Gastrointestinal neuroendocrine tumors (NET) frequently present with unresectable hepatic metastases, which poses a barrier for curative treatment. Resection of the primary tumor and subsequent orthotopic liver transplantation (OLT) has been proposed as a treatment approach but available data in this regard is limited. We present a clinical case of an otherwise asymptomatic 44-yo man complaining of abdominal pain and dyspepsia that was diagnosed of a 10 cm duodenal tumor with multiple hepatic metastases. A CT-guided biopsy confirmed a NET. He underwent first a Whipple’s procedure, and then was listed for liver transplantation. During the waiting time a multimodal therapeutic approach was used including the use of radioactive 177lutetium-labeled somatostatin analogues, long-acting somastostatin analogues and antiangiogenic antibodies (bevacizumab) in order to keep neoplastic disease under control. Two years after Whipple’s procedure and given disease stability he underwent OLT with an uneventful postoperative evolution. Patient condition and graft function are optimal after a 4-year follow-up period with no evidence of recurrence. This case report underscores how a multimodal approach involving careful patient selection, resective surgery as well as use of somatostatin analogues and antiangiogenic biological therapy followed by liver transplantation can achieve excellent long-term results in this difficult patient population.
Neuroendocrine tumors (NETs) are a rare and wide group of neoplasms that arise from neuroendocrine cells with 85% of them arising from the gastrointestinal tract and pancreas. Gastrointestinal NETs frequently (46-93%) have liver metastases at diagnosis and this feature represent a major prognostic factor as they often are multiple and unresectable.1,2 When liver metastases are not treated, overall 5-year survival rate is approximately 30-40%.3
Orthotopic liver transplantation (OLT) has been used to treat hepatic involvement from NETs when metastases are unresectable or for palliation of medically uncontrollable symptoms. A 70% post-transplant 5-year survival rate from the time of diagnosis has been reported after analyzing more than 700 cases worldwide.4 However, patient selection criteria and the role of concomitant treatment modalities such as peptide receptor radionuclide therapy, long-acting somastostatin analogues and antiangiogenic antibodies are still matter of debate.1,5 In fact, neoadjuvant and adjuvant approaches seem attractive given the substantial recurrence rates reported after surgical treatment.1 Here, we present a case of a duodenal NET with liver metastases treated with a multimodal approach involving surgical resection of the primary tumor followed by peptide receptor radionuclide therapy with radioactive 177lutetium-labeled somatostatin analogues and the use of long-acting somastostatin analogues and antiangiogenic antibodies (bevacizumab) while waiting an OLT which was performed successfully 2 years after diagnosis.
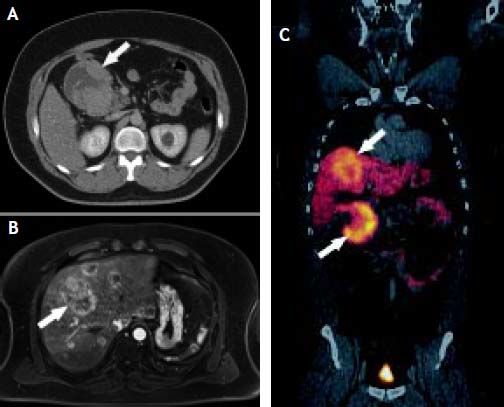
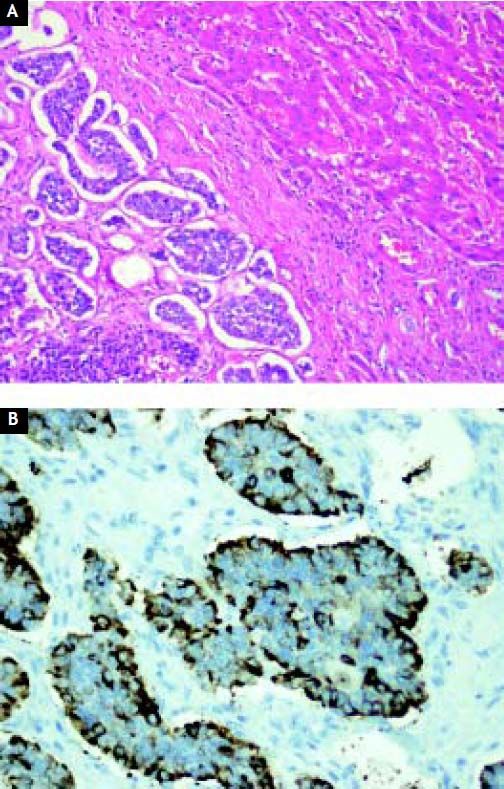
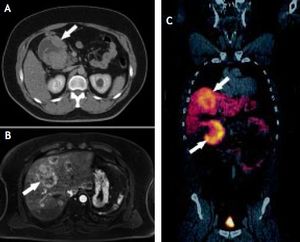
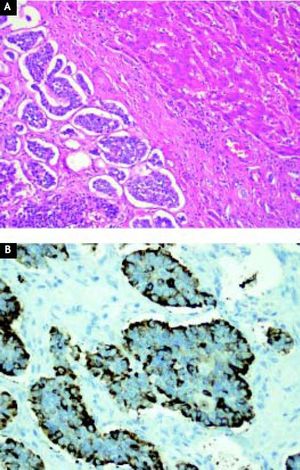
