Immune Checkpoint Inhibitors (ICI) have shifted the paradigm of cancer therapy treatment. Despite their efficacy, ICIs may induce immune-related adverse events (irAE), which can affect various organs, namely the liver. This study intends to perform a comprehensive clinical description of the hepatic irAEs associated with ICI in a Portuguese population of a tertiary hospital centre.
Materials and methodsA retrospective analysis of patients who developed immune-mediated liver injury (IMLI), among a cohort of patients treated with ICIs between March 15th of 2015 and December 15th of 2019 in a tertiary hospital. We used both Common Terminology Criteria for Adverse Events (CTCAE) and Drug‐Induced Liver Injury Network (DILIN) criteria to define liver injury.
ResultsAmong 151 patients, eight (5.3%) patients developed liver injury grade ≥3, of which five had hepatic metastasis. As such, only 3 cases were classified as IMLI. All IMLI presented with cholestasis pattern; the median duration from ICI initiation to IMLI was 84 days and/or 4 ICI cycles; one patient registered IMLI one month after nivolumab suspension; all were treated with steroids and one was successfully submitted to ICI re-challenge; a favourable outcome was seen in all patients; the median time to hepatic biochemistries normalization was 150 days. Among 10 patients with previous hepatic conditions, only one developed liver injury grade 2.
ConclusionsClinically significant ICI-related hepatotoxicity was uncommon; Immune-mediated liver injury may present a cholestatic pattern predominance. There was a low rate of liver injury of any kind in patients with previous hepatic disease while on ICI.
Immunotherapy has shifted the paradigm from directly treating tumour cells to enhancing the hosts’ immune system and has recently been a source of promising new cancer treatments especially for metastatic disease. Immune checkpoint blockade (ICB) increases antitumor immunity by blocking intrinsic downregulators, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1) [1]. Immune checkpoint inhibitors (ICI) are monoclonal antibodies against these immunity downregulators: nivolumab and pembrolizumab (anti-PD-1); atezolizumab (anti-PD-L1); ipilimumab and tremelimumab (anti-CTLA-4).
Immune-related adverse events (irAE) is the term for ICB's inflammatory side effects consequential to its increase in the activity of the immune system. These irAE regularly involve the gastrointestinal tract, endocrine glands, skin and liver. However, they may affect any organ system [2]. Immune-mediated liver injury (IMLI) has a reported incidence of 2–9% in monotherapy, and may be up to 18% in patients treated with combination of anti-PD-1 and anti-CTLA-4 [3,4].
The pathophysiology underlying irAE is unknown but is theorized that it relates to the role of immune checkpoints in immunologic homeostasis maintenance. It is hypothesized that in addition to T-cell–mediated immunity, anti–PD-1 or anti–PD-L1 treatment modulates humoral immunity, enhancing pre-existing auto-antibodies [5]. The extent to which autoantibodies (rather than autoreactive T cells) contribute to irAE remains unknown and may differ between different toxic effects. Cytokines may also play a role on development of this adverse effects [1].
IrAEs may warrant therapy's cessation or the administration of immunosuppressive agents [6]. No prospective trials have defined strategies for effectively managing specific irAE. Further clarification of irAE's characteristics is warranted, considering the consequences of interrupting a potentially life-saving treatment and the adverse effects of long-term corticosteroids in high-doses. In this study we perform a comprehensive clinical description of the hepatic irAEs associated with ICI in a Portuguese population of a tertiary centre hospital.
2Materials and methodsThis retrospective study was conducted at the Centro Hospitalar Universitário do Porto with approval of the institution's Ethics Committee.
2.1PatientsWe included patients older than 18 years old, who were (1) exposed to one or more ICIs between March 15th of 2015 and December 15th of 2019 that (2) were submitted at least to a one cycle of ICI in our hospital. Nineteen patients were excluded due to lack of clinical information or loss of follow-up in this institution.
2.2Clinical informationClinical data was obtained retrospectively from the electronic medical records.
Data collected included demographic information (age at the time of ICI initiation and sex); smoke history; comorbid illnesses (based on the Charlson index) [7]; and presence of an auto-immune disease. Alcohol use was not obtained because of the heterogeneity of its characterization according to each oncologist. Clinical notes were also collected.
