Infected hepatic echinococcosis (IHE), defined as a cystic infection, and the development of a liver abscess may be a complication in the natural history of hepatic echinococcosis. The aim of this study was to review the evidence available related to clinical, therapeutic, and prognostic aspects of IHE.
We conducted a systematic review. Trip Database, BIREME-BVS, SciELO, LILACS, IBECS, PAHO-WHO; WoS, EMBASE, SCOPUS and PubMed were consulted. Studies related to IHE in humans, without language restriction, published between 1966 and 2020 were considered. Variables studied were publication year, geographical origin of the samples, number of patients, therapeutic and prognosis aspects, and methodological quality (MQ) for each article. Descriptive statistics was applied. Subsequently, weighted averages (WA) of the MQ of each article were calculated for each variable of interest. 960 related articles were identified; 47 fulfilled selection criteria, including 486 patients with a median age of 48 years, 51.6% being male. The largest proportion of articles were from Spain, India, and Greece (36.1%). Mean cyst diameter was 14.1 cm, and main location was right liver lobe (74.0%). WA for morbidity, mortality, hospital stay, and follow-up were 28.5%, 7.4%, 8.5 days and 14.8 months, respectively. The most common causative microorganisms of superinfection isolated were Enterobacteriaceae. An association with cholangitis was reported in 13.4% of cases. Mean MQ of the 47 articles included was 7.6 points.
We can conclude that the information related to IHE is scarce and scattered throughout articles of small casuistry and poor quality, and consequently does not provide strong evidence.
Cystic echinococcosis (CE) is characterized by cystic lesions, most commonly in the liver and lungs, which can be fatal if left untreated. CE can be found worldwide, and the World Health Organization considers it as one of the 17 neglected tropical diseases [1,2].
Different complications may occur during the natural history of CE, of which cystic superinfection is of note. This condition is recognized as infected hepatic echinococcosis (IHE) and includes diagnoses such as hepatic hydatid abscess, liver abscess of hepatic origin or superinfection of liver hydatid cysts. Superinfection may occur as a complication of bacteremia by any cause, but in most cases it develops when the integrity of the cyst membrane has been lost and communication is established between the cyst and the bile ducts [3,4]. In fact, as early as 1891 there is evidence that infection of hydatid cysts could lead to subsequent rupture [5]. Thus, it is estimated that superinfection may complicate ruptured hepatic echinococcosis in up to 25% of cases, leading to the development of a liver abscess [6].
In contrast to the usual clinical course of a liver abscess, IHE usually appears with nonspecific clinical manifestations. These manifestations may be due to the presence of a pericystic membrane, that is, an inflammatory reaction of the host against the Echinococcus which generates a nonspecific defense mechanism in the host. Hence, it is possible that clinical features and abnormal lab results may not derive from IHE (which explains the difficulty in achieving an adequate diagnosis). Early diagnosis is important since it reduces high postoperative morbidity (POM) and mortality which have been estimated at 24.4% and 2.0%, respectively [6–8].
The general objective of this review was to summarize the evidence available related to the clinical, therapeutic, and prognostic aspects of IHE. The specific objectives were to describe the different treatments used, POM and mortality associated, reported in the primary articles. To determine results of emergency treatment adopted versus elective surgery. To describe other therapeutic resources reported in the primary articles.
2Material and methodsThis manuscript was written following the guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [9], and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines.
2.1Protocol and registrationSystematic review registration number PROSPERO CRD42020143171.
2.2DesignSystematic Review (SR).
2.3Eligibility criteriaStudies related to IHE in an adult human population published between 1966 and 2020 were included. No language or design type restriction was applied. Articles contaminated with other types of liver abscesses; echinococcosis alveolaris, multilocularis and vogeli were excluded. Narrative reviews, consensus documents and discussion papers were also not considered.
2.4Information sourcesThe following Meta search engines, libraries and databases were reviewed: Trip Database, BIREME-BVS, SciELO, LILACS, IBECS, PAHO-WHO; Web of Science, EMBASE, SCOPUS and PubMed. The closing date for article search and recruitment was March 30, 2020.