Case ReportA 44-year-old male complaining of abdominal pain and dyspepsia lasting for 3 weeks underwent an upper gastrointestinal endoscopy and an abdominal ultrasound due to the coexistence of obesity (BMI 40 kg/m2) and suspected fatty liver. While the endoscopy was normal the abdominal ultrasound revealed the presence of a duodenal mass with at least two hepatic masses. A computed-tomography (CT) showed a 10 cm lesion compromising the second portion of the duodenum and a 7.6 cm focal lesion in the right hepatic lobe. Due to the presence of steatosis, that can make difficult diagnosis of other liver masses on CT, a magnetic resonance imaging (MRI) was performed. The latter exam showed fifteen bilateral metastatic images on the liver. A CT guided biopsy confirmed a NET. A positron emission tomography (PET-CT) with Gallium (68Ga)-DOTATATE showed that both primary tumor and metastatic lesions were positive for somatostatin receptors. No other secondary lesions were found (Figure 1). Despite massive metastatic involvement of the liver, a multidisciplinary meeting decided to treat the patient with a curative intention, thus involving the participation of a team of specialists including surgeons, oncologists, hepatologists and nuclear medicine physicians. The patient underwent an exploratory laparotomy and, as no other secondary lesions were found, a radical en-bloc pancreaticoduodenectomy with partial transverse colon resection was performed. Additionally, a liver specimen was resected. The pathology report described a tumor of 9.2 by 7.7 by 7 cm with hemorrhagic and necrotic areas in the peri-duodenal and peri-pancreatic region, confirming a moderately differentiated NET. Proliferation activity estimated by the Ki-67 index6 was 2-5%. The liver specimen confirmed the metastatic involvement (Figure 2). The postoperative course was uneventful. Three months after surgery the patients received treatment with a somatostatin radiolabeled analogue (Lu177-DO-TATATE, 4 doses). The latter resulted in a significant reduction of the size of the metastatic lesions. Then treatment with long-acting somastostatin analogues was started (Sandostatin LAR® 20 mg, every three weeks) and the patient was listed for liver transplantation which was agreed to be performed upon confirmation of disease stability or mild progression. After 7 months of follow-up and due to a mild growth of some of the hepatic lesions Bevacizumab 15 mg/kg every 3 weeks was added to therapy, resulting in a new reduction in the size of the metastases. Two years after Whipple’s procedure, the patient underwent an OLT with a cadaveric donor with an uneventful postoperative course. He has been on regular follow-up during 43 months and remains asymptomatic under standard immunosuppression. Serial (68Ga)-DOTA-TATE-PET-CT studies every 9-12 months have shown no evidence of recurrent disease.
A. CT-scan showing a 10-cm of diameter, exocentric, well-defined tumor (arrow) with an exocentric cystic degeneration region compromising the 2nd portion of the duodenum. B. MRI showing multiple bilateral hyper vascular lesions (arrow) consistent with a metastatic involvement. Size of the major lesion was 6.5 cm. C. Ga68-DOTATATE PETCT showing a duodenal tumor (lower arrow) with an overexpression of somatostatin receptors and multiple metastatic lesions of similar characteristics on the liver (upper arrow).