Oncologic information such as underlying cancer type and the presence of hepatic metastasis based on imagiological data, was included. The type, and duration of ICI therapy, as well as occurrence of nonhepatic irAEs, were noted.
Biological data included aspartate aminotransferase (AST, normal range 10-30 U/L for women and 10-34 U/L), alanine aminotransferase (ALT, normal range 10-36U/L for women and 10-44 U/L for men), total bilirubin (Bil-T, normal range, <1mg/dL), alkaline phosphatase (ALP, normal range 35-104 U/L for women and 40-129 U/L for men), gamma-glutamyltransferase (GGT, normal range 6-39U/L for women and 10-66 U/L for men). Blood tests were performed per protocol before each immunotherapy injection.
2.3Liver injury definitionBaseline liver biochemistries of serum AST, ALT, ALP and Bil-T were defined as those results obtained immediately prior to the first ICI dose.
Currently, adverse events in oncology trials are often graded using the US National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). These criteria grade adverse events (including irAEs) on a scale of 1–5, denoting: 1- mild reactions; 2- moderate reactions; 3- severe reactions; 4- life-threatening events and 5- death [8].
According to CTCAE version 4.03 hepatoxicity is defined as grade 3 if: (1) AST or ALT ≥5-20 times upper limit of normality (x ULN) and/or (2) T-Bil>3-10 x ULN; and grade 4 if: (1) AST or ALT >20 x ULN and/or (2) Bil-T >10 x ULN.
According to Drug‐Induced Liver Injury Network (DILIN) [9] study criteria, drug-induced liver injury is defined as: (1) serum ALT ≥ 5x ULN; and/or (2) serum ALP ≥ 2x ULN; and/ or (3) Bil-T ≥ 2.5 mg/dL (or > 2x baseline if baseline > ULN).
In patients with abnormal liver tests prior to starting treatment with the implicated drug, ULN is replaced by the mean baseline values obtained prior to irAE onset and increases should be proportionate to this modified baseline.
In this paper, we considered patients with IMLI those having hepatotoxicity grade 3 or 4 according to CTCAE plus patients with ALP ≥2 X ULN and/or Bil-T ≥ 2.5 mg (according to DILIN) while on ICIs or after cessation of ICI therapy. Given the lack of specific biomarkers, the diagnosis of IMLI was an exclusion diagnosis, so exclusion of other reasons for its occurrence were considered, namely hepatic metastasis, evaluation of auto antibodies (antinuclear antibodies, anti-smooth muscle antibodies, anti-actin antibodies, namely anti-F-actin, anti-soluble liver antigen/liver pancreas antibodies, anti-neutrophil cytoplasmic antibodies, anti-mitochondrial antibodies, anti-DNA antibodies, anti-liver-kidney microsomal-1 and 3 antibodies, anti-liver cytosol antibody-1) and virologic serology (namely for hepatitis A, B, C and E).
The pattern of liver injury (LI) was classified using the R value (ratio of serum activity of ALT to ALP): ‘hepatocellular’ when there is a 5-fold or higher rise in ALT alone or when R is 5 or more; ‘cholestatic’ when there is a 2-fold or higher rise in ALP alone or when R is 2 or less; ‘mixed’ when R is between 2 and 5.
The peak liver biochemistry test results were recorded as the maximum values after meeting LI criteria; normalisation of liver biochemistries was determined when the abnormal lab test returned to ALT < 5 x ULN, ALP <2 x ULN or/and Bil-T < 2.5 x ULN. Laboratory results performed within the 3 months preceding ICI initiation were reviewed to exclude patients who had pre-ICI ALT ≥ 3 X ULN (CTCAE grade 1 or higher), typically used by oncologists as a threshold to determine eligibility for ICI initiation.
Clinical signs and symptoms at the time of hepatotoxicity were noted (abdominal pain, fever, jaundice and ascites). When available, liver histology's were assessed.
Previous hepatic disease was defined using evidence from imagiological, histological or analytical data; hepatic disease was then categorized according to medical records, imagiological, endoscopic, fibroscan, analytical and clinical criteria. All patients had an abdominal ultrasound or computed tomography before ICI initiation, to determine the presence of focal lesions in the liver or biliary tracts.