2.5SearchThe search was carried out according to PECO components (population study [P], exposure [E], comparator [C], and outcome [O]). Studies were searched for subjects with hepatic echinococcosis [P], complicated with a secondary infection [E], without comparing [C], clinical features [O]. Sensitive searches were performed using MeSH terms, free words, truncated terms and Boolean connectors (AND and OR); with strategies adapted to each database (Table 1). A systematic and sensitive literature search was performed. Therefore, search strategies were developed, using MeSH terms, free words, truncated terms and Boolean connectors (AND, OR and NOT); using strategies adapted to each source of information consulted. In addition, a manual cross-reference search, and a directed search in infectious disease and parasitology journals was performed.
Search strategies for information source and results achieved. (N = 960).
| Information sources | Search strategies |
|---|---|
| Trip Database (n = 9) | ("Echinococcosis, Hepatic" OR “hepatic hydatid cyst” OR “hepatic hydatidosis” OR “liver echinococcosis” OR “liver hydatid cyst” OR “liver hydatidosis” OR "Echinococcosis, Hepatic/complications") AND ("Liver Abscess" OR "Liver Abscess, Pyogenic") |
| BIREME-BVS (n = 38) | (mh:("Equinococosis Hepática/CO" OR "Equinococosis Hepática/DI" OR "Equinococosis Hepática/MO" OR "Equinococosis Hepática/SU") AND mj:("Absceso Hepático")) |
| SciELO (n = 49) | ((hepatic echinococcosis) OR (liver hydatidosis) OR (hepatic hydatidosis) OR (liver hydatid cyst) OR (hepatic hydatid cyst)) AND (liver abscess) |
| LILACS (n = 6) | Hepatic echinococcosis [Words] or Liver hydatid cyst [Words] and Liver abscess [Words] |
| IBECS (n = 0) | "Hepatic echinococcosis" [Palabras] and "Liver hydatid cyst" [Palabras] and "Liver Abscess" [Palabras] |
| PAHO- MA WHO (n = 94) | “Cystic echinococcosis” NOT alveolar NOT multilocularis |
| Web of Science (n = 6) | #1 (Ti=(Hepatic Echinococcosis) OR Ti=(liver hydatid cyst) OR Ti=(Echinococcosis, Hepatic) OR Ti=(Hepatic hydatidosis)) |
| #2 (Ti=(Liver Abscess) OR Ti=(Liver Abscess, Pyogenic)) | |
| #3 (#1 AND #2) | |
| EMBASE (n = 204) | ('liver hydatid cyst'/exp OR 'liver hydatid cyst' OR 'liver echinococcosis'/exp OR 'liver echinococcosis' OR 'hepatic echinococcosis'/exp OR 'hepatic echinococcosis') AND 'liver abscess'/exp AND [1967−2020]/py |
| SCOPUS (n = 335) | ("Hepatic Echinococcosis" OR "liver hydatid cyst" OR "Hepatic hydatidosis") AND "Liver Abscess" AND (LIMIT-TO (DOCTYPE, "ar")) AND (LIMIT-TO (SRCTYPE, "j")) |
| PubMed (n = 219) | ("Hepatic echinococcosis" OR "Echinococcosis, Hepatic" OR “hepatic hydatid cyst” OR “hepatic hydatidosis” OR “liver echinococcosis” OR “liver hydatid cyst” OR “liver hydatidosis” OR "Echinococcosis, Hepatic/complications") AND ("Liver Abscess" OR "Liver Abscess, Pyogenic" OR “Hepatic Abscess”) |
BIREME: Latinamerican and Caribbean Center on Health Science Information; BVS: Virtual Health Library; SciELO: Scientific Electronic Library Online, IBECS: Spanish Bibliographic Index of the Health Sciences.
Articles identified in each source of information, were filtered by duplication between databases. Three review authors (CM, SC, CR) independently screened the titles and abstracts of all the reports identified as a result of each search. Then, full-text study reports were retrieved, and two review authors (CM, SC) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. Disagreements were resolved by consensus or if required, a third review author (LG) was consulted. Duplicate articles identified were excluded.
