Introduction and aim. Indo is widely one of the non-steroidal anti-inflammatory drugs and one of the common toxic effects of this drug is hepatic failure. Thymol is a monoterpene phenol with many different pharmacological activities. However, up to now its hepatoprotective effects on Indo-induced gastric ulcer model in rats have not been explored yet.
Material and methods. Thirty five Sprague-Dawley rats were divided into seven groups: control, ulcer control (30 mg/kg Indo), Indo + reference standard (50 mg/ kg Rantidine), Indo + Thymol (75, 100, 250 and 500 mg/kg) groups. 10 minutes after the induction of ulcer with Indo; Thymol was orally administered to the rats. Liver function enzymes (AST, ALT and LDH) were measured from serum samples. TOS/TAC, TNF-α and PGE2 levels, eNOS and Caspase-3 activity were assessed from tissue homogenate samples. In addition, histopathologic analysis on liver sections was performed.
Results. Indo significantly increased the levels of hepatic enzymes, TNF-α and eNOS, and caspase-3 activation, while decreased PGE2 levels. Furthermore, it induced oxidative stress as evidenced by elevated TOS and decreased TAC levels. However, Thymol treatment induced a significant improvement in these parameters, especially in 250 mg/ kg dose. On the other hand, treatment with Thymol 500 mg/kg dramatically affected the parameters much worse than the Indo treated group.
Conclusion. The findings of the current study demonstrated that Thymol administration significantly ameliorated liver injury due to Indo toxicity. This effect of Thymol (250 mg/kg) may be mediated by its anti-oxidative or anti-inflammatory effect, and up-regulation the synthesis of PGE2.
NSAIDs are widely used worldwide and are the most common drugs associated with drug induced liver injury.1 Although there are many studies attempting to decrease the toxicity of NSAIDs, to date there are no effective drugs and therapeutic strategies for the treatment or prevention of hepatic damage caused by NSAIDs. Indo, is one of the most commonly used NSAIDs with potential hepatotoxicity.2 It is used to relieve fever, pain, stiffness, and swelling in musculoskeletal disorders. Indo is rapidly absorbed from the gastrointestinal tract after oral administration and its hepatic clearance is low.3
Thymol, a monoterpene phenolic compound, is found in the oils of thyme and produced from different plant species such as Thymus vulgaris, Thymbra spicata, Thymus ciliates, Origanum vulgarae, Trachyspermum ammi species, Monarda fistulosa and Nigella sativa seeds. It has many pharmacological biological activities such as antimicrobial, anti-inflammatory, antioxidant,4 antiapoptotic,5 anti-hepatotoxic,6 anti-hyperlipidemic and anti-hyperglycemic,7 neuroprotective8 and radioprotective activities.9 Many phenolic compounds in various plants have been reported to have potent antioxidative and hepatoprotective activities.10 So, the aim of the present study was to evaluate the hepatoprotective role activity of Thymol on Indo-induced gastric ulcer models and to identify the molecular pathways involved in this pathophysiology.
Material and MethodsAnimalsThirty Sprague-Dawley rats, female and weight ranging 250-300 g, were used in the study. Constant environmental conditions were maintained with a temperature of 22 ± 1 °C, humidity of 55% and a 12-h light/dark cycle. Experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996). All experimental procedures in this study were approved by the Atatürk University Local Ethics Committee for Animal Experiments (No. 104, 02.06.2015).
ChemicalsIndo were purchased from DEVA Holding A.S. (Istanbul, Turkey). Certified chemical standard of Thymol (> 99.5%) and the other all chemicals were purchased from Sigma-Aldrich International (St. Louis, MO, USA).
Experimental DesignThe rats were divided into seven groups containing 5 rats in each group: control, ulcer control (30 mg/kg Indo), Indo + reference standard (50 mg/kg Ranitidine hydrochloride), Indo + Thymol (75, 150, 250 and 500 mg/kg). Doses of Thymol were determined according to our preliminary studies based on the dose patterns of this agent and the previous studies.11 The animals were fasted for 24 h prior to the administration of each of control, standard and test compounds. The control rats received 1 mL distilled water orally by gavages. All substances used were dissolved in distilled water. Firstly, Indo (30 mg/kg, 0.5 mL) was administered orally to the above mentioned groups. After 10 minutes of this treatment, Thymol (1 mL/ rat) were given orally at the studied doses. Six hours after Indo treatment, the rats were anesthetized using Sevoflurane (3.5vol% and 30% oxygen: 70% N2O) for a 30-min experimental period using a rat-adapted masks. A longitudinal incision was made 2 cm to in the upper abdomen and livers were dissected.
Estimation of serum biochemical parametersLiver function enzymes including serum AST, ALT, and LDH were determined in accordance with methods provided by the diagnostic kits (Bioclinica).12
Preparation of tissue homogenatesFresh tissues were rinsed with ice-cold saline and immediately stored at -80 °C. The tissue specimens were weighed and then homogenized in a PBS at pH 7.0. Homogenized liver specimens were then centrifuged at 10,000 rpm at 4 °C for 15 min to isolate the supernatant for subsequent analysis.
Determination of TNF-α, Caspase-3, PGE2, and eNOS levelsThe commercially available ELISA kits were used according to the manufacturers’ recommendation: Rat TNF-α kit (Biolegend San Diego, CA, USA), Caspase-3 activity kit (Beyotime Institute of Biotechnology, Haimen, China), PGE2 Express kit (Cayman Chemical Company, Ann Arbor, MI, US), eNOS kit (Korain Biotech, Junjiang Internatioanl Bldg., SH. China).
Determination of oxidative stressThe automated TAC and TOS assays were carried out in tissue homogenates using commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey).13
Histopathological examinations and assessmentsLiver sections were fixed in 10% formalin and then embedded in paraffin to form blocks. Later, the samples were then serially-sectioned (5 -μM thick) using a Leica RM2135 microtome (Leica, Berlin, Germany), mounted on glass slides and then stained using H&E solution. In order to determine the liver glycogen content, some slides were stained by PAS base following the manufacturers’ instruction (Sigma-Aldrich, PAS kit). The presence of an amyloid fibril protein was detected using Congo red-staining kit and then sections were examined under a light microscope by an experienced pathologist who was blinded to the treatment. The high-resolution pictures of samples (x200 and x400) were taken under bright field using an Olympus BX60 microscope.
Statistical analysisResults are expressed as the mean ± SD. Firstly, to see whether all data are normally distributed or not, they were analysed by Kolmogorov-Smirnov tests and then the differences in variance were analysed statistically using a one-way analysis of variance (ANOVA) test (GraphPad Prism 6.01, GraphPad Software, Inc.). Tukey’s test was used as a post. p < 0.05 was considered as statistically significant. The superscripts of a and b were used to compare the control and Indo groups with other studied groups and the degree of statistical significance was denoted such as a1: p < 0.05, a2: p < 0.01, a3: p < 0.001, a4: p < 0.0001.