Outcomes assessed included ICI (1) treatment modifications, namely a) definitive suspension, b) transitory suspension, c) dose modification; (2) treatment with steroids and/or (3) need for other immunosuppressive treatments (4) requirement for hospitalization or intensive care unit admission; (5) occurrence of liver failure (6) recurrence of liver injury after ICI rechallenge; (6) death related to IMLI.
2.4Statistical analysisDescriptive statistics were calculated using mean (standard deviation [sd]) or median (range) for normally and nonnormally distributed data, respectively.
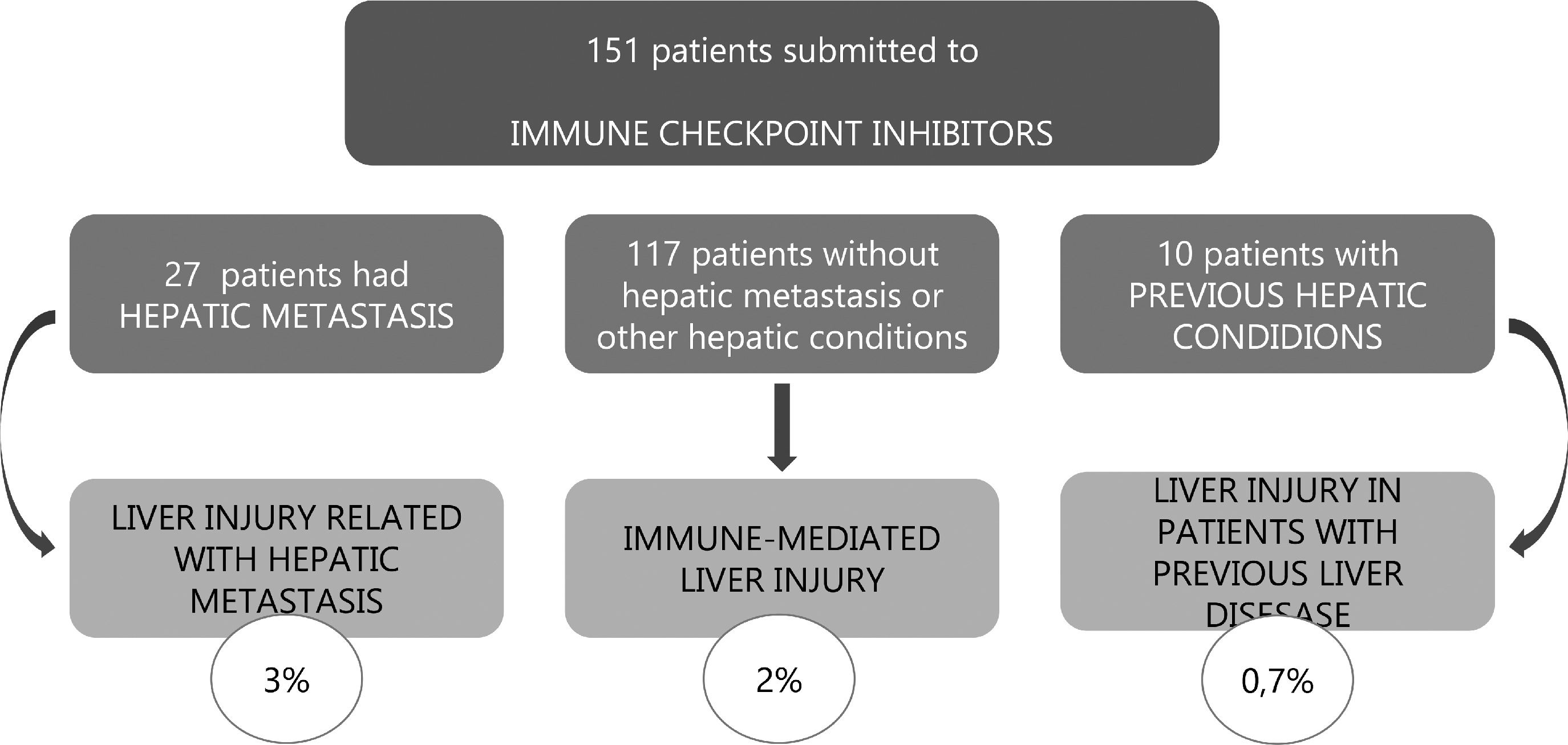
3Results3.1General characteristicsThere were 151 ICI recipients during the study period, the majority were men (76,8%). Of these, 5 received anti–CTLA-4 therapy, 145 received anti–PD-1/PD-L1 therapy, and 1 received combination therapy (anti-CTLA-4 plus anti-PD1). Overall population characteristics are detailed in Table 1. Patients had a median age at the beginning of treatment of 64 years (IQR 16). Lung cancer was the most common underlying malignancy (48%). Forty-seven all grade nonhepatic irAEs were recorded, with skin toxicities being the most common (28%), followed by endocrine (26%).
