Hepatitis B virus (HBV) reactivation is a well-established complication of severe immunosuppression in patients with hematologic malignancy and positive hepatitis B surface antigen (HBsAg). Patients who receive high-dose chemotherapy, corticosteroids, rituximab, or have a bone marrow transplant are particularly at increased risk for HBV reactivation. However, limited information is available in the literature regarding HBV reactivation in patients with isolated anti-HBc, particularly in the setting of multiple myeloma (MM). We report two cases of HBV reactivation in MM patients with isolated anti-HBc positive with a rather atypical presentation. In conclusion, our cases highlight that clinicians need to be cognizant about this potentially fatal but preventable complication of chemotherapy and immunosuppression.
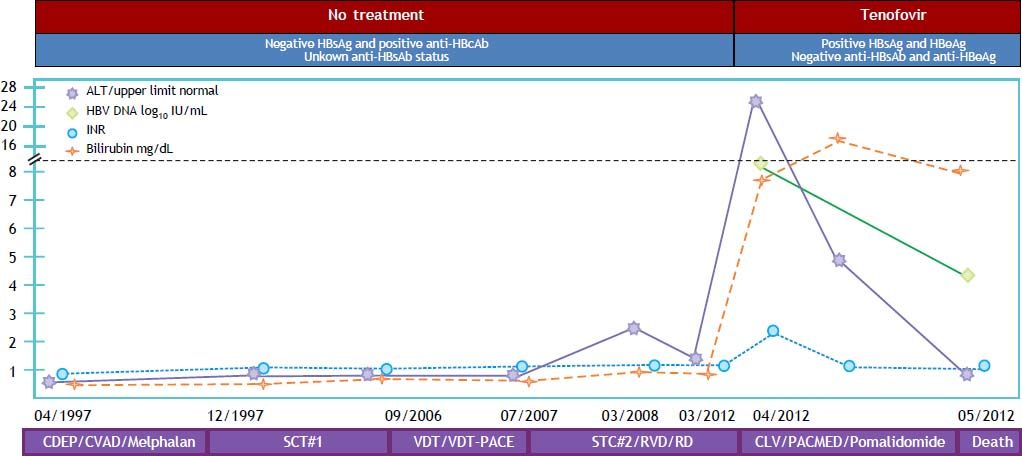
A 56-year-old Caucasian man was diagnosed with an IgG kappa MM, stage IIA, back in 1997. He received multiple chemotherapeutic regimens and two autologous peripheral stem cell transplant in 1997 and 2008 (Figure 1). Patient continued on multiple different chemotherapies after stem cell transplants and was started on monotherapy with pomalidomide when he was referred to our gastroenterology clinic for elevated aminotransferases with jaundice in April 2012. Mildly elevated aminotransferases had been observed for the first time at the time of second stem cell transplant, back in 2008. At that time, he was found to have a positive anti-HBc, along with a negative HBsAg; anti-HBs status and HBV DNA were not investigated. Other causes of liver disease were ruled out, and abnormalities in liver function tests were deemed to be an adverse effect from chemotherapy. Subsequently, aminotransferases remained mostly within the normal range, with brief and intermittent elevations up to 3 times the upper limit of normal. When he was referred to gastroenterology clinic for futher evaluation of abnormal liver function test and jaundice (April/2012), laboratory tests were significant for ALT of 820 IU/mL, AST of 1,001 IU/mL, total bilirubin of 7.5 mg/dL, INR of 1.8 with positive HBsAg, hepatitis B e antigen (HBeAg), and HBV DNA > 8.2 log10 IU/mL. HBV infection was thought to be sexually transmitted in the remote past. There was no evidence of hepatic encephalopathy. Treatment with tenofovir 300 mg/d was started, and other viral hepatitis, including hepatitis delta, were ruled out. There was no evidence of cirrhosis or portal hypertension by means of imaging and endoscopic studies. After four weeks of antiviral treatment a decrease in HBV DNA to 4.3 log10 IU/mL was observed, along with normalization of aminotransferases and INR, and a marked decrease in total bilirubin. Patient showed a remarkable clinical improvement, but had to be readmitted to hospital due to a sudden onset of exertion dyspnea. He was found to have severe pulmonary hypertension and S. aureus bacteremia. During hospitalization he developed hypotension responsive to vasopressors, acute kidney injury with metabolic acidosis, which resulted in pulseless electrical activity from which he was resuscitated. Patient was found to have hypoxic-ischemic encephalopathy with poor response to stimuli. Support was withdrawn and patient expired 11 days after admission.