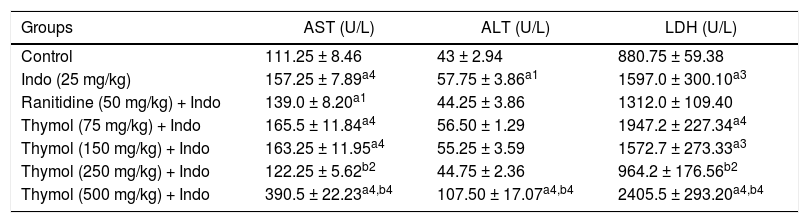
ResultsEffect of Thymol on the serum AST, ALT and LDH levelsThe levels of AST, ALT and LDH in serum of the control and experimental groups were shown in table 1. The levels of AST, ALT and LDH were significantly elevated in the Indo-treated rats. In rats treated with Thymol at doses of 75 and 150 mg/kg, no significant differences in the levels of AST, ALT, and LDH were observed in comparison with Indo group. However, treatment with Thymol 250 mg/kg significantly decreased these marker enzymes. On the other hand, treatment with Thymol 500 mg/kg remarkably increased the levels of the enzymes more than Indo treated group.
The effects of Thymol on serum AST, ALT and LDH in Indomethacin-induced liver injury in rat.
| Groups | AST (U/L) | ALT (U/L) | LDH (U/L) |
|---|---|---|---|
| Control | 111.25 ± 8.46 | 43 ± 2.94 | 880.75 ± 59.38 |
| Indo (25 mg/kg) | 157.25 ± 7.89a4 | 57.75 ± 3.86a1 | 1597.0 ± 300.10a3 |
| Ranitidine (50 mg/kg) + Indo | 139.0 ± 8.20a1 | 44.25 ± 3.86 | 1312.0 ± 109.40 |
| Thymol (75 mg/kg) + Indo | 165.5 ± 11.84a4 | 56.50 ± 1.29 | 1947.2 ± 227.34a4 |
| Thymol (150 mg/kg) + Indo | 163.25 ± 11.95a4 | 55.25 ± 3.59 | 1572.7 ± 273.33a3 |
| Thymol (250 mg/kg) + Indo | 122.25 ± 5.62b2 | 44.75 ± 2.36 | 964.2 ± 176.56b2 |
| Thymol (500 mg/kg) + Indo | 390.5 ± 22.23a4,b4 | 107.50 ± 17.07a4,b4 | 2405.5 ± 293.20a4,b4 |
Data are presented as means ± SD (n = 5). a denotes significant differences between other studied groups and control (a1: p < 0.05, a3: p < 0.001, a4: p < 0.0001), b denotes significant differences between other studied groups and Indo group (b2: p < 0.01, b4: p < 0.0001) by Tukey’s multiple range tests.
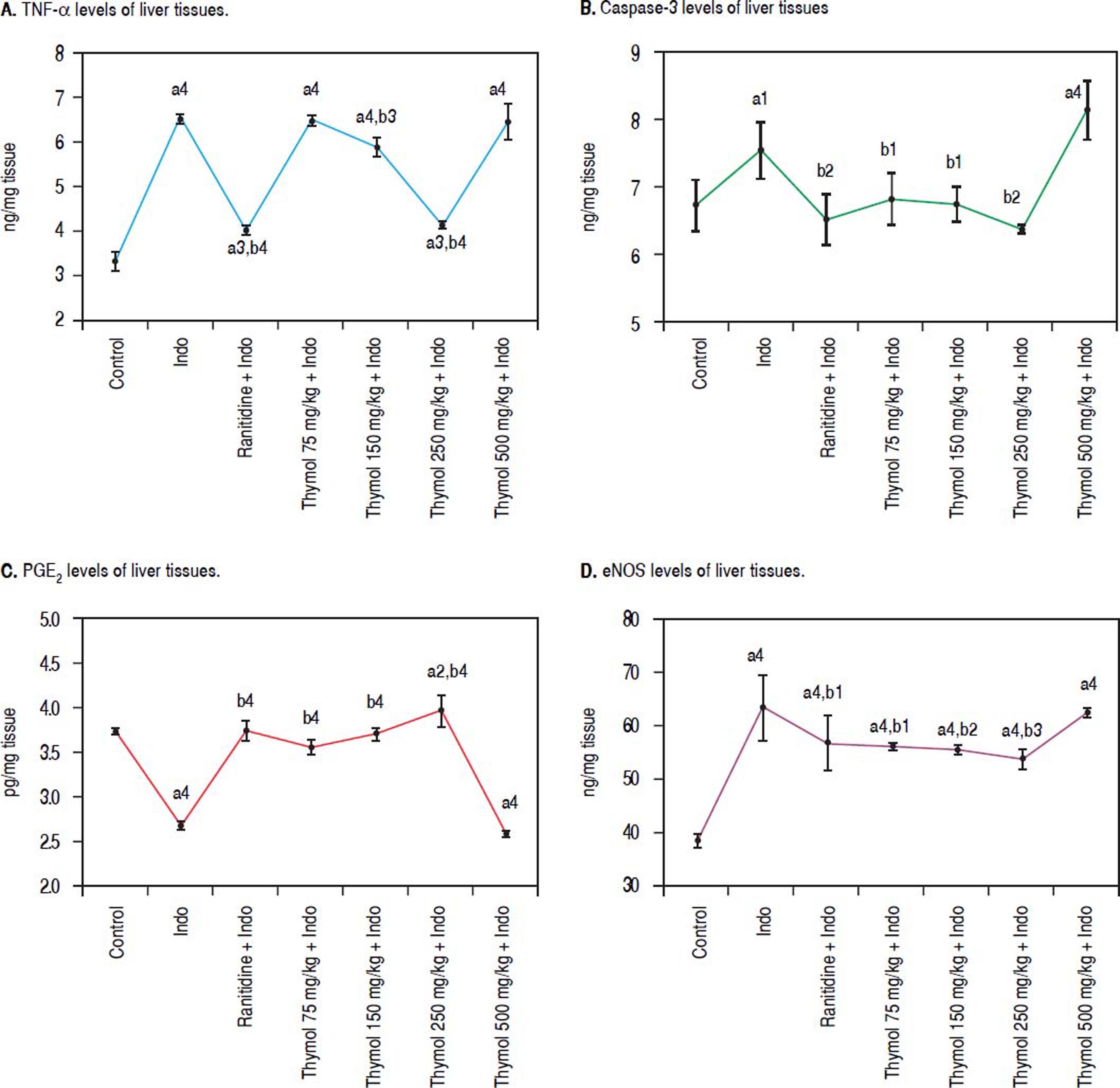
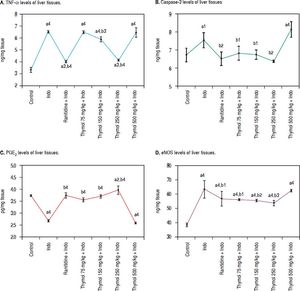
Compared to control, the levels of TNF-α showed significant increase in Indo-treated group. On the other hand, Thymol treatment at dose of 250 mg/kg also markedly decreased the of TNF-α level (p < 0.0001). Moreover, Thymol at the dose of 75 mg/kg rats did not alter the TNF-α evel when compared with Indo group. However, Thymol at the dose of 150 mg/kg slightly decreased this level. Also, the 500 mg/kg Thymol treatment did not affect the level of TNF-α stimulated by Indo (Figure 1A).