- Selected clinical characteristics of patients submitted to ICIs (N =151 patients)
Legend, ALP - alkaline phosphatase; ALT - alanine aminotransferase; AST - aspartate aminotransferase; Bil-T - total bilirubin; GGT - gamma-glutamyltransferase; IQR - interquartile range; irAE - immune-related adverse event; n – number; sd - standard deviation; yr – years;
Eight patients developed liver injury during or after ICI treatment, however 5 were not considered to have IMLI because they had hepatic metastasis (figure 1). Detailed clinical information of patients who developed liver injury is available on supplementary table 2.
3.2Immune-mediated liver injuryRegarding the 3 IMLI patients, 2 were men; mean age of 63.7 years. General characteristic of IMLI are presented in table 2. None had pre-existing liver injury. Concerning their baseline hepatic biochemistries they presented a median ALT 17 U/L, AST 22 U/L, ALP 82 U/L and GGT 26 U/L. The median duration from ICI initiation to IMLI was 84 days and/or 4 cycles of ICI. One patient registered IMLI one month after nivolumab suspension. Peak levels of laboratory tests included a median ALT 254 U/L (5 x ULN), ALP 627 U/L (4 x ULN) and T-Bil 0.67 mg/dL. No patient presented clinical signs or symptoms, namely fever, abdominal pain or jaundice. All patients presented a cholestatic pattern of IMLI and none was submitted to liver biopsy. Two patients suspended ICI treatment and all were submitted to steroid therapy. No patient required further immunosuppressive or other additional therapy. A favourable outcome was seen in all patients and the median time to hepatic biochemistries normalization was 150 days.
No patient presented clinical symptoms, and there was no need for hospital admittance in any case. No liver failure occurred and no IMLI death-related was reported.
One patient was submitted to ICI rechallenge with the same therapy and dosage with no recurrence of the liver injury.
3.3Hepatic metastasis and liver injuryRegarding the 5 patients with hepatic metastasis and liver injury, 2 were men; median age of 53 years. Its characteristic are detailed in Table 4. Their baseline hepatic biochemistries were median ALT 56 U/L, AST 34 U/L, ALP 128 U/L and GGT 148 U/L.
The median duration from ICI initiation to liver injury was 45 days and/or 2 cycles of ICI. Peak levels of laboratory tests included a median ALT 232 U/L (5 x ULN), ALP 750 U/L (6 x ULN) and Bil-T 0.9 mg/dL. Four patients presented a cholestatic and one hepatocellular pattern of liver injury. Four patients suspended ICI treatment and 2 were submitted to steroid therapy.
No patient presented clinical signs or symptoms, namely fever, abdominal pain or jaundice.
We would like to individualize a specific case (patient 7 on supplementary table 1) of a 62-year-old female patient with lung cancer that developed a hepatocellular liver injury 2 cycles after pembrolizumab. There was no evidence of hepatic metastasis before treatment. She was considered to have IMLI since other aetiologies were excluded and absence of hepatic metastasis was reassured with abdominal ultrasound, which prompt the beginning of steroids. The absence of response to steroids prompted a hepatic biopsy, which complicated with haemorrhagic shock and diffuse liver metastasis was diagnosed intra-operatively.
Table 4 is a comparative presentation of features of IMLI versus liver injury in patients with liver metastasis.