2.7Data collection processA critical review by 3 researchers (SC, CM and LG) of each selected article was applied. The selected data were extracted by three authors (SC, CM and CR), and then collected in an Excel spreadsheet (Mac Excel, version 15.24; 2016 Microsoft Corporation®). All studies meeting the inclusion criteria underwent analysis of methodological quality (MQ) according to an ad-hoc designed scale [10]. This scale with content and construct validity is composed of 3 domains: design, sample size and methodological aspects; and its final score is the sum of the 3 domains; the score may range between 6 and 36 points, with a cut-off point of MQ of 18 points.
2.8Data itemsThe following variables were considered: year of publication, geographic origin of the article, number of cases, main clinical manifestation, therapeutics, and prognosis (POM and mortality).
2.9Risk of bias in individual studiesTwo review authors (CM, LG) independently assessed the risk of bias for each study applying a MQ scale [10].
2.10Summary measuresDescriptive statistics were applied, with calculation of percentages and averages.
2.11Synthesis of resultsWeighted averages (WA) of each variable were determined by the sum of the product of the study variable by the MQ score of where the article came from, divided by the sum of MQ scores of all the study articles. For this purpose, the following formula was applied (WA=ΣXi*eiΣei), in which WA is weighted average; Xi, the value achieved for the outcome in the study i (for all outcomes); ei, represents the MQ score obtained in study I; and Σei, correspond to the sum of the scores obtained in all the studies [11].
2.12EthicsIn order to reduce possible biases in selection and analysis, masking of authors and study centers was implemented.
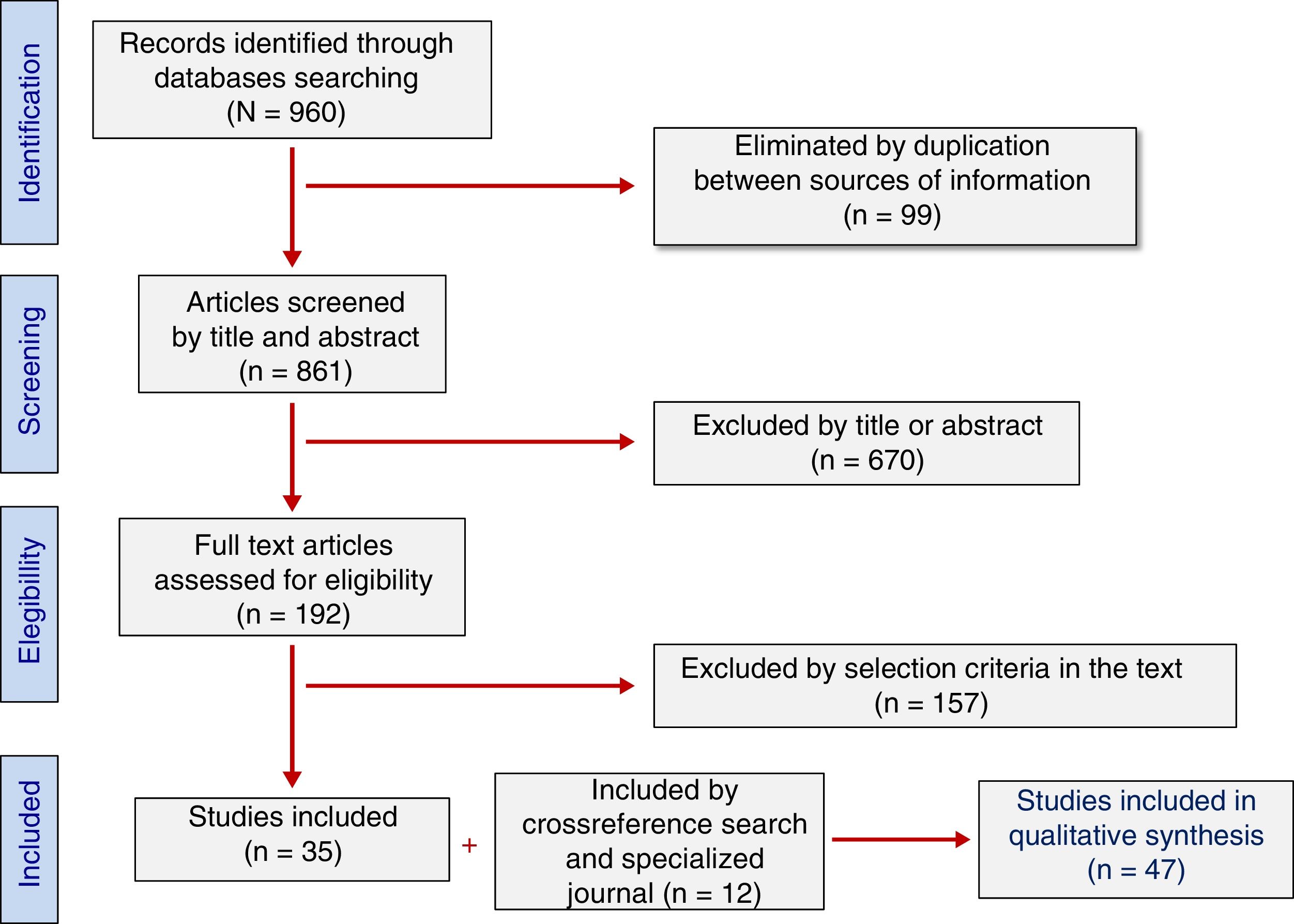
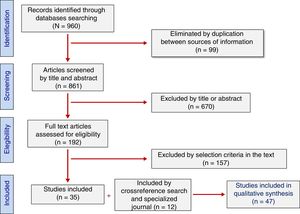
3Results3.1Study selectionA total of 960 studies were retrieved from the search (Table 1). Of these, 99 were excluded for duplication of information sources and 670 were discarded as being considered “irrelevant” after reading titles and abstracts. In-depth analysis of the 191 remaining articles allowed the exclusion of 156 studies that did not meet the selection criteria. Furthermore, 11 studies collected by cross-reference search and 1 collected by directed search in specialized journals (n = 12) were added. Finally, 47 studies were included in the study sample of this review (Fig. 1).
3.2Study characteristicsAll the studies analyzed were case reports or series including 486 patients [median age of 48 years (WA of 45.9 years), 51.6% males]. The largest series were those of Prousalidis (n = 77) [12] and Symeonidis (n = 75) [13]. The remainder were reports that included between 1 and 65 cases [14–58].