Reactivation of HBV in case no. 1. CDEP: cyclophosphamide, dexamethasone, etoposide, and cysplatin. CLV: carfilzomib, lenalidomide, and vorinostat. CVAD: cyclophosphamide, vincristine, doxorubicin, and prednisolone. PACMED: cisplatin, cytarabine, cyclophosphamide, mesna, etoposide, and dexamethasone. RD: lenalidomide and dexamethasone. RVD: lenalidomide, bortezomib, and dexamethasone. SCT: stem cell transplant. VDT: velcade, dexamethasone, and thalidomide. VDT-PACE-VDT: plus cisplatin, doxorubicin, cyclophosphamide, and etoposide.
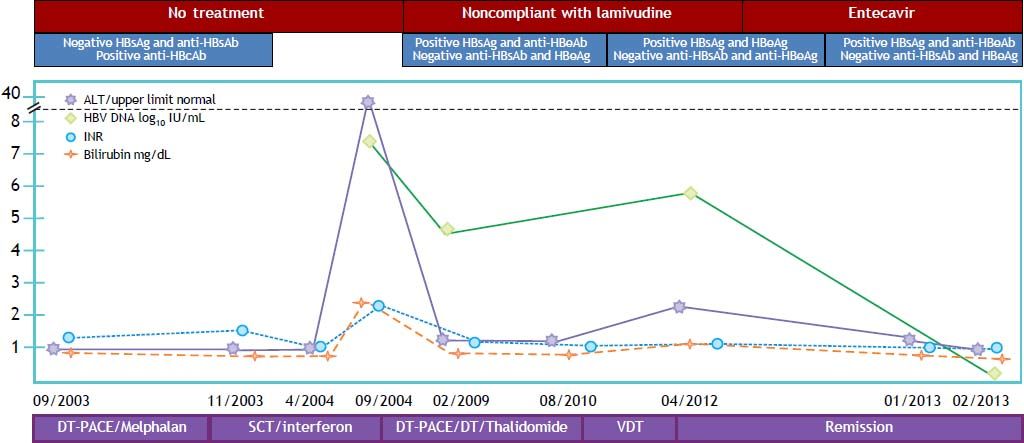
A 77-year-old Caucasian man was diagnosed with IgG kappa MM, stage IIIA in September 2003, and treated with an autologous peripheral stem cell transplant in November 2003 (Figure 2). Patient continued on consolidation and maintenance chemotherapy. After confirmed progressive MM in July 2010, he received re-induction and consolidation chemotherapy and remained free from any chemotherapy since 2011. MM is currently considered to be in near-complete remission. Prior to any chemotherapy and stem cell transplant, patient had negative HBsAg and anti-HBs, but positive anti-HBc with completely normal liver enzymes. The patient developed a self-limited hepatitis flare with peak ALT of 1,323 IU/mL, AST of 987 IU/mL, total bilirubin of 2.1 mg/dL, and INR of 2.3 with HBV DNA at 7.5 log10 IU/mL in September 2004. He remained with intermittently elevated aminotrans-ferases no more that 2 times the upper limit of normal. Positive HBsAg was first documented in February 2009, but patient did not start treatment (lamivudine 100 mg/d) until May 2010 due to compliance issues. Multiple sexual partners and transfusions were identified as main risk factors for HBV infection. By the time when treatment was started HBeAg was negative, anti-HBe positive, and HBV DNA was at 4.69 log10 IU/mL. In April 2012 patient had experienced HBeAg seroreversion, and HBV DNA increased to 6.0 log10 IU/mL due to poor compliance with lamivudine treatment. He was switched to entecavir 1 mg/d with an initial favorable response characterized by normalization of liver enzymes, and HBeAg seroconversion after three months of therapy. Patient continues on antiviral treatment with no complications and no evidence of cirrhosis or portal hypertension, with last available HBV DNA at < 1 log10 IU/mL.