3.4Previous liver disease and liver injuryThere were 37 patients with liver conditions before ICI initiation: 27 had liver metastasis, 3 had hepatic steatosis defined by imaging, 2 had previous exposure to hepatitis B virus but no active infection and no current treatment, 4 had a past of chronic hepatitis C virus infection, of those 4 with fibroscan ≤9,5 and 1 with FS>9,5. One patient presented with non-treated chronic C hepatitis; one patient had cirrhosis of other causes and one patient had hepatocellular carcinoma (which was not the tumour undergoing treatment). All patients with cirrhosis were Child-Pugh A.
Only one patient with previous hepatic disease presented with grade 2 liver injury (figure 1): 50-year-old male with untreated chronic C hepatitis, who also had previous exposure to hepatitis B virus (negative HBs-Ag, positive anti-HBc and positive anti-HBs) but no active infection. He was submitted to pembrolizumab in the context of lung cancer. In the beginning of treatment, the patient presented detectable HCV viral load, and developed grade 2 liver injury after 4 cycles of therapy as well as an increase in HCV virus load, leading to treatment suspension during 2 cycles, which resulted in an improved analytical. He was rechallenged in the 7th cycle and completed a total of 16 cycles without any relapsed increase in liver enzymes.
4DiscussionIn this retrospective, single‐centre study, we describe a comprehensive serological, chronological, and clinical data of a set of patients with checkpoint inhibitor irAE. Similar real-world evaluations have been made over the last years in different regions of the globe (Table 4). Over a 4-year follow up, 2% of ICI patients developed hepatotoxicity ICI-related grade 3 or 4 according to both CTCAE and DILIN criteria, one patient under pembrolizumab and two under nivolumab. This data confirms the uncommon occurrence of this Drug Induced Liver Injury (DILI), which has been reported in 1–10% of patients during ICI monotherapy [10–13]. IMLI occurs less often with anti-PD-1 ICI alone, with a reported incidence of 1–4% of patients [4], which is the likely explanation of our low prevalence of IMLI, since most patients were treated with this drug class. Marie-Léa Gauci et al. [14] reported the occurrence of immune related hepatoxicity ≥grade 3 on 21 of 339 patients (6%) with advanced melanoma. Of them, seven (8%) were under anti-CTLA, 4 (1.7%) under anti-PD-1 (nivolumab n = 3, pembrolizumab= 1) and 10 (38%) under combination (ipilimumab plus nivolumab).
Given the lack of specific biomarkers, the diagnosis of DILI remains an exclusion diagnosis. In our population all liver injury cases were diagnosed based on analytics since no patients presented clinical symptoms. In fact, asymptomatic liver biochemical anomalies are the most common presentation of IMLI [15]. However, the presentation of anti-CTLA-4-related liver injury remains highly heterogeneous, ranging from a mild rise in AST levels to fulminant liver failure [16].
IMLI characterization varies according to the type of liver injury criteria used. CTCAE and DILIN criteria disagree on what is conventionally considered a serious liver injury. Consequently, ICI-induced hepatoxicity's approach to monitoring and managements is a challenge for hepatologists and oncologists. Grading systems’ discrepancies require clear identification. Furthermore, it underlines that while ICI-induced hepatotoxicity is a form and probably represents a new subset of DILI, since elevated bilirubin is seldom observed. In this paper, we opted to use a conjugation of both CTCAE and DILIN criteria. As such, we included all patients with CTCAE hepatotoxicity grade 3-4 and patients with FA ≥ 2 x ULN e/ ou Bil-T ≥2,5 x ULN.
Despite not having a standard biochemical signature the biochemistry pattern of IMLI has typically been reported as a hepatocellular injury. [17] In a Japanese population treated with ICI [13] with all grade liver injury, cholestatic and mixed-type liver injuries were more frequent than the hepatocellular type, and one case showed bile duct dilation on imaging tests. In our population, all 3 patients with IMLI presented with a cholestatic pattern, and in one case there was bile duct dilation on imaging tests (MRCP and CT), with no other features suggesting biliary obstruction. ERCP exposed common bile duct and intrahepatic bile duct dilation without an obstructive cause. He was submitted to steroids and evolved favourably with normalization of hepatic biochemistry. Hepatic irAEs with abnormal image findings in the bile ducts have been reported alongside with ICI-associated cholangitis, but their incidence is rare [18–21].