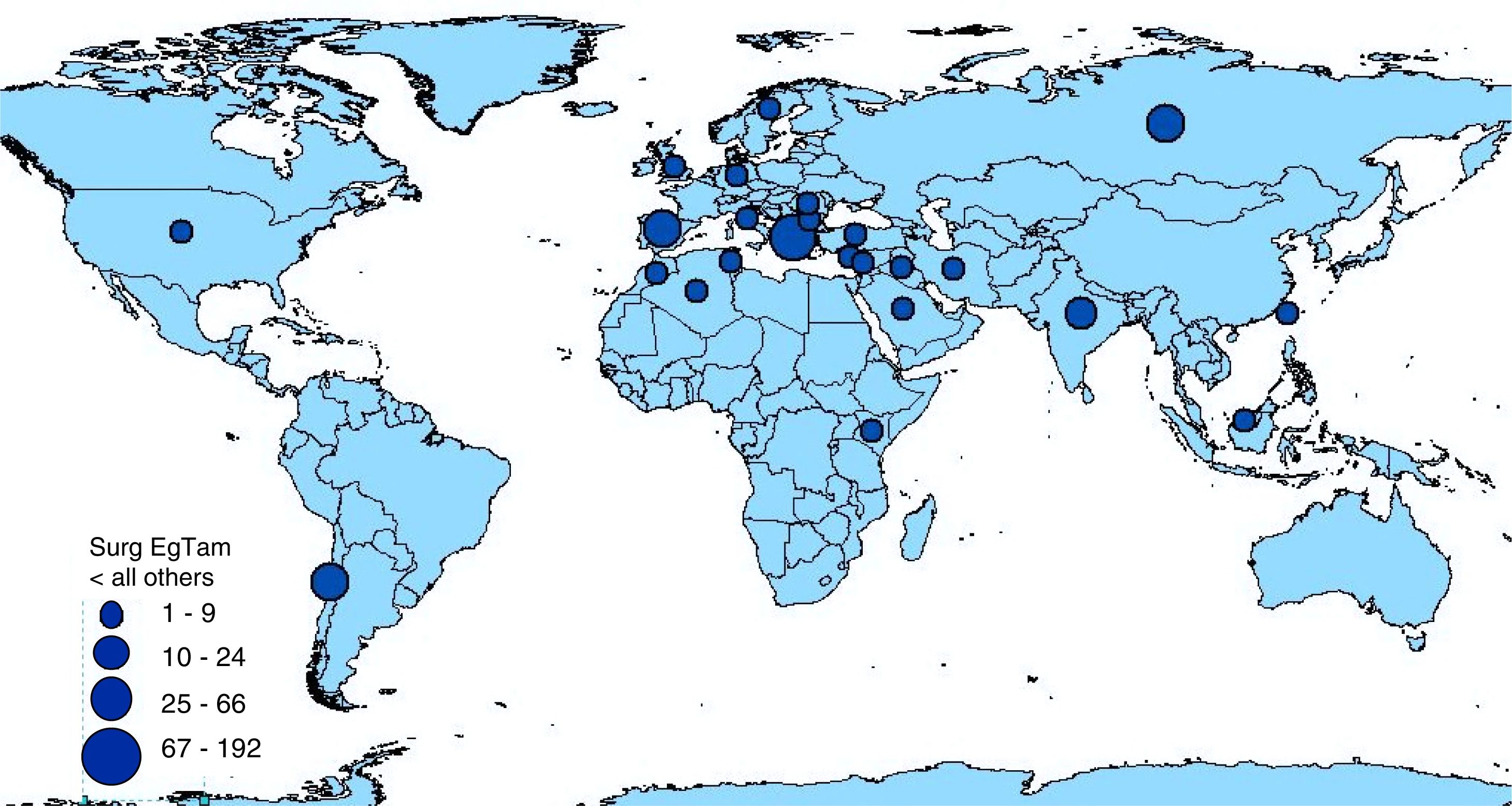
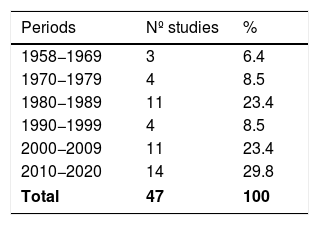
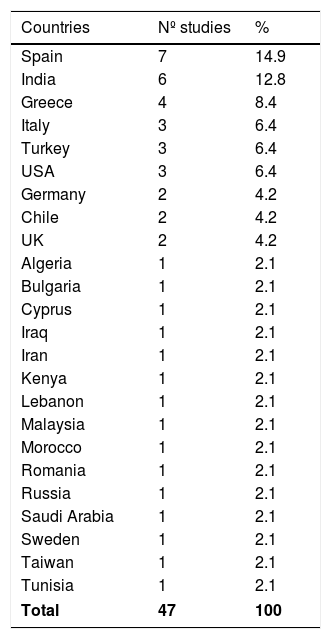
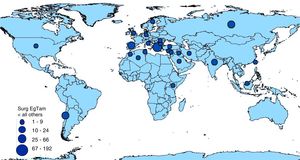
3.3Synthesis of resultsThe highest percentage (29.8%) of articles was published during the period 2010–2020 (Table 2). The countries with the highest number of publications were Spain, India, and Greece [14.9%, 12.8% and 8.4%, respectively (Table 3 and Fig. 2)].
Country origin of analyzed patients.
| Countries | Nº studies | % |
|---|---|---|
| Spain | 7 | 14.9 |
| India | 6 | 12.8 |
| Greece | 4 | 8.4 |
| Italy | 3 | 6.4 |
| Turkey | 3 | 6.4 |
| USA | 3 | 6.4 |
| Germany | 2 | 4.2 |
| Chile | 2 | 4.2 |
| UK | 2 | 4.2 |
| Algeria | 1 | 2.1 |
| Bulgaria | 1 | 2.1 |
| Cyprus | 1 | 2.1 |
| Iraq | 1 | 2.1 |
| Iran | 1 | 2.1 |
| Kenya | 1 | 2.1 |
| Lebanon | 1 | 2.1 |
| Malaysia | 1 | 2.1 |
| Morocco | 1 | 2.1 |
| Romania | 1 | 2.1 |
| Russia | 1 | 2.1 |
| Saudi Arabia | 1 | 2.1 |
| Sweden | 1 | 2.1 |
| Taiwan | 1 | 2.1 |
| Tunisia | 1 | 2.1 |
| Total | 47 | 100 |
Mean cyst diameter reported in primary articles was 14.1 cm (WA of 12.5 cm). Regarding the location of IHE, 54.5% were found in the right liver lobe, 11.7% in the left liver lobe and in 7.4% were detected in both liver lobes. This data was not available in 128 cases.