Effects of Thymol administration on liver treated with Indo. A. Level of TNF-α in liver was determined using an ELISA kit specific for rat. B. Level of caspase-3 in liver was determined using an ELISA kit specific for rat. C. Level of PGE2 in liver was determined using an ELISA kit specific for rat. D. Level of eNOS in liver was determined using an ELISA kit specific for rat. Data are presented as mean ± SD (n = 5).a denotes significant differences between other studied groups and control (a1: p < 0.05,a2: p < 0.01,a3: p < 0.001,a4: p < 0.0001), b denotes significant differences between other studied groups and Indo group (b1:p < 0.05, b22:p < 0.01, b3: p < 0.001, b4: p < 0.0001) by Tukey’s multiple range tests.
Administration of Indo significantly elevated the caspase-3 activity in the liver homogenates. On the other hand Thymol treatment at the tested doses except for 500 mg/kg significantly inhibited Indo-induced caspase-3 activation. Especially, this enzyme activity with 250 mg/kg dose of Thymol treatment was found to be similar to the control and Ranitidine treated groups (Figure 1B). On the other hand, Thymol at the dose of 500 mg/kg remarkably increased the caspase-3 activity more than Indo treated group (p < 0.0001).
Evaluation of PGE2 Level in LiverIndo administration resulted in a significant decrease in PGE2 level as compared with control animals (p < 0.0001). Controversially, Ranitidine treatment remarkably increased PGE2 levels with respect to the Indo treated group. Except for 500 mg/kg dose of Thymol, other tested doses increased PGE2 level in a dose-dependent manner. Indeed; 250 mg/kg dose of Thymol treatment led to a significant increase in PGE2 level (p < 0.0001). This dose exhibited more profound effect on PGE2 level than the effect of Ranitidine treatment. On the contrary, Thymol at the dose of 500 mg/kg significantly decreased PGE2 level which similar to Indo treatment (Figure 1C).
Effect of Thymol on Liver eNOS activityA significant increased eNOS level was observed with Indo administration. Treatment with Thymol at all doses prevented the elevation of the eNOS level as compared to the control group. A slight decreased eNOS level was observed with Ranitidine treatment. Moreover, the 250 mg/kg dose of Thymol treatment significantly prevented the elevation of the eNOS level with respect to the Ranitidine group. However, this dose could not reverse this level to the normal one. Oppositely, a significant increased levels of eNOS by the 500 mg/kg dose of Thymol administration was obtained which was similar to Indo group (Figure 1D).
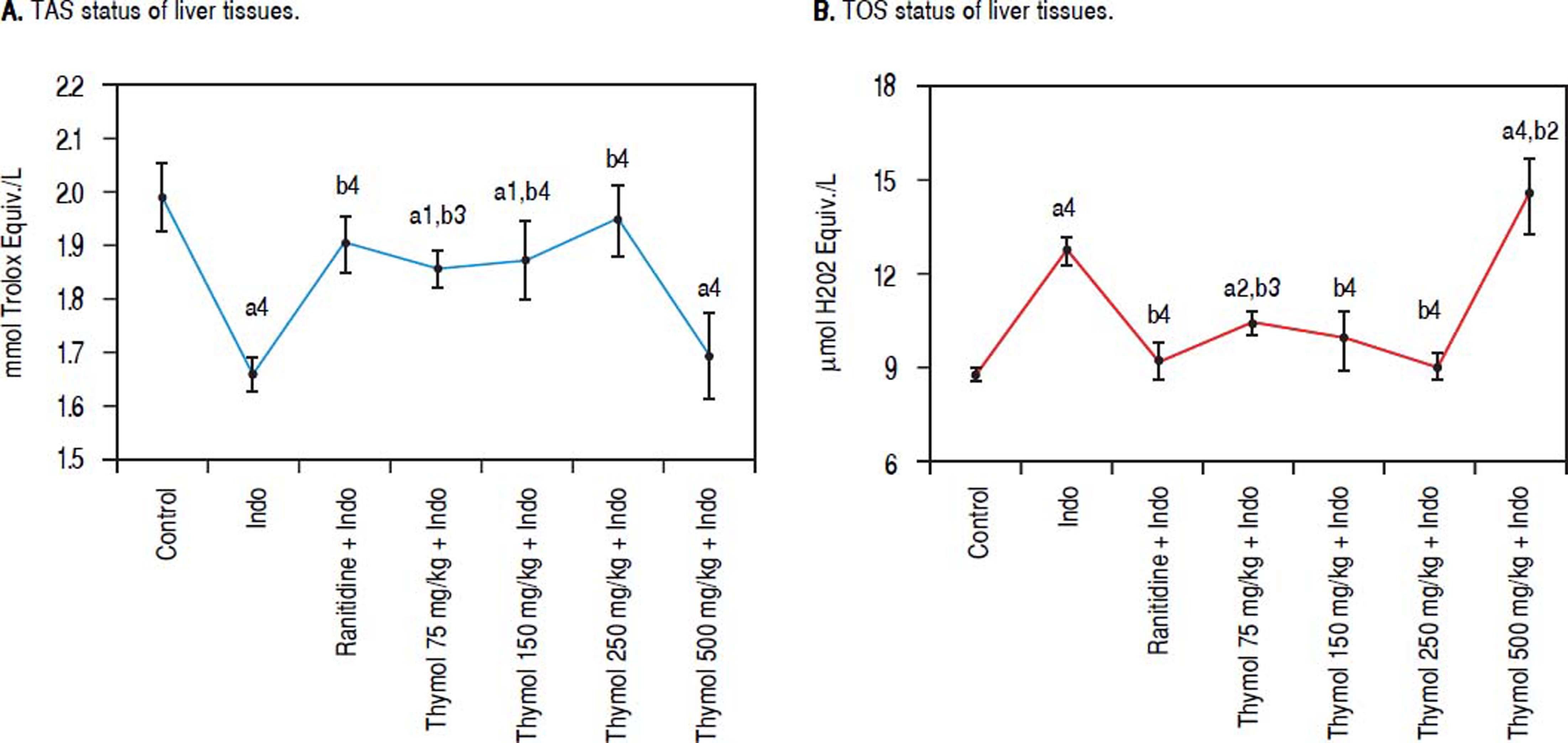
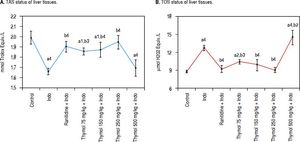
Effect of Thymol on antioxidant/oxidant capacity in liver tissueThe antioxidant/oxidant capacity of Thymol on Indotreated rats was determined by measuring TAC and TOS levels in liver homogenate (Figure 2A-B). Indo administration increased the TOS level while markedly decreased TAC level compared with the control rats. Treatment with Thymol at doses of 75, 150 or 250 mg/kg significantly reduced the elevated TOS level. Especially, the 250 mg/kg dose of Thymol significantly restored the TOS levels which were similar to the control one. On the contrary, TOS levels at the dose 500 mg/kg of Thymol were found to be statistically higher than that of Indo group. Moreover, a significant reduction in the TAC level was observed in the 500 mg/kg dose of Thymol treated group when compared with the control (p < 0.0001).