A case report on biliary obstruction in a patient under nivolumab, reported obstruction resolution after high-dose prednisone administration, favouring its immune mediated origin. Similar to our report, no gallstone or other aetiology were detected on MRCP or ERCP; there was no history of hepatic/biliary/pancreatic diseases or abdominal surgery and no bacterial infection was documented; however in that reported case, biliary obstruction persisted after treatment with antibiotics and elevation of AST, ALT, and ALP levels also recurred until initiation of prednisone [19]. Vincent Cheung etal. [22] also reported a case of cholestatic IMLI, the patient underwent magnetic resonance cholangiopancreaticogram as well as liver biopsy, that demonstrated bilobar intrahepatic bile duct structuring and ectasia, and portal based mixed inflammation with minimal lobulitis. The cholestasis was responsive both to steroid and ursodeoxycholic acid. Al Hepatic irAEs-related bile duct disorders might occur at the microscopic level. PD-1 ligands (PD-L1 and PDL2) are expressed by biliary epithelial cells which does not occur for CLTA-4 ligands (B7 (CD80 or CD86) molecules [23]. Cholestatic liver injury was more frequent in our patients, probably due anti-PD1 use in all cases. Such findings may indicate that anti-PD-1 or PD-L1’s inhibition of the immune checkpoint pathway may induce biliary disorder in the hepatic irAE. On the other hand the pathogenesis of ICP-related biliary injury is unknown and the lack of immune mediated liver injury features have been reported [21], what could suggest involvement of mechanisms distinct from those of immune-mediated hepatitis. It would be useful to correlate the biochemical patterns to histologic features, to more fully characterize patterns of possible liver injury due to ICI therapy.
The timeframe for irAE development after treatment initiation is within the first few weeks. Even so, they may present at any time including posterior to ICB therapy's interruption [1] as happened with one of our patients that presented IMLI one month after ICI nivolumab suspension. The terminal half-life of nivolumab has been reported to be 12–20 days [24]. Previous report shows that prolonged nivolumab binding to T lymphocytes was detected more than 20 weeks after the last infusion, regardless of the total number of nivolumab infusions [25]. Considering this, these patients’ careful follow-up should extend beyond ICIs administration is finalized. On our cohort, one patients developed IMLI 3 and other 6 cycles after ICI, which in accordance to Ethan D. Miller et al. [12] who reported a median of 3 ICI infusions to hepatoxicity development.
In our cohort, liver biopsy was obtained in only 1 patient who presented a refractory liver injury to steroids. This low rate may reflect concerns about risks of biopsy, or that a biopsy and referral to a hepatologist were not considered. This patient's liver histology presented no inflammatory infiltrated and no portal fibrosis was identified; diffuse intralobular macrovesicular steatosis with hepatocyte ballooning and occasional Mallory hyaline bodies without neutrophilic lobulitis. Steatosis and features of sustained cholestasis were identified, without the most common findings of immune mediated liver injury. The liver biopsy complicated with haemorrhagic shock and a diffuse liver metastization was diagnosed intraoperatively, despite normal previous imaging exams. As such, poor response to steroid therapy might also be a criterion for a more detailed study, especially before implementing new immunosuppressing therapies. In other reports there was evidence that hepatotoxicity caused by anti-CTLA-4 drugs showed a specific pattern of granulomatous hepatitis associated with severe lobular necrotic and inflammatory activity, fibrin deposits and central vein endothelitis. For anti-PD1/PD-L1 immunotherapy recipients, liver damage was more heterogenous, involving lobular and periportal activity. ICI's liver injury differs to the typically observed in autoimmune hepatitis, since characteristic features are lacking such as plasma cell infiltration, severe interface hepatitis, piecemeal necrosis and rosette formation. As such, the term autoimmune-hepatitis should be dismissed in favour of immune-mediated hepatitis. Adding to its role in diagnosis, histological assessment may inform on the severity of liver damage and impact therapeutic decisions [11].