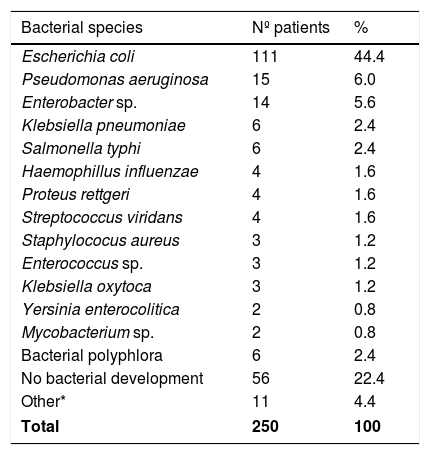
Samples for microbiology were only reported in 250 out of 486 cases (49.3%). In 56 cases (11.6%) the cultures were negative. In positive cases, enterobacterias were the microorganisms most frequently isolated (Table 4).
Bacterial species reported in 250 patients.
| Bacterial species | Nº patients | % |
|---|---|---|
| Escherichia coli | 111 | 44.4 |
| Pseudomonas aeruginosa | 15 | 6.0 |
| Enterobacter sp. | 14 | 5.6 |
| Klebsiella pneumoniae | 6 | 2.4 |
| Salmonella typhi | 6 | 2.4 |
| Haemophillus influenzae | 4 | 1.6 |
| Proteus rettgeri | 4 | 1.6 |
| Streptococcus viridans | 4 | 1.6 |
| Staphylococus aureus | 3 | 1.2 |
| Enterococcus sp. | 3 | 1.2 |
| Klebsiella oxytoca | 3 | 1.2 |
| Yersinia enterocolitica | 2 | 0.8 |
| Mycobacterium sp. | 2 | 0.8 |
| Bacterial polyphlora | 6 | 2.4 |
| No bacterial development | 56 | 22.4 |
| Other* | 11 | 4.4 |
| Total | 250 | 100 |
Other*: Acinetobacter baumannii, Aeromonas hydrophila, Citrobacter, Haemophilus parainfluenzae, M. Gordonae, M. morganii, Salmonella paratyphi B, Staphylococcus epidermidis, Streptococcus bovis, Streptocuccus Milleri, and Streptococcus salivarius (one of each).
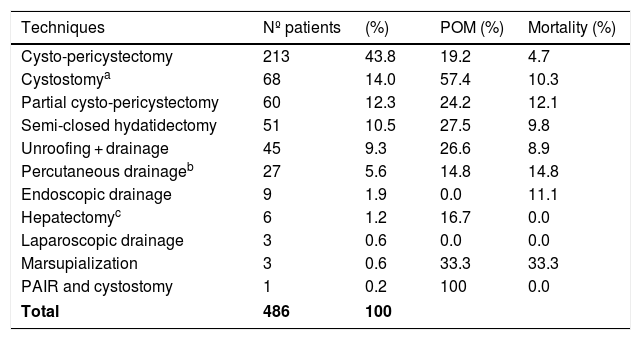
All cases were surgically treated. The surgical procedure most frequently used was cysto-pericystectomy, total or partial (56.1%). Techniques, POM and associated mortality are summarized in Table 5.
Surgical techniques, pom and mortality associated reported.
| Techniques | Nº patients | (%) | POM (%) | Mortality (%) |
|---|---|---|---|---|
| Cysto-pericystectomy | 213 | 43.8 | 19.2 | 4.7 |
| Cystostomya | 68 | 14.0 | 57.4 | 10.3 |
| Partial cysto-pericystectomy | 60 | 12.3 | 24.2 | 12.1 |
| Semi-closed hydatidectomy | 51 | 10.5 | 27.5 | 9.8 |
| Unroofing + drainage | 45 | 9.3 | 26.6 | 8.9 |
| Percutaneous drainageb | 27 | 5.6 | 14.8 | 14.8 |
| Endoscopic drainage | 9 | 1.9 | 0.0 | 11.1 |
| Hepatectomyc | 6 | 1.2 | 16.7 | 0.0 |
| Laparoscopic drainage | 3 | 0.6 | 0.0 | 0.0 |
| Marsupialization | 3 | 0.6 | 33.3 | 33.3 |
| PAIR and cystostomy | 1 | 0.2 | 100 | 0.0 |
| Total | 486 | 100 | ||
POM: Postoperative morbidity.