Effects of Thymol on liver oxidative stress level after treated with Indo. A. Level of TAS. B. Level of TOS. Data are presented as mean ± SD (n = 5).a denotes significant differences between other studied groups and control (a1:p < 0.05,a2:p < 0.01,a4:p < 0.0001), b denotes significant differences between other studied groups and Indo group (b2: p < 0.01, b3: p < 0.001, b4: p < 0.0001) by Tukey’s multiple range tests.
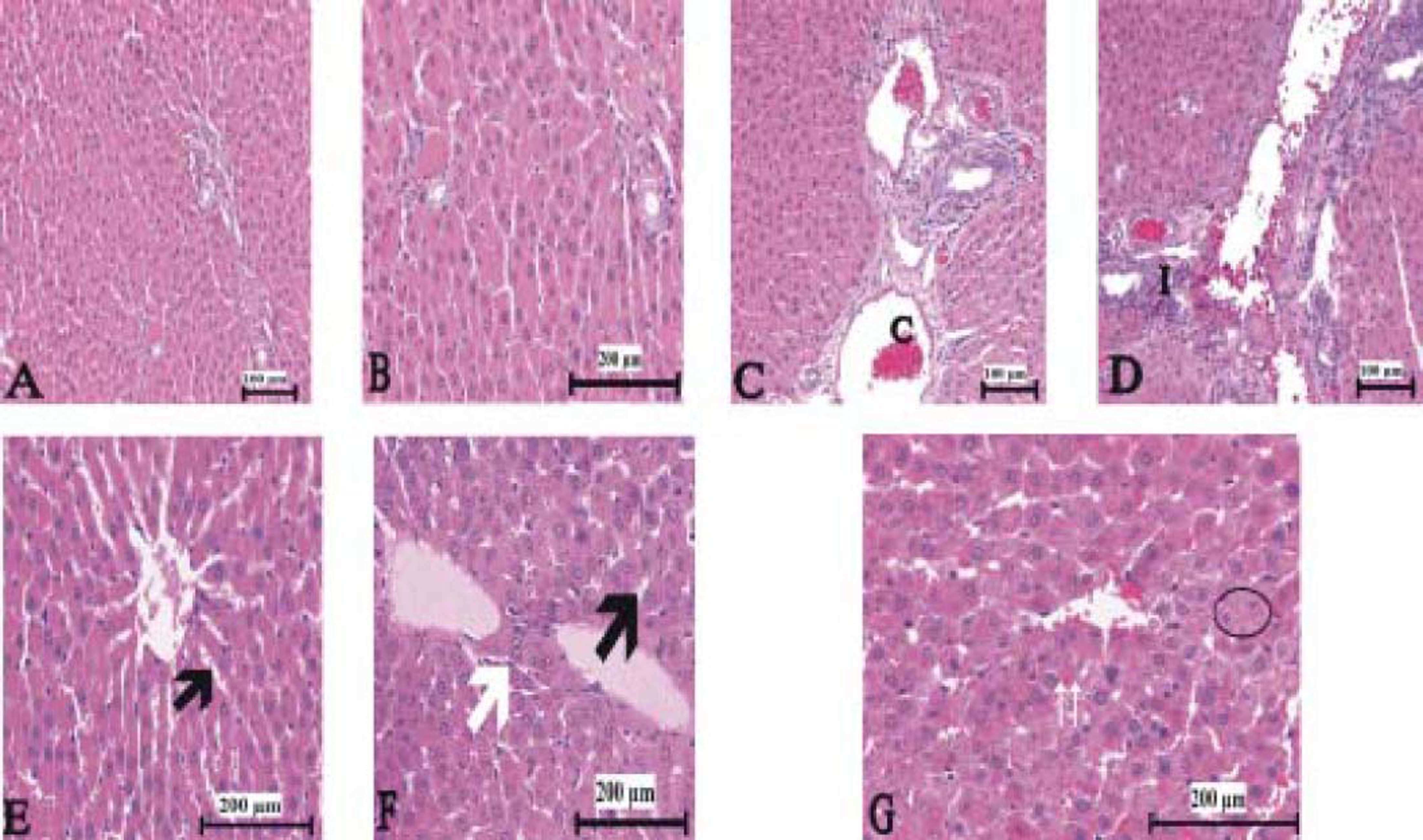
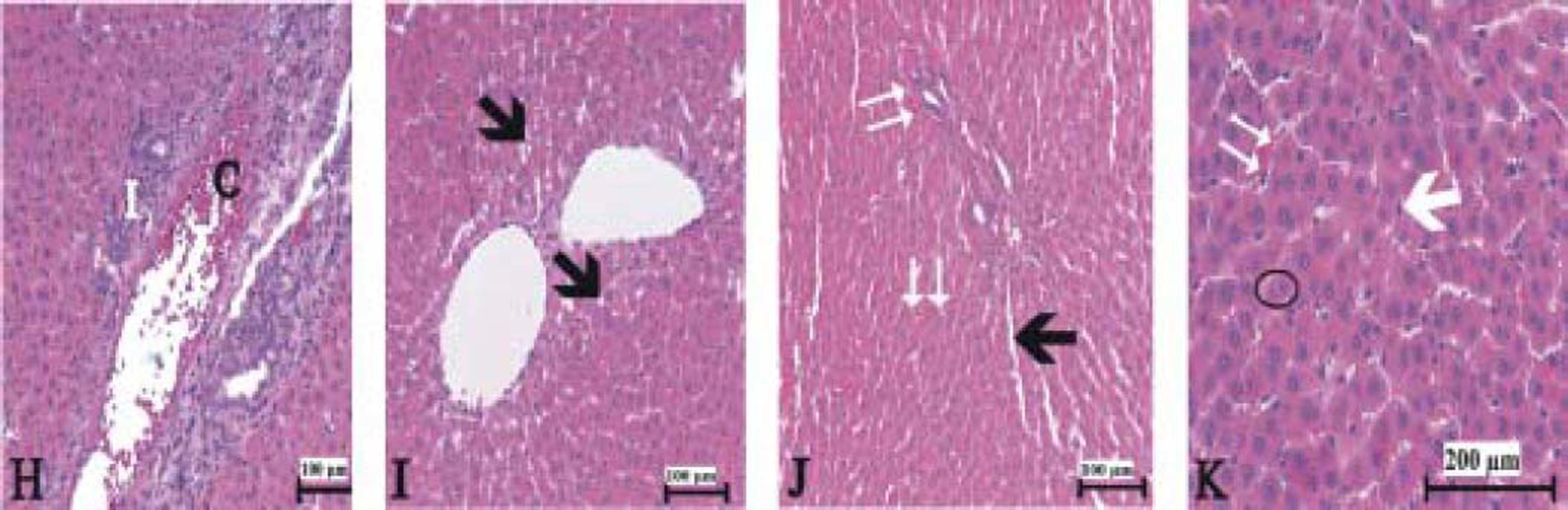
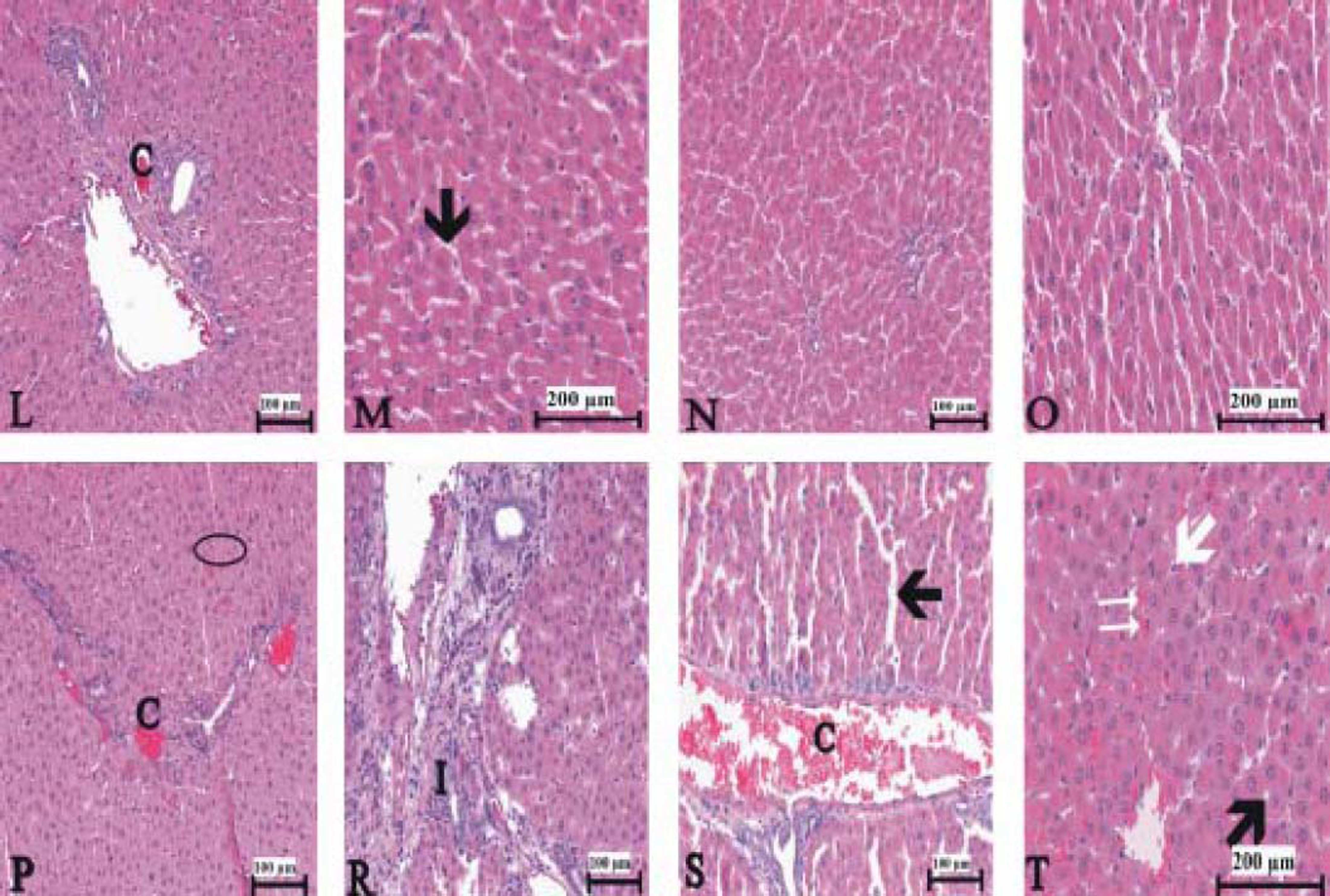
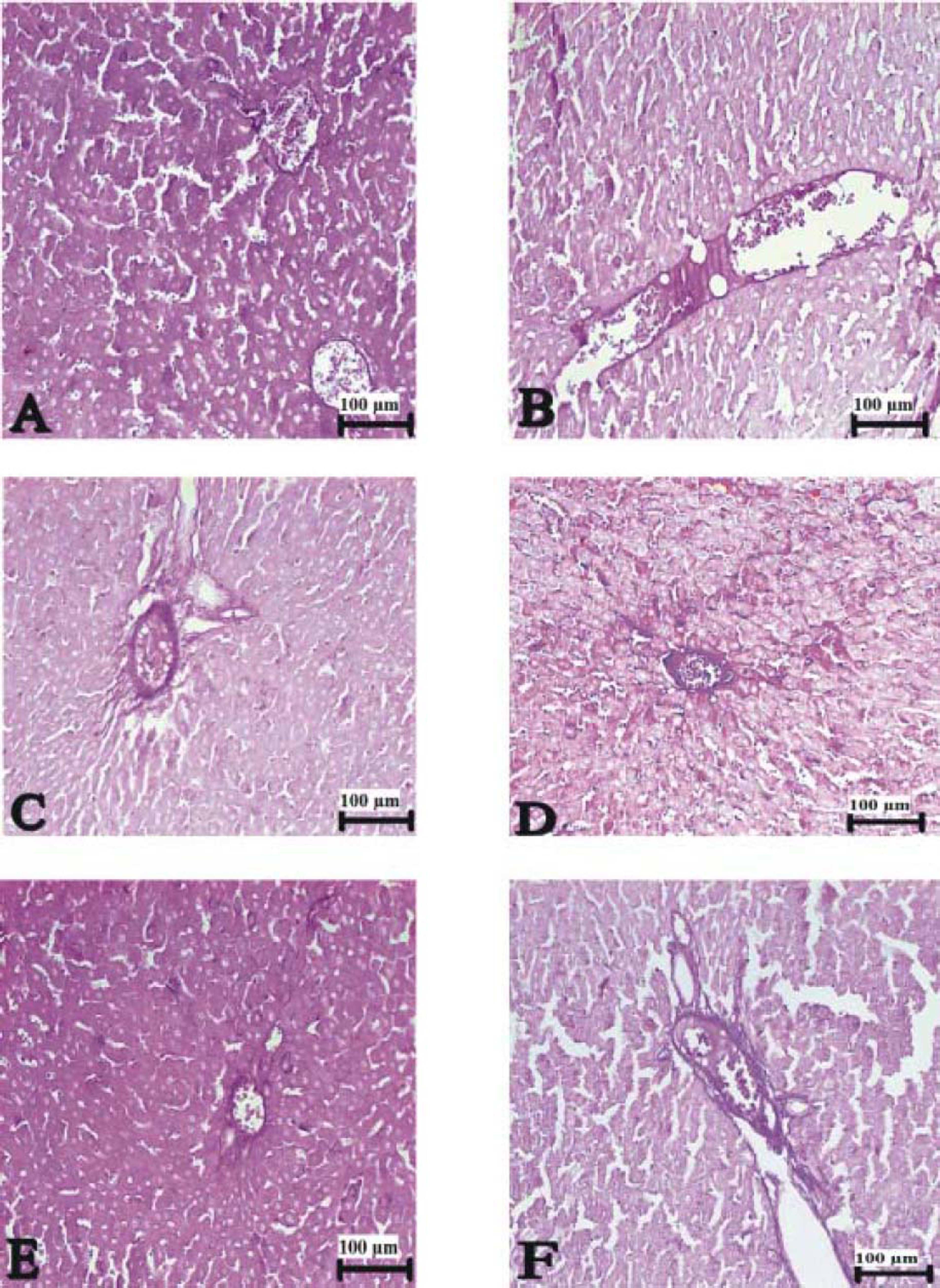
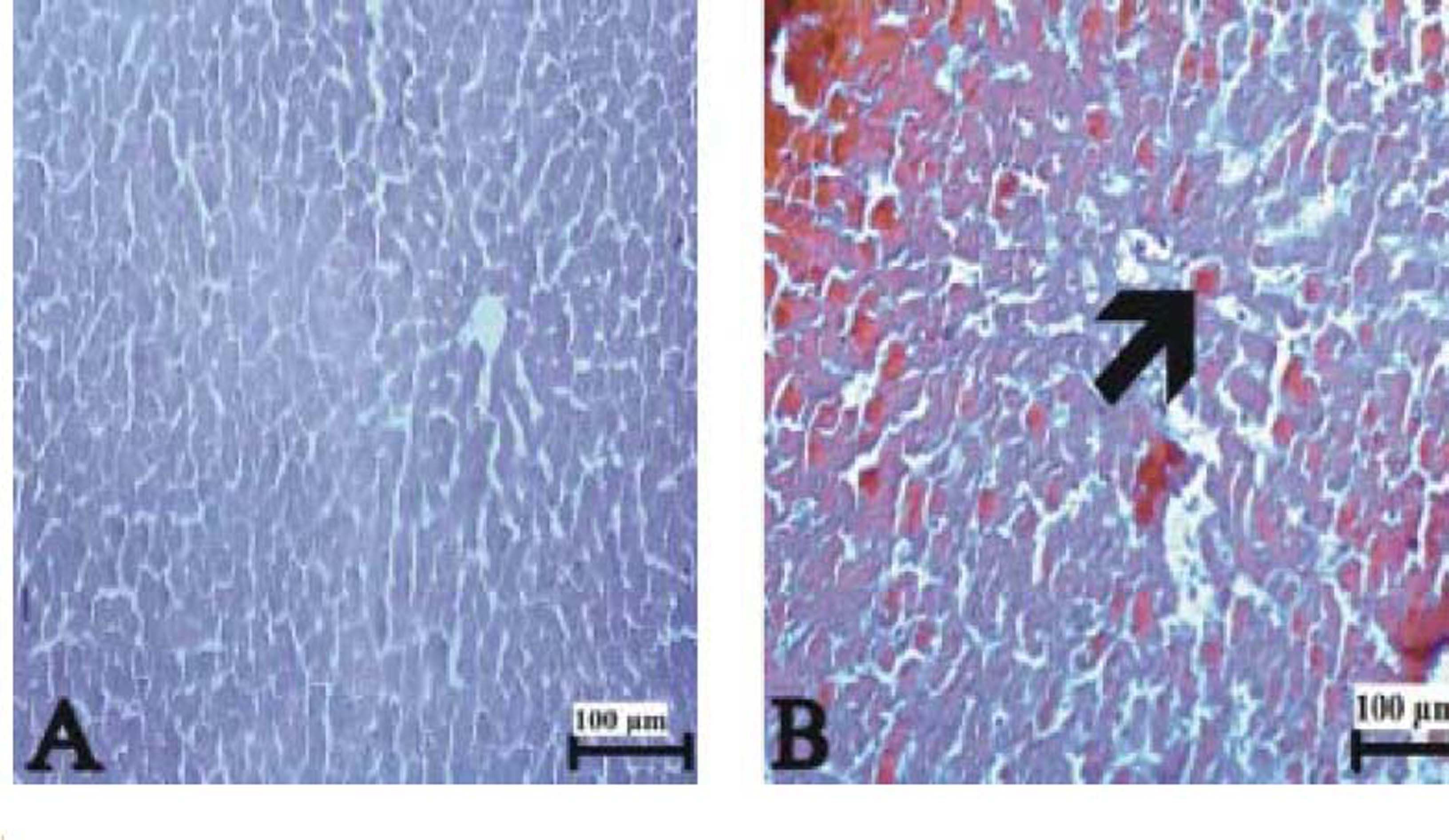
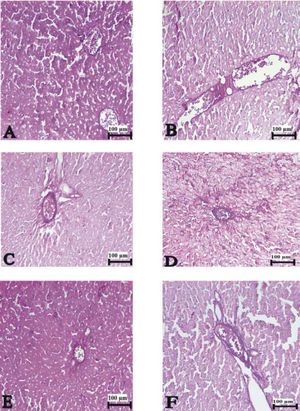
Figure 3(A-T), 4(A-F) and 5(A and B) demonstrated the histopathological analysis of control and experimental rat liver by H & E, PAS and Amyloid staining, respectively. The livers of the control rats showed normal hepatic