In our cohort, all three patients with IMLI were treated with steroid therapy. One patient permanently suspended treatment after IMLI; one suspended treatment briefly and after favourable evolution was rechallenged; and a third patient maintained treatment with ICI. As shown, patient's approach was not uniform but did not result in different outcomes, since all patients improved. This reflects that the optimal management of irAEs, including hepatotoxicity, is yet to be determined. According to current recommendations for grade 3 or 4 hepatotoxicity, checkpoint inhibitor therapy should be permanently discontinued, and corticosteroids started. If there is no response to corticosteroids within 2–3 days, a second immunosuppressant may be added [10]. However some articles advocate a more conservative approach, arguing that spontaneous improvement in liver tests may happen after suspending immunotherapy without any corticosteroid administration [11,15].
As stated above, one patient was submitted to rechallenge with the same ICI (nivolumab at the same dosage). Other similar series have demonstrated a safer rechallenge approach [26]. In Marie-Léa et al. cohort immunotherapy was resumed in 8 patients after grade 1 achievement without hepatitis relapse: the same immunotherapy for 2 patients (9.5%) and another class of immunotherapy for the 6 remaining patients (28.6%). Ethan D. Miller et all [12] reported rechallenge in 31 patients after hepatoxicity ≥3. Those who initially received monotherapy were either given the same regimen or received a medication from a different class but no one who initially received monotherapy was rechallenged with combination therapy. Only 8 of the patients (26%) who reinitiated ICI therapy exhibited recurrent hepatotoxicity; all 8 initially had grade 3 hepatotoxicity. Currently, limited data is available on rechallenge, which is explained by the usual prohibition of rechallenge on clinical trials providing most data [1]. In Pollack et al.’s retrospective study, among recipients of combination ICI therapy discontinued due to a range of irAEs (36% had hepatotoxicity and 24% of those had CTCAE grade 3 or grade 4 hepatotoxicity), anti-PD1 therapy rechallenge led to hepatitis recurrence in 17% (7% were CTACAE grade 3 or grade 4) [27]. An observational, cross-sectional, pharmacovigilance cohort study examined individual case safety reports from theWorld Health Organization database VigiBase. This cohort study found a 28.8% recurrence rate of the same irAE associated with the discontinuation of ICI therapy after a rechallenge with the same ICI. The recurrence rates for colitis, hepatitis, and pneumonitis were higher after rechallenge compared with other irAEs [28]. Santini et al. [29] retrospectively identified 68 patients with advanced non–small cell lung cancer who were treated with anti–PD-1 or anti–PD-L1 either as monotherapy or in combination with anti–CTLA-4. Of these patients, 38 (55.9%) received a rechallenge with an anti−PD-1 or anti−PD-L1 therapy alone. Over a median follow-up of 14.3 months, the same irAE occurred in 26% of the patients, and any type of irAE occurred in 52% of the patients after the ICI rechallenge. The question of whether to administer ICI rechallenge is crucial. Practical guidelines for irAE management are based on clinical observations and expert consensus, but they do not discuss the possibility of a rechallenge. Patients who present cancer progression after the discontinuation of an ICI for irAE occurrence may find an ICI rechallenge to be beneficial. In the absence of specific recommendations, the decision to rechallenge must be discussed in each case and may be considered for select patients.
Irene Tsung et al evaluated the cause of liver injury in patients under pembrolizumab treatment, and they found only a minority of the liver injury cases were attributed to pembrolizumab hepatotoxicity (29%) while cancerous replacement of the liver accounted for most of the other patients with benign or malignant biliary obstruction identified in 5.7%. They demonstrate that patients who present with an acute hepatocellular or mixed injury pattern are more likely to be experiencing immune‐mediated liver injury due to pembrolizumab but there was no particular serum ALT, ALP, or bilirubin level that reliably differentiated patients with DILI from other causes of liver injury [1,30] In our cohort we find 8 patients with liver injury grade 3 or 4 and only 3 patients attributable to immune mediated adverse effects, being the majority associated with hepatic metastasis. This data indicates that careful clinical assessment of the cause of liver injury is critical including contrast enhanced cross‐sectional imaging of the liver to assess for tumour progression and to help ensure that the appropriate actions are undertaken, namely the institution of immunosuppressive therapy.