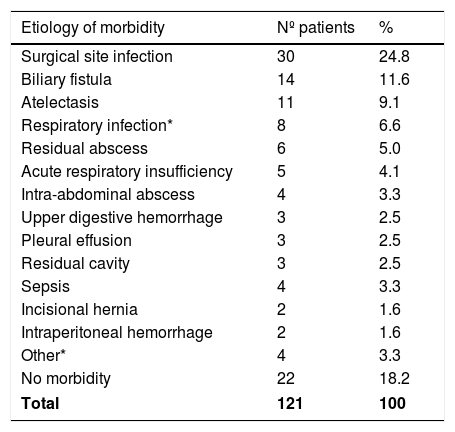
The WA for POM and mortality for all the articles were 28.5% and 7.4%, respectively. A description of POM causes was only available in 121 patients (Table 6), highlighting surgical site infection (30/121 cases, 24.8%) The causes of mortality were sepsis (19/36 cases; 52.8%), respiratory infections (7/36 cases; 19.4%), pulmonary thromboembolism (4/36 cases; 11.1%), myocardial infarction and cardiac arrhythmia (4/36 cases; 11.1%) and cerebral stroke (2/36 cases; 5.6%). The WA for hospital stay and follow-up were 8.5 days and 14.8 months, respectively.
Morbidity reported in 121 patients.
| Etiology of morbidity | Nº patients | % |
|---|---|---|
| Surgical site infection | 30 | 24.8 |
| Biliary fistula | 14 | 11.6 |
| Atelectasis | 11 | 9.1 |
| Respiratory infection* | 8 | 6.6 |
| Residual abscess | 6 | 5.0 |
| Acute respiratory insufficiency | 5 | 4.1 |
| Intra-abdominal abscess | 4 | 3.3 |
| Upper digestive hemorrhage | 3 | 2.5 |
| Pleural effusion | 3 | 2.5 |
| Residual cavity | 3 | 2.5 |
| Sepsis | 4 | 3.3 |
| Incisional hernia | 2 | 1.6 |
| Intraperitoneal hemorrhage | 2 | 1.6 |
| Other* | 4 | 3.3 |
| No morbidity | 22 | 18.2 |
| Total | 121 | 100 |
Respiratory infection*: Pneumonia and pneumonitis.
Other*: Cardiac arrhythmia, pericarditis, pulmonary thromboembolism, and subphrenic abscess (one of each).
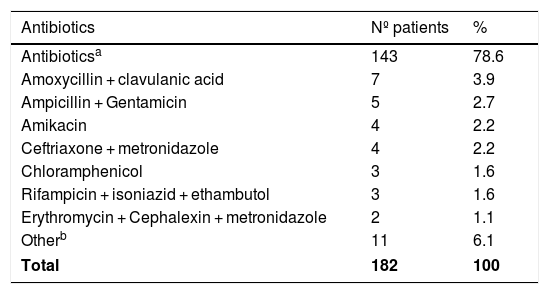
The use of an antibiotic scheme was described in 37.4% of cases (n = 182), and the regimens used were heterogeneous (Table 7). An association with cholangitis secondary to cholangiohydatidosis was described in 65 cases (13.4%).