The case presented above exemplifies a highly specialized and multimodal approach for the treatment of a resectable gastrointestinal NET with unresectable liver metastases, a difficult clinical scenario for which there is no evidence-based treatment guidelines.2,7 Thus, therapeutic decisions were made by a multidisciplinary team of specialists considering the age of the patient, the absence of significant comorbidities and the poor prognosis of palliative treatment. Each treatment option was carefully considered and instituted sequentially aiming to control the hepatic disease burden or guided by the clinical course (i.e. disease stability) and progression in the waiting list for liver transplantation. In particular, and in spite of inadequate evidence to support its use, we decided to use neoadjuvant therapy with antiangiogenic antibodies due to tumor growth during follow-up. Our case provides the opportunity to briefly review the current evidence on each treatment modality employed.
Indeed, surgical excision of both primary NET and liver metastases is the preferred approach when possible but unresectability of hepatic masses is frequent and only 10-25% of liver metastases can be removed curatively with clear margins and no microscopic residue.8 Thus, OLT is becoming increasingly accepted in this setting although assessment of its real benefit is complex due to the lack of randomized studies comparing outcomes with transplantation vs. other non-surgical treatments.4,9 A recent report from a panel of experts concluded that OLT should be offered to selected patients employing rigorous selection criteria.7 Factors such as age, functional status, tumor histology, tumor localization, feasibility of primary resection before transplantation, hepatic tumor burden, tumor growth during follow-up, mitotic activity using the Ki-67 index and local transplantation timing need to be considered in each case. A recent consensus report state that Minimal requirements for consideration of OLT should be the following: mortality less than 10%, absence of extrahepatic disease as determined by PET/CT, primary tumor removed prior to transplantation and well-differentiated NET. The consensus also indicate that patients less than 50 years old who are free of extrahepatic tumor and have low (less than 10%) Ki-67 proliferation index are those who are most likely to benefit from OLT.10 However, no prospective validation of these factors has been carried out. Among the contraindications for OLT transplantation are the presence of poorly differentiated tumors, tumors with non-portal system drainage, extra-hepatic metastases and severe carcinoid heart disease. Based in available data the expected 5-year survival rate for OLT in the setting of the case under discussion can reach 69-84%. As mentioned above, OLT likely benefits only a subgroup of patients with NET and hepatic metastases and late recurrences can occur.11 Prospective collection of radiological, biological and clinical data of patients undergoing OLT in this setting will help to identify those patient that benefit with this therapeutic option.4
Although data on the improvement of outcome of OLT for NET liver metastases by the use of neoadjuvant therapies is scarce or inadequate we decided to use this approach in our case given the long waiting time in our country. Thus, we first opted for the use of peptide receptor radionuclide therapy with radioactive177lutetium-labeled somatostatin analogues which have shown promising results in the setting of our case.12 Then we used long-acting somastostatin analogues since its use in patients like ours have shown a significant improvement in progression-free survival.13 Finally, due to a mild tumor growth we decided to use additional molecular targeted therapies, aiming to control disease progression. The use of the vascular endothelial growth factor receptor inhibitor with bevacizumab was decided based on expert opinion and a significant reduction in liver masses was seen under this treatment. Bevacizumab efficacy in this setting has not been definitively demonstrated although some positive data was already available when our patient was seen.14 Several promising trials evaluating the efficacy of the combination of this antibody with both long-acting somastostatin analogues and with mammalian target of rapamycin (mTOR) inhibitors have been published more recently.1,13 An overview of the clinical experience with the use of bevacizumab-based therapies in the treatment of NETs is provided by a recent systematic review.15
The multimodal approach used in our patient allowed a potentially curative treatment. Although the approach used in this patient was successful, given the scarcity of data on the topic, the current management of gastrointestinal NET with liver metastases must be addressed on a case-by-case basis.
Abbreviations- •
BMI: body mass index.
- •
CT: computed-tomography.
- •
MRI: magnetic resonance imaging.
- •
NET: neuroendocrine tumor.
- •
OLT: orthotopic liver transplantation.
- •
PET-CT: positron emission tomography.
None.