Cirrhosis-related mortality is underestimated and is increasing; extrahepatic factors may contribute. We examined trends in cirrhosis mortality from 1999-2017 in the United States attributed to liver-related (varices, peritonitis, hepatorenal syndrome, hepatic encephalopathy, hepatocellular carcinoma, sepsis) or extrahepatic (cardiovascular disease, influenza and pneumonia, diabetes, malignancy) causes, and compared mortality trends with congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) populations.
Materials and methodsA national mortality database was used. Changes in age-standardized mortality over time were determined by joinpoint analysis. Average annual percentage change (AAPC) was estimated.
ResultsCirrhosis cohort: From 1999-2017, both liver-related (AAPC 1.3%; 95% confidence interval [CI] 0.7-1.9) and extrahepatic mortality (AAPC 1.0%; 95% CI 0.7-1.2) increased. Cirrhosis vs other chronic disease cohorts: changes in all-cause mortality were higher in cirrhosis (AAPC 1.0%; 95% CI 0.7-1.4) than CHF (AAPC 0.1%; 95% CI -0.5- 0.8) or COPD (AAPC -0.4%; 95% CI -0.6- -0.2). Sepsis mortality was highest in cirrhosis (AAPC 3.6%, 95% 3.2- 4.1) compared to CHF (AAPC 0.6%, 95% CI -0.5- 1.7) or COPD (AAPC 0.8%, 95% CI 0.5- 1.2). Cardiovascular mortality increased in cirrhosis (AAPC 1.3%, 95% CI 1.1- 1.5), declined in CHF (AAPC -2.0%, 95% CI -5.3- 1.3) and remained unchanged in COPD (AAPC 0.1%, 95% CI -0.2- 0.4). Extrahepatic mortality was higher among women, rural populations, and individuals >65 years with cirrhosis.
ConclusionsExtrahepatic causes of death are important drivers of mortality and differentially impact cirrhosis compared to other chronic diseases.
Worldwide, cirrhosis accounts for approximately two million deaths yearly and the burden may be underestimated and increasing [1–10]. Like congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), cirrhosis can cause multi-organ damage and may be subject to similar precipitants of mortality. Although deaths in cirrhosis are traditionally considered to be liver-related, there may be a substantial impact from extrahepatic causes including cardiovascular disease (CVD), extrahepatic infections, or non-hepatocellular carcinoma malignancies. [2]
If deaths are also driven by extrahepatic causes in the current era, then a change in management is needed beyond the historically singular focus of preventing portal hypertension (PH)- related complications. Further, if extrahepatic causes differentially impact cirrhosis compared to other chronic diseases, a realignment of healthcare policy may be required. There is also limited understanding of how demographic and regional disparities affect mortality across cirrhosis, COPD and CHF populations.
We hypothesized that trends in cirrhosis mortality over the last two decades have skewed towards extrahepatic causes which may contribute to excess deaths when compared to other chronic diseases. Additionally, certain demographic subgroups with cirrhosis may have excess mortality compared to other chronic disease populations.
2MethodsWe used national death data to describe trends in annual mortality within a cohort of US decedents with cirrhosis stratified by liver-related or extrahepatic causes of death. Trends in cirrhosis mortality were also compared to trends among decedents with CHF and COPD.
Data source: The Multiple Cause of Death database is a public-use data file generated by the National Center for Health Statistics to enumerate deaths in the US [11]. The data set extracts de-identified death certificate data for > 99% of all deceased residents in all 50 states and the District of Columbia from 1999 to 2017. Part I of the death certificates contain the single condition leading to death, the underlying cause of death. Certificates also contain additional causes occurring at the time of death, referred to as multiple causes of death. Diagnosis listed on death certificates are coded based on the International Classification of Diseases, Tenth Revision (ICD-10).