DiscussionThe two patients we are presenting developed HBV reactivation while receiving chemotherapy after autologous peripheral stem cell transplant for MM, one of them characterized by a severe HBV hepatic flare that likely could have contributed to his death to some extent. Interestingly, both of them had isolated positive anti-HBc with negative HBsAg before reactivation. It is well known that patients with chronic HBV infection (i.e. positive HBsAg) can have HBV reactivation while or after receiving chemotherapy, immunosuppressive medicine, or anti-TNF medication.1 In oncological patients exposed to chemotherapy, HBV reactivation occurs in about 34% of those with a positive HBsAg.2 Although less frequently described, patients with isolated anti-HBc antibodies (i.e. with a negative HBsAg) can also develop HBV reactivation, as this has been observed in 0.7 to 3% of patients after chemotherapy.3 Likely, a positive anti-HBs antibody in this group of patients confers protection against HBV reactivation, particularly when present at high titers.4 In cases with allogeneic hematopoietic stem cell transplantation protection from anti-HBs antibodies (or lost of) is driven by serology of the do-nor.5 On rare instances, anti-HBs may be the only marker disclosing a remote and resolved HBV infection. Time frame for HBV reactivation after initiation of chemotherapy has been reported from 4 to 36 weeks.3,6 It is remarkable, however, that our patients presented with HBV reactivation 1 and 11 years after initial treatment for MM.
Mortality from HBV reactivation is estimated to be around 7%, and it supervenes as a consequence of acute liver failure.2 However, antiviral prophylaxis with lamivudine in patients with a positive HBsAg undergoing chemotherapy decreases the chance of reactivation by at least 79%, and reduces mortality to 2%. Prophylactic lamivudine also decreases interruptions of chemotherapy and reduces cancer-related mortality.2 In spite of this, HBV testing prior to chemotherapy is still not a common practice, and it is performed in < 20% of oncological patients according to some studies.7,8
Some of the main risk factors identified for HBV reactivation in patients undergoing chemotherapy are hematologic malignancies (particularly lymphoma), long-term corticosteroid therapy, use of rituximab, and bone marrow transplantation. HBV reactivation in patients with isolated anti-HBc has been particularly described after treatment with rituximab. In one study which included 104 patients with diffuse large B cell lymphoma, 46 of whom (44.2%) were HBsAg negative/anti-HBc positive, a total of 25 of patients were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), and none had HBV reactivation. In contrast, among the 21 patients treated with rituximab plus CHOP, five developed HBV reactivation, including one patient who died of hepatic failure.9 A recent meta-analysis which included 183 cases of rituximab-associated HBV reactivation showed that rituximab-based therapy had 5.7 times increased risk of HBV reactivation compared with nonrituximab-treated group among patients with isolated anti-HBc.10
Several studies from Asian countries showed cases of HBV reactivation in MM patients with negative HBsAg and positive/negative anti-HBc. A recent case report described a patient with MM with negative HBsAg, anti-HBc, and HBV DNA, but positive anti-HBs, who developed HBV reactivation while on salvage treatment with bortezomib. In this report, the patient had monthly monitoring of HBV DNA, and serum HBV DNA level became detectable after 10 cycles of bortezomib treatment. HBV DNA became undetectable 3 weeks after entecavir was started.11 Another study showed two cases of HBV reactivation in a single center retrospective and prospective combined study. Retrospective chart review identified one patient with negative HBsAg and antiHBc, but positive anti-HBs who developed HBV reactivation after the initial treatment of autologous peripheral stem cell transplant followed by ranimustine, cyclophosphamide, and prednisolone salvage treatment after the initial recurrence of MM. The prospective part of the study identified HBV reactivation in 1 out of 15 patients (6.6%) with negative HBsAg, positive anti-HBc and anti-HBs prior to treatment. Serial monitoring of HBV DNA yielded a detectable viral load 10 months after autologous peripheral stem cell transplant. HBV DNA turned un- detectable promptly after the initiation of entecavir treatment.12 A single center study from Singapore with 15 HBV patients with MM showed that 20% of patients with lamivudine prophylaxis developed HBV reactivation. High-dose chemotherapy with stem cell transplant followed by maintenance thalidomide was a significant risk factor for HBV reactivation.13