architecture. H&E staining revealed that hepatic inflammatory cell infiltration, congestion, dilatation of sinusoids, haemorrhage, hepatocyte apoptosis and an increased Kupffer cells in Indo-treated rats (Figure 3A-G). While the administration of Thymol at dose of 75 mg/kg showed no histopathological amelioration of these effects (Figure 3H-K), at increased doses was observed reduction of congestion and dilatation of sinusoids (Figure 3L-M). Especially, treatment with the 250 mg/kg dose of Thymol clearly improved these histological changes in the liver (Figure 3N-O). In contrast, Thymol at the dose of 500 mg/ kg caused more serious damage than Indo in the rat liver (Figure 3P-T). Compared with controls, PAS staining showed a profound decreased in the amount of glycogen in hepatocytes with Indo treatment (Figure 4A-B). The 150 mg/kg dose of Thymol treatment caused a mild increase liver glycogen compared with Thymol at the dose of 75 mg/kg (Figure 4C-D). Moreover, the increased doses of Thymol elevated the amount of glycogen fairly similar to control. However, the highest dose of Thymol led to a significant depletion of glycogen (Figure 4E and F). Furthermore, no significant changes in the amyloid storage of the liver were observed in Indo treated group (data not shown). Conversely, Thymol at the dose of 500 mg/kg treatment caused to a significant accumulation of this protein in hepatocytes (Figure 5A-B).
Photomicrographs of liver sections stained with hematoxylin and eosin. A-B. Control rat liver. C-G. Indo treated [C: congestion. I: Infiltration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.
The liver sections stained with hematoxylin and eosin in 75 mg/kg Thymol + Indo treated rats. [C: congestion. I: Infilitration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.