Liver related and extrahepatic causes of death: The methods used in this study are like those previously described ([1-6,8,9]). The underlying cause of death was classified as cirrhosis, CHF, or COPD. Additional causes occurring at the time of death were classified as liver-related or extrahepatic. Liver-related causes included those due to PH (i.e. variceal bleeding, hepatic encephalopathy [HE], hepatorenal syndrome [HRS], ascites, peritonitis), hepatocellular carcinoma (HCC), and sepsis. Extrahepatic causes were national drivers of mortality defined as the top 15 leading causes of death in the US by the CDC: cardiovascular diseases (CVD) (i.e. myocardial infarction, strokes, CHF, and hypertension), malignancy (non-HCC, hepatobiliary, other), accidents (unintentional injuries including motor vehicle collisions, accidental drug overdose), diabetes, and influenza and pneumonia. Extrahepatic causes outside of this list were not assessed.
Case ascertainment: We first identified decedents with cirrhosis of all ages (Supplemental Table 1) using validated ICD-10 codes with positive predictive value of 93% for predicting underlying cirrhosis in the medical record [7]. To preserve coding accuracy, other coding definitions of cirrhosis (e.g K70.4, K74.0, K74.1, K74.2) were excluded from this study. Next, within the cirrhosis cohort, we identified decedents with liver-related causes of death also with validated codes (positive predictive value approaching 90%) [7]. Following this, we identified decedents with extrahepatic causes of death. Cirrhosis was categorized as the underlying cause of death while liver-related or extrahepatic manifestations were categorized as additional causes occurring at the time of death. We then identified decedents with various etiologies of cirrhosis related to hepatitis C (HCV) or hepatitis B virus (HBV) infection, alcohol associated liver disease (ALD), or non-alcoholic fatty liver disease (NAFLD) using diagnostic coding as previously described [2,6]. We then identified decedents with CHF and COPD using coding definitions like those used in recent studies [12–14]. Within the CHF and COPD cohort, we next identified decedents with extrahepatic causes of death and used the approach as described above. We performed several sensitivity analyses: 1) using various definitions of liver disease 2) considering each extrahepatic or liver-related manifestation as the underlying cause, 3) calculating rates standardized to the 2010 US population and 4) considering sepsis as an extrahepatic cause of death (Supplemental Table 2 and 3). Trends in mortality did not differ considerably between various methods of data acquisition. Due to limitations of death certificate dataset, it was difficult to parse out individuals with compensated versus decompensated cirrhosis. However, we defined decompensated cirrhosis using codes for cirrhosis and portal hypertension-related complications (varices, peritonitis, ascites, hepatic encephalopathy, hepatorenal syndrome) and sepsis as the underlying cause of death. Compensated cirrhosis was defined using validated codes for cirrhosis as the underlying cause of death (Supplemental Table 1). Patients with overlapping chronic conditions (i.e cirrhosis as the underlying cause of death and CHF as additional, comorbid cause occurring at the time of death) were considered; overall, less than 10% of total cirrhosis deaths occurred in decedents with both cirrhosis and other chronic disease listed on death certificates and these were excluded from the overall analysis. Additional consideration was given for individuals <18 years; given that deaths in this subgroup account of 0.03% of all cirrhosis deaths, they were included in the overall analysis.
2.1Statistical analysisAge-specific mortality rate per 100,000 persons was calculated by dividing the number of liver-related or extrahepatic deaths by the total US census population for each calendar year. Similar to previously published studies, [6] rates were standardized to the age distribution of the 2000 US standard population using the direct method. We also conducted a sensitivity analysis by repeating the analysis standardized to the 2010 US standard population. We analyzed mortality in the year 2017 as well as annual trends from 1999-2017. Trends were stratified by demographic subgroups of the population. We used the 2013 National Center for Health Statistics Urban-Rural classification scheme to categorize deaths as urban or rural deaths [15]. First, trends in liver-related and extrahepatic causes of death were analyzed in the cirrhosis cohort. Next, extrahepatic causes were compared across cirrhosis, CHF and COPD cohorts. Lastly, extrahepatic causes were compared among demographic subgroups across each chronic disease cohort.
Joinpoint regression analysis (National Cancer Institute's Joinpoint regression software version 4.7.0.0; http://surveillance.cancer.gov/joinpoint) was utilized to assess changes in trends over time [16]. Changes in age-standardized mortality rates (ASMR) were plotted over time and points of significant breaks in trend (joinpoints) were identified. Statistically distinct trend segments were fitted with a log-linear model. We calculated annual percentage change (APC) to describe the slope of each trend segment and average APC (AAPC) to describe trends from 1999-2017 [17]. A maximum of three jointpoints were allowed. At least two years were required from a joinpoint to either end of the data and in between consecutive jointpoints. Statistically significant differences between trend segments were tested using 95% confidence intervals (CIs) and t-test using a p-value of <0.05 [18]. The study was approved by the institutional review board.
