the regimen of coformulated OBT/PTV/r with DSV and RBV to result in 100% SVR 12 rate in HCV GT1b-infected patients with compensated cirrhosis.
Similar results have been reported previously by Feld, et al.10 and Andreone, et al,22 Feld, et al.10 reported the 100% SVR12 rate in phase IIIb study conducted on 60 patients with GT 1b-infection and cirrhosis who received OBV/ PTV/r and DSV without RBV for 12 weeks. Andreone, et al.22 reported 96.6% SVR12 rate in phase III trial including 91 patients with GT 1b-infection and compensated cirrhosis who received the 3-DAAs with RBV for 12 weeks. However, to the best of our knowledge this is the first study reporting a 100% SVR12 rate in patients with HCV GT 1b and compensated cirrhosis treated with OBT/PTV/r, DSV and RBV. Previous studies also reported lower response rates among patients who were previously treated with PegIFN/RBV in comparison to treatment naive patients.17,22-27 However, in our study we report 100% of SVR12 both among patients previously treated with PegIFN/RBV as well as treatment naive patients. In addition, the rates of SVR12 in our study were consistently high, even in the presence of baseline characteristics that have been previously identified as determinants of poor rates of response. Recent systematic review published by Cavalcante, et al.28 identified male gender, genotype I, high grade of fibrosis and high initial viral load to be associated with poor SVR in both Interferon free and Interferon based regiments. Zeusem, et al. emphasized the negative predictive role of genotype I, presence of cirrhosis and previously relapsed or null response to IFN in patients treated with PODr regimen.29 However similar characteristics, such as high baseline HCV RNA levels, high Child Pugh score and presence of signs of portal hypertension (platelet count, LFTs, serum albumin) did not influence high SVR12 rates in our study. The high rate of success we explain with good adherence to the therapy, same viral genotype (GT 1b) in all patients and the fact that we included only patients with compensated liver cirrhosis.
Since the approval of OBT/PTV/r with DSV and RBV for HCV GT1b infected patients with compensated liver cirrhosis, two cases of drug discontinuation due to adverse events were reported in the literature.30,31 Both cases presented grade 4 hyperbilirubinemia and ascites, but their follow-up testing revealed the achievement of SVR, regardless of premature discontinuation. Our results are in line with these findings as one patient from previously untreated group in our study developed hyperbilirubinemia associated with grade III hepatic encephalopathy at week 6 of treatment. As these are considered to be serious adverse events, study drug was prematurely discontinued in this patient. However her viral load at SVR12 was undetectable, regardless of only 6 weeks of treatment.
The major limitation of our study is that we didn’t conceived it as a placebo-controlled study, justifying it with the risk of hepatic decompensation among untreated patients. Second, there was no active comparator group owing to the toxic effects of standard interferon containing regimens. Also the small number of patients in each study group limited the power to detect differences between groups. Finally, although the fibrosis stage was assessed at baseline by means of serum biomarkers, we didn’t have data about the fibrosis stage at the end of the treatment. However we find our findings valuable as we report the first results of treatment with novel therapeutic regimen for HCV patients in Romania. Our findings can be useful for others functioning in similar healthcare settings.
In conclusion, an all-oral regimen of co-formulated OBT/PTV/r with DSV and RBV results in high rate of sustained virologic response at post-treatment week 12 among HCV GT 1b infected patients associated with compensated cirrhosis, regardless of previous treatment experience with PegIFN/RBV.
Conflict of InterestThe authors declares that there is no conflict of interest regarding the publication of this article.
Source FundingNone declared.
Abbreviations- •
AASLD: American Association for the Study of Liver Diseases.
- •
ALK: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI:body mass index.
- •
DAAs: direct acting antivirals.
- •
DSV: dasabuvir.
- •
EASL: European Association for the Study of the Liver.
- •
EOT: end of treatment.
- •
GGT: gamma glutamyl transpeptidase.
- •
GT: genotype.
- •
Hb: haemoglobin.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
INR: international normalized ratio.
- •
LFT: liver function tests.
- •
OBV: ombitasvir.
- •
PegIFN/RBV: pegylated interferon and ribavirin.
- •
Plt: platelet count.
- •
PTV: paritaprevir.
- •
r: ritonavir.
- •
RBV: ribavirin.
- •
SD: standard deviation.
- •
SVR: sustained virologic response.
- •
TBIL: total bilirubin.
- •
WBC: white blood cell count.