150 μmg/kg Thymol + Indo treated rat liver [C: decreased congestion; small black arrow: decreased sinusoidal dilatation]. N-O. Normal liver histology in 250 μmg/kg Thymol + Indo treated rats. P-T. 500 ßmg/kg Thymol + Indo treated rat liver [The significant histopathological findings in hepatic tissue]. [C: congestion. I: Inflitration; Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm;. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.
The liver sections stained with PAS. (A) Control rat liver, (B) Pale and slightly stained hepatocytes in Indo treated, (C) 75 mg/kg Thymol + Indo treated (D) increased glycogen content in 150 ßmg/kg Thymol + Indo treated (E) PAS-positive glycogen stores in 250 ßmg/kg Thymol + Indo treated group, and (F) decreased glycogen content in liver of 500 μmg/kg Thymol + Indo treated rats. Bars: 100 μm.
Indo is one of the most commonly used NSAIDs to treat pain, inflammation, fever and to create gastric ulcer models in animals. Moreover, hepatotoxic effects of this drug have been reported.14 At present, the prevention and treatment of hepatic injury is of key importance and remains a major challenge in modern hepatology. Up to now, there is no efficient protective and preventive therapy for Indo-induced hepatotoxic effects. Therefore, the present study investigated the hepatoprotective effects of Thymol with a broad dosage range (75-500 mg/kg) on gastric ulcer models caused by oral administration of Indo in rats. To provide a better understanding of the possible mechanisms underlying the therapeutic effects of Thymol, the levels of proinflammatory cytokines was assessed. Thymol was chosen since it has protective effects on different organs including the stomach, heart and kidneys.15–17
The results of the present study showed that the administration of Indo (30 mg/kg) caused a significant elevation of serum hepatic enzymes levels, an elevation of TNF-α level, eNOS and caspase-3 activation, an induction of hepatic oxidative stress and histopathological changes. These effects indicated a severe liver dysfunction in rats with Indo treatment. Similarly, previous studies also indicated that the single application of Indo dose (25-40 mg/kg) orally caused liver toxicity.18,19 ALT is present prevalently in cytosol, whereas AST is present in both cytosol and mitochondria of hepatocytes.20 In the present study, AST levels were found higher than ALT level, implying the release of mitochondrial AST due to increased mitochondrial oxidative stress.21 In contrast to Indo administration, Thymol treatment markedly reduced the levels of AST, ALT and LDH (Table 1). In agreement with our results, Thymol (150 mg/kg) administration has been reported to prevent the Paracetamol (NSAIDs)-induced rises in serum enzymes in rats.6 Moreover, Indo significantly increased the levels of TNF-α and eNOS, and caspase-3 activation in tissues. In addition, Indo decreased the PGE2 level. Furthermore, it induced oxidative stress as evidenced by elevated TOS and decreased the TAC level. However, treatment with Thymol exhibited a significant improvement in these parameters. This improvement was more pronounced in the group treated with Thymol 250 mg/kg. Treatment with Thymol 75 and 100 mg/kg did not succeed to reverse the parameters. On the other hand, treatment with Thymol 500 mg/kg dramatically affected the parameters much worse than Indo treated group.
TNF-α is one of the potent pro-inflammatory cytokines secreted by activated numerous cell types in response to an inflammatory stimuli and plays a causal role in the development of liver injury.22 In the present study, the increased TNF-α level indicated an inflammatory reactions induced by Indo treatment. However, Thymol administration could prevent the enhancement of TNF-α levels (Figure 1A). In agreement this result, it has been reported that Thymol has strong ameliorative effects against hydrocortisone-induced oxidative stress injury in hepatic tissues by modulating and decreasing reverse levels of TNF-α and TOS.23 In addition, Thymol has been shown to protect HepG2 cells from Acetaminophen-induced apoptosis by down-regulating TNF-α level.24 This suggested that protective effects of Thymol on Indo-induced liver injury are at least in part, associated with inhibition of TNF-α production.
The hepatic damages of Indo may be due to Indo-induced apoptosis in hepatocytes. To evaluate this processes, the levels of Caspase-3 was measured since Caspase-3, the effector in the apoptotic cascade, plays a key role to trigger hepatocyte apoptosis.25 Caspase-3 is activated through two alternative pathways: 1) death receptors such as Fas/FasL and TNF-R1, and 2) intrinsic mitochondrial pathways.26 In the present study, caspase-3 activity significantly increased due to Indo treatment with respect to the control group (Figure 1B), implying that apoptosis triggered the development of liver damage. In contrast, treatment with Thymol resulted in a remarkable inhibition of Caspase-3 activities, suggesting that Thymol has a potential antiapoptotic effect on hepatocyte and this may mainly rely on the death receptor TNF-α pathway. Indo-increased TNF-α level may be related to the inhibition of PGE2 synthesis since Indo inhibited the synthesis of prostaglandin endoperoxide, that catalyze the first committed step in prostaglandin synthesis.27 On the other hand, the decreased TNF-α levels might be associated with a decreased release of TNF-a due to an elevated PGE2 synthesis since PGE2 has been reported to inhibit TNF-α release from macrophages.28 Therefore, Thymol-enhanced PGE2 level may prevent TNF-α release and cause a reduction in its level. These results suggested that Thymol may up-regulate PGE2 synthesis, thereby inhibiting TNF-α release, and thus may alleviate hepatic damage.