In our population, the presence of chronic liver disease or known liver injury (excluding liver metastasis) was not risk factors for IMLI onset. However, IMLI's low incidence is likely a limiting factor for this conclusion. Defining pre-existing liver disease's accountability when assessing DILI is uncertain. This prompts discussion on the best criteria to mark acute/overlying injury in the background of some degree of chronic injury [31]. Hepatology consults and liver biopsy in those with possible pre-existing liver injury, should be both considered in future studies. We report a patient with past HBV infection and untreated chronic HCV infection developing a grade 2 liver injury. The adopted approach was to temporarily suspend treatment, leading to favourable evolution. The patient was then rechallenged and completed 16 ICI cycles without any increase in liver enzymes. ICI therapies’ clinical trials usually excluded patients with history of chronic viral infections, namely HBV, HCV and HIV. Nevertheless, some reports on ipilimumab administration for advanced melanoma suggest that its use in patients with pre-existing HBV and HCV infection might be effective and safe [32,33]. Such studies had patients with HBV infection treated with ipilimumab while simultaneously given either entecavir or tenofovir. HCV patients, on their turn, were not under HCV treatment [34]. A retrospective review of 23 Chinese patients with advanced melanoma under either ipilmumab, pembrolizumab or combination therapy, reported similar safety data. Prior HBV infection was reported in eight patients, and active HBV infection on three patients who being treated with concomitant entecavir [35].
We conclude that clinically significant ICI-related hepatotoxicity was uncommon. There was a cholestasis pattern predominance in liver injuries. ICI proved to be safe in patients with previous hepatic diseases in our population.
Limitations to this retrospective paper are: consideration only to exposure time without reporting ICI doses; ICI clinical response was not assessed; the lack of histological studies; the absence of alcohol intake which is a possible underlying factor por hepatic abnormalities.
– Immune-mediated liver injury (IMLI) and liver injury-metastasis related features
Legend ALP - alkaline phosphatase; ALT - alanine aminotransferase; AST - aspartate aminotransferase; Bil-T - total bilirubin; IQR - interquartile range; irAE - immune-related adverse event; LI – liver injury; n – number; sd - standard deviation; yr – years;
– Immune-mediated liver injury (IMLI) features
Legend, ALP - alkaline phosphatase; ALT - alanine aminotransferase; ICI – Immune checkpoint inhibitors;
–Real world data on immune mediated liver injury in patients under immune checkpoint inhibitors
| Reference | Patients, n | Geographic location | Study period | IMLI Incidence (grade 3-4) | Latency to IMLI | ICI discontinuation n (%) | Steroids use | Time to hepatic biochemistries normalization | Data on rechallenge |
|---|---|---|---|---|---|---|---|---|---|
| Marie-Léa Gauci et al[1] | 339 | Europe (France) | April 2012 to December 2017 | 6% | 63 days | 20/21 | 13/21 | 37.5days | 8/21 without hepatitis relapse (same immunotherapy for 2 patients and another class for the 6 remaining patients |
| Ethan D. Miller et al[2] | 5762 | USA | January 2010 to March 2018 | 2% | 62 days for patients submitted to steroids; 44 days for patients not submitted to steroids | 69/100 | 67/100 | 35 for patients submitted to steroids; 19 days for patients not submitted to steroids | 31/100 Only 8 of the patients who reinitiated ICI therapy exhibited recurrent hepatotoxicity; |
| Vincent Cheung et al[3] | 453 | Europe (UK) | December 2011 to 2018 | 4% | 120 days | - | 18/20 | - 20 days in patients submitted to steroids | 4/20. No recurrence. |
| Mar Riveiro-Barciela et al[4] | 414 | Europe (Spain) | January 2016 to December 2018 | 7% | 12 weeks | 28/28 | 28/28 | 1.5 months | 6/11, four of them continued to be treated with an anti-PD-1/PD- L1 agent and two others previously treated with an anti-CTLA-4 were treated with an anti-PD-1/PD- L1. None presented recurrence of the irH |
| Koji Imoto et al[5] | 387 | Japan | January 2014 to February 2019 | 3% | 45.5 days | - | 5/11 | 4-6 weeks | - |
Legend ICI – immune checkpoint inhibiotors; IMLI – immune mediated liver injury;
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.