On a provisional clinical opinion from the American Society of Clinical Oncology (ASCO), it was sug gested that there was insufficient data to test for HBV markers as a routine in all patients with cancer prior to initiation of cytotoxic or immunosuppressive therapy. Personalized screening with HBsAg determination based on high risk for HBV exposure or risk of reactivation was advised.14 The Centers for Disease Control and Prevention (CDC) and the American Association for the Study of Liver Diseases (AASLD) have included the determination of anti HBc along with HBsAg in their recommendations,15,16 although the CDC also favored universal screening rather than limiting screening only to patients presenting risk factors for HBV infection.17 A recent cost-effectiveness analysis on HBV screening with HBsAg in lymphoma patients before chemotherapy has shown a small but robust benefit when the universal-screening strategy was compared to the recommended targeted screening for high-risk patients. The predicted benefit was comparable to other well-established oncology screening practices (i.e. mammogram or prostate-specific antigen) and was associated with a 10-fold decrease in the risk of HBV reactivation. The study did not address a possible role for screening with anti-HBc as well.18
Although routine prophylaxis with lamivudine or an antiviral with a high barrier to resistance (when treatment is planned for ≥ 12 months) is a standard recommendation for patients with a positive HBsAg at the time of initiating chemotherapy, the AASLD has stated that there is not enough information to advise on the use of routine prophylaxis for patients with isolated anti-HBc. Thus, current recommendation is to closely monitor these patients with serial HBV DNA determinations, in order to start antiviral therapy pre-emptively as soon as HBV DNA turns positive, or immediately after HBV reactivation becomes manifest.15 There is, however, no known safe interval for HBV DNA monitoring although it has been suggested to have HBV DNA checked every 1-3 months. For this reason, some favor the use of prophylactic treatment with antivi-rals, just like in HBsAg positive patients. Given a prevalence of isolated anti-HBc of about 5% in the US,19 and the low risk of HBV reactivation in this particular group, this strategy will end up in over treating a significant number of patients. No cost-effectiveness analyses are available to better guide such decision. As the majority of patients respond to treatment when this is started early in the course of the hepatitic flare, vigilance of HBV DNA and aminotransferases sounds reasonable for isolated antiHBc patients undergoing chemotherapy.
The MM patients represent a particular subpopulation of hematologic malignancies undergoing aggressive cytotoxic and immunosuppressive therapy, along with multiple stem cell transplantations. Given the dreadful consequences of HBV reactivation we consider it will be prudent to check for HBsAg, and anti-HBc in all MM patients before starting chemotherapy. Those with positive HBsAg should undergo antiviral prophylaxis, as per AASLD guidelines, whereas patients with isolated anti-HBc should enter a close HBV DNA monitoring with monthly determinations during peaks of immune suppression, and otherwise trimonthly. Due to the clinical course of MM, it is likely that most patients would benefit more from having antiviral prophylaxis with an agent with a high barrier to resistance (i.e. entecavir or tenofovir), than from lamivudine. However, compliance with medications and risk for development of resistance to antivirals needs to be weighed when taking this decision. AASLD, and CDC are putting their efforts together to gain better knowledge on what the proper care of patients at risk for HBV reactivation should be. In the meantime, and while some more evidence becomes available, clinicians taking care of patients with MM need to be cognizant about this potentially fatal but preventable complication of chemotherapy and immunosuppression.