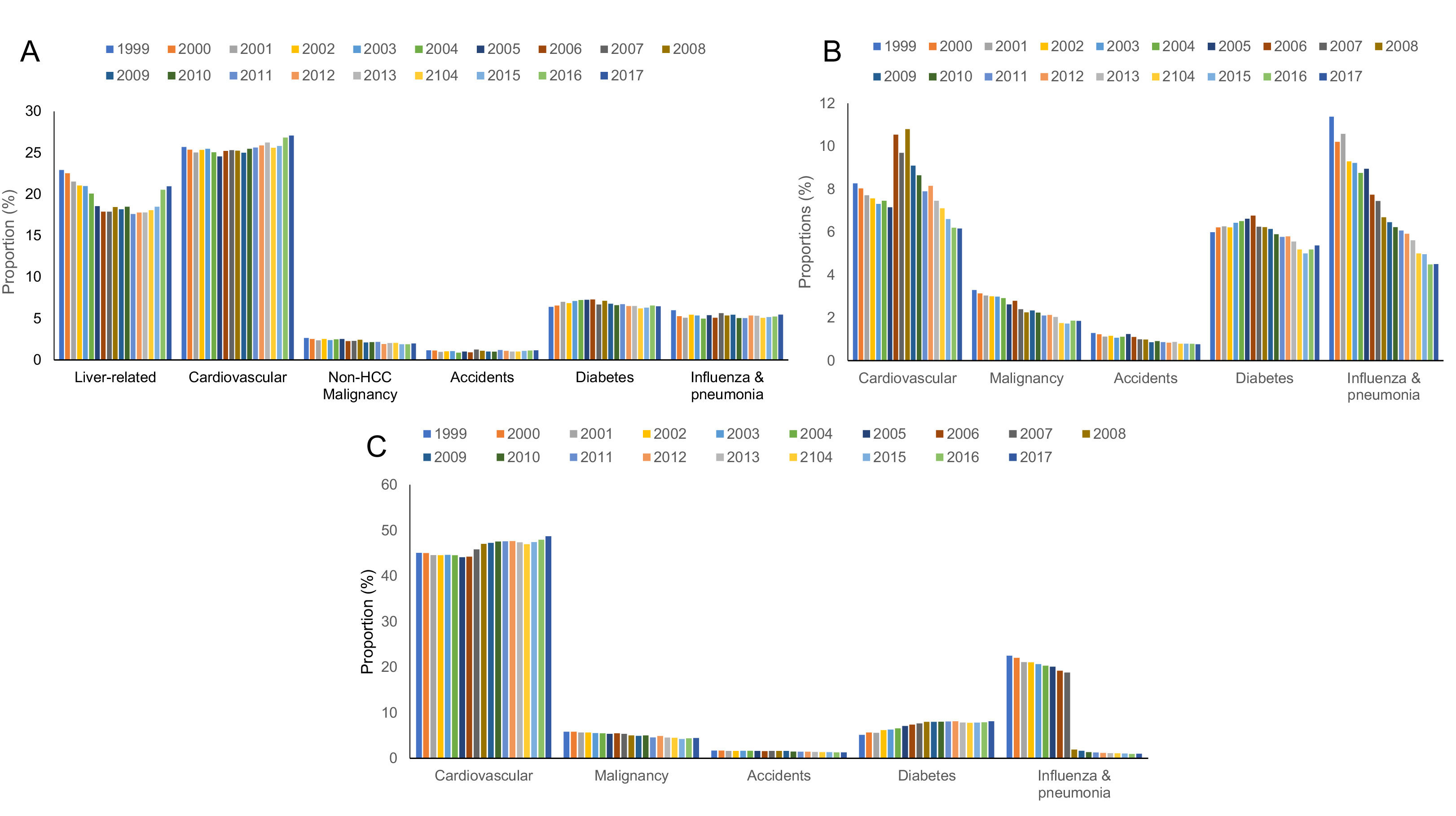
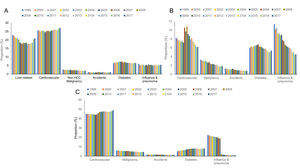
3Results3.1Cirrhosis related all-cause mortality, 2017Age-standardized mortality from all causes in the cirrhosis cohort was 9.3/100,000 (n= 35,960) (Table 1). Rates were similar when standardized to the age distribution of the 2010 US standard population (9.3/100,000 vs 10.3/100,000). Among liver-related causes of death, sepsis (1.1/100,000), varices (0.5/100,000) ascites (0.5/100,000), and HRS (0.5/100,000) had the highest ASMR followed by peritonitis (0.3/100,000), HE (0.1/100,000), and HCC (0.1/100,000). Among extrahepatic causes, ASMR was highest for CVD (2.5/100,000) followed by diabetes (0.6/100,000) influenza and pneumonia (0.5/100,000), non-HCC malignancies (0.2/100,000) and accidents (0.1/100,000).