In addition to TNF-α, Caspase-3, and PGE2 levels, to assess the hepatic injuries of Indo and hepatoprotective activities of Thymol, e-NOS level was measured. NO plays an important role in regulating various physiological processes such as platelet inhibition, neurotransmission, apoptosis, blood vessel relaxation, immunity, and inflammatory.29 However, overproduction of NO is cytotoxic since it triggers formation of oxidative free radicals. Therefore, NO plays an important role in various kinds of liver injury.30 NO is generated by three isoforms of NOS: nNOS, iNOS, and eNOS.31 In the liver, eNOS is present in the hepatocytes, endothelium of hepatic arteries, terminal hepatic venules, and sinusoids.32 eNOS-derived NO has been known to be beneficial for liver function regulating, while iNOS- derived NO may be both beneficial and detrimental effects for liver homeostasis.33 It has been previously reported that hepatic damage may be caused by excessive NO production through iNOS; however, there is little information about the effects of NO production by increased eNOS on the liver and the role of high eNOS in the development of liver injury remains unclear.34 In the current study, the increased eNOS activity may be the reason of Indo-induced harmful effects on the liver. Thymol treatment decreased eNOS level, indicating that Thymol could attenuate Indo-induced liver injury (Figure 1D). This was also confirmed by attenuation of congestion in the hepatic histopathological examination. Moreover, Thymol (250 mg/kg) was able to keeps blood flowing properly. This is consistent with previous studies that the regular consumption of the polyphenol rich food may reduce incidence of vascular disorders and improve flow-mediated dilation.35,36
Oxidative stress; another factor contributing to liver damage, represents an imbalance between the production of ROS and the activity of antioxidant defence systems.37 ROS has been reported to be an important cytotoxic and signalling mediator in the initiation and progression of inflammatory liver diseases processes.38 Indo has been shown to increase the production of ROS in the hepatic tissue. Besides, the enhanced ROS level, Indo decreased the activity of antioxidant enzymes.39 In this study, the TOS has markedly increased in the liver treated with Indo in comparison with the control group (Figure 2B). This situation could be related to Indo-induced production of ROS. However, treatment with Thymol effectively restored the antioxidant/oxidant balance by preventing the increased TOS and rising TAS. These effects might be due to antioxidant and free radical scavenging properties of Thymol. It is well known that natural antioxidants play an important role in preventing the deleterious effects of toxic agents in hepatic tissue by scavenging for free radicals and/or by inhibiting free radical generation or by modulation of the inflammatory response.40,41 Thymol has been shown to act as scavengers of reactive oxygen species such as superoxide anion radical, hydroxyl radical and singlet oxygen.42 Moreover, the antioxidant effects of Thymol have been reported.43,44 This effect was related to an enhanced antioxidant enzyme activities such as superoxide dismutase, glutathione peroxidase and an increased levels non-enzymatic antioxidants such as vitamin C, vitamin E and reduced glutathione (GSH).16 Related to this, a high antioxidant enzyme activities and total antioxidant status were demonstrated in thymol-fed rats.45 In addition, the anti-inflammatory activity of Thymol may rely on its ability to prevent the inhibition of PGE2 or to reduce the production of several inflammatory factors in the hepatocytes, including TNF-α. Thymol has been also reported to inhibit evaluation of some proinflammatory mediators and prevent hepatocyte apoptosis.17,46
In addition to biochemical parameters, to assess Indo-induced structural damages on liver, histopathological changes were detected. A hepatic inflammatory cell infiltration, congestion, dilatation of sinusoids, haemorrhage, hepatocyte apoptosis and an increase in number of Kupffer cells were observed in Indo-treated group (Figures 3, 4, and 5). Conversely, Thymol treatment markedly ameliorated the hepatic damage induced by Indo. The liver of rats treated with Thymol at dose of 250 mg/kg showed a normal structure. It has been know that Kupffer cell function plays an important role in the pathogenesis induced by hepatotoxic compounds. Moreover, Kupffer cells has been demonstrated to increase drug-induced liver injury through the generation of superoxide anion by macrophage and cytokines.47 Therefore, a blocking of Kupffer cells function by Thymol administration could significantly reduce the hepatotoxicity. However, the treatment with the highest dose of Thymol led to significant pathological changes and the protein aggregations in liver. An amyloid deposition in hepatocytes was clearly observed in the Thymol group at the dose of 500 mg/kg. The main reasons of this amyloid plaque formations may elevated oxidative stress due to the highest dose of Thymol treatment since a higher TOS levels at the dose 500 mg/kg of Thymol was found as compared to the Indo-treated group.48 In addition to amyloid storage, the results obtained from PAS staining clearly demonstrated that treatment with Thymol (except for the overdose) significantly increased the glycogen accumulation, which reduced by Indo treatment. This suggests that mechanism of its hepatoprotective effect may be related to the increased production of glycogen. Thus our findings are an important pharmacological basis for the therapeutic potentials of Thymol in Indo-induced hepatic damages when compared with standard reference drug.
Consequently, the hepatoprotective mechanisms of Thymol may rely on its anti-inflammatory activity by inhibiting the release of proinflammatory mediators including TNF-α and eNOS, on its anti-oxidative activity by reducing the level of TOS and on its antiapoptotic activity, which probably depends on down-regulation of activated caspase-3. Therefore, Thymol can be an impressive hepatoprotective in drug toxicity; however, the high dose of this molecule should be taken into account for further examination.
Abbreviation- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
eNOS: endothelial nitric oxide synthase.
- •
FasL: fas ligand.
- •
H&E: hematoxylin and eosin.
- •
HepG2: human hepatocellular carcinoma.
- •
Indo: indomethacin.
- •
iNOS: inducible NOS.
- •
LDH: lactate dehydrogenase.
- •
nNOS: neuronal NOS.
- •
NO: nitric oxide.
- •
NOS: nitric oxide synthase.
- •
NSAIDs: non-steroidal anti-inflammatory drugs.
- •
PAS: periodic acid-Schiff.
- •
PBS: phosphate buffered saline.
- •
PGE2: prostaglandin E2.
- •
ROS: reactive oxygen species.
- •
TAC: total antioxidant capacity.
- •
TNF-R1: tumor necrosis factor-receptor 1.
- •
TNF-α: tumor necrosis factor-alpha.
- •
TOS: total oxidant status.
This work was supported by BAP (No. 2015/344) from Atatürk university.
Conflicts of InterestThe author declare that there is no conflict of interest regarding the publication of this article.




![Photomicrographs of liver sections stained with hematoxylin and eosin. A-B. Control rat liver. C-G. Indo treated [C: congestion. I: Infiltration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm. Photomicrographs of liver sections stained with hematoxylin and eosin. A-B. Control rat liver. C-G. Indo treated [C: congestion. I: Infiltration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.](https://static.elsevier.es/multimedia/16652681/0000001700000006/v1_201906150939/S1665268119310609/v1_201906150939/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![The liver sections stained with hematoxylin and eosin in 75 mg/kg Thymol + Indo treated rats. [C: congestion. I: Infilitration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm. The liver sections stained with hematoxylin and eosin in 75 mg/kg Thymol + Indo treated rats. [C: congestion. I: Infilitration. Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.](https://static.elsevier.es/multimedia/16652681/0000001700000006/v1_201906150939/S1665268119310609/v1_201906150939/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![150 μmg/kg Thymol + Indo treated rat liver [C: decreased congestion; small black arrow: decreased sinusoidal dilatation]. N-O. Normal liver histology in 250 μmg/kg Thymol + Indo treated rats. P-T. 500 ßmg/kg Thymol + Indo treated rat liver [The significant histopathological findings in hepatic tissue]. [C: congestion. I: Inflitration; Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm;. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm. 150 μmg/kg Thymol + Indo treated rat liver [C: decreased congestion; small black arrow: decreased sinusoidal dilatation]. N-O. Normal liver histology in 250 μmg/kg Thymol + Indo treated rats. P-T. 500 ßmg/kg Thymol + Indo treated rat liver [The significant histopathological findings in hepatic tissue]. [C: congestion. I: Inflitration; Small black arrow: sinusoidal dilatation. White arrow: kupffer cells. Big black arrow: vacuolar changes in cytoplasm;. Circle symbol. Double white arrow: hemorrhage]. Bars: 100 and 200 μm.](https://static.elsevier.es/multimedia/16652681/0000001700000006/v1_201906150939/S1665268119310609/v1_201906150939/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![The liver sections stained with Congo red. (A) Control rat liver, (B) amyloid deposits in liver of 500 μmg/kg Thymol + Indo treated rats. [Arrow: amyloid accumulation]. Bars: 100 μm. The liver sections stained with Congo red. (A) Control rat liver, (B) amyloid deposits in liver of 500 μmg/kg Thymol + Indo treated rats. [Arrow: amyloid accumulation]. Bars: 100 μm.](https://static.elsevier.es/multimedia/16652681/0000001700000006/v1_201906150939/S1665268119310609/v1_201906150939/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