Antibiotics used reported in 182 patients.
| Antibiotics | Nº patients | % |
|---|---|---|
| Antibioticsa | 143 | 78.6 |
| Amoxycillin + clavulanic acid | 7 | 3.9 |
| Ampicillin + Gentamicin | 5 | 2.7 |
| Amikacin | 4 | 2.2 |
| Ceftriaxone + metronidazole | 4 | 2.2 |
| Chloramphenicol | 3 | 1.6 |
| Rifampicin + isoniazid + ethambutol | 3 | 1.6 |
| Erythromycin + Cephalexin + metronidazole | 2 | 1.1 |
| Otherb | 11 | 6.1 |
| Total | 182 | 100 |
Other: Includes single drug cefotaxime, ceftazidime, ciprofloxacin, gentamicin (one case of each). And schemes: Cefazolin + gentamicin, cefazolin + metronidazole, cefuroxime + metronidazole, mefoxin + tetracycline, penicillin + streptomycin + tetracycline, piperacillin + tazobactam + imipenem, Imipenem + tobramycin + metronidazole (one case of each).
All the articles selected were at high risk of bias. All had less than 15 points in the MQ scale used (average of 7.6 points, median of 6.0 points; minimum 6 points and maximum 14 points).
3.5Potential biases in the review processWe requested additional information from several authors to expand and/or verify some study data, unfortunately, with no response. Thus, missing data may introduce bias to this review.
3.6Risk of bias across studiesThere may be a publication bias, given the scarcity of studies from certain geographic areas and countries, which could represent variants not verified in this review.
4Discussion4.1Summary of evidenceThis SR is based on the evidence available regarding IHE. The information was retrieved from systematic searches of 10 sources of information over the last 60 years and represents a compilation of 47 primary articles (all are case series and case reports) including 486 patients diagnosed and treated worldwide. There are no other SR related to this topic.
Regarding the results obtained, it was of note that over 27% of the articles were published in the last decade. These percentages may be associated with the increase in diagnostic suspicion and better access to diagnostic technology. The origin of the studies may be associated with publication bias because cystic echinococcosis is endemic in many areas throughout the world, some of which are not represented in this review.
Concerning the clinical features of IHE, a large diameter of the infected cysts and a higher frequency in the right lobe of the liver are verified. It was verified that infected cysts have a large diameter and are more frequent in the right lobe of the liver. Surgical treatment was universal, with significant POM and mortality rates, being surgical site infection and organ dysfunction and sepsis the most common causes, respectively. Consequently, a long hospital stay was reported. These data justify not only timely treatment of liver hydatid cysts but also strict monitoring of patients awaiting surgery and those who refuse treatment.
Microbiological sampling policies and the antibiotic schemes administered were very heterogeneous and did not allow conclusions to be drawn. The microorganisms most frequently isolated were Enterobacteriaceae (E. coli, Pseudomonas, Enterobacter and Klebsiella). Additionally, in some cases a cholangitis secondary to cholangiohydatidosis was diagnosed (13.4%). Since infection of the surgical site is the most common cause of POM and sepsis/organ failure, the most common cause of death in the postoperative period, it seems that common sense would dictate the administration of a prophylactic antibiotic regimen against Gram-negative bacteria in patients who are to be operated for liver hydatidosis, and even continue this regimen as treatment for a short period of time following surgery [59].
Unfortunately, the MQ of the primary studies was low and had a high risk of bias. In this context, it should be noted that the scale used to assess MQ (MInCir-therapy) has been validated in inter- and intra-observer reliability [60,61] and content and criterion validity studies [10,61,62]; and has been used to measure MQ in different scenarios. Therefore, the results reported should be considered with caution.
4.2Answer to the specific objetives- 1
The different treatments used, the postoperative morbidity and associated mortality reported in the primary articles, was informed in detail in the Table 5.
- 2
There was no comparison in the primary articles between emergency and elective treatments.
- 3
Other applied therapeutic resources, reported in the primary articles (percutaneous, endoscopic, and laparoscopic drainage, etc.), was infomed in Table 5.
The evidence available regarding IHE is limited, because it was obtained from level 4 studies (report and retrospective case series of reduced casuistry).
5ConclusionsThe evidence of IHE available at present is scarce and is dispersed among articles of reduced casuistry, poor methodological quality and low level of evidence; making it difficult to sustain facts supported by an adequate grade of recommendation. Furthermore, new studies with appropriate internal validity and adequate number of patients are required to clarify the uncertainty.
FundingFunded by projects DI19-0030, Universidad de La Frontera (Chile) and CONICYT-MEC 80190026 (Chile).
Conflict of interestThe authors have no conflicts of interest to declare