Age-standardized mortality from liver-related and extrahepatic causes of death in cirrhosis.
| Deaths †(proportion) | ASMR (2017) | AAPC (1999-2017) | L95% | U95% | Year | APC | |
|---|---|---|---|---|---|---|---|
| Cirrhosis | 504,192 | 9.3 | 1.0%* | 0.7 | 1.4 | 1999-20092009-20152015-2017 | -0.3%*3.5%0.6% |
| Liver-related | |||||||
| All liver-related‡ | 130,954(26.0%) | 2.7 | 1.3%* | 0.7 | 1.9 | 1999-20072007-20122012-2017 | -2.5%*2.7%*6.1%* |
| Varices | 29,942 (5.9%) | 0.5 | -1.3%* | -2.1 | -0.4 | 1999-20062006-2017 | -6.9%*2.5% |
| Peritonitis | 12,743 (2.5%) | 0.3 | 1.5%* | 0.6 | 2.5 | 1999-20072007-2017 | -3.6%*5.9%* |
| Ascites | 20,006 (4.0%) | 0.5 | 2.9%* | 1.6 | 4.3 | 1999-20092009-20142014-2017 | -3.0%*2.8%25.6%* |
| HE | 6,402 (1.2%) | 0.1 | 4.0%* | 3.0 | 5.0 | ||
| HRS | 30,688 (6.1%) | 0.5 | -0.3%* | -0.9 | 0.3 | 1999-20092009-2017 | -3.2%*3.5%* |
| HCC | 5,362 (1.1%) | 0.1 | 3.2%* | 1.8 | 4.5 | 1999-2008 2008-2017 | 0.9%5.5%* |
| Sepsis | 46,759 (9.3%) | 1.1 | 3.6%* | 3.2 | 4.1 | 1999-20092009-2017 | 1.4%*6.4%* |
| Extrahepatic | |||||||
| All extrahepatic§ | 177,303 (35.2%) | 3.3 | 1.0%* | 0.7 | 1.2 | 1999-20082008-2017 | -0.8%*2.8%* |
| CVD¶ | 129,377 (25.7%) | 2.5 | 1.3%* | 1.1 | 1.5 | 1999-2008 2008-2017 | -0.7%*3.3%* |
| Non-HCC Malignancy | 11,124 (2.2%) | 0.2 | -1.1%* | -1.5 | 0.6 | ||
| Accidents (unintentional injuries) | 5,363 (1.1%) | 0.1 | 1.7%* | 0.3 | 3.2 | ||
| Diabetes Mellitus | 33,821 (6.7%) | 0.6 | 0.9%* | 0.2 | 1.7 | 1999-20032003-20102010-2017 | 2.4%-1.0%2.0%* |
| Influenza and pneumonia | 26,684 (5.3%) | 0.5 | 1.1%* | 0.3 | 1.8 | 1999-20102010-2017 | -0.94.2%* |
Asterix (*) indicates significant (p<.05) trend
No significant joinpoint trends were identified in HE, non-HCC malignancy, and accident-related mortality
: Total number of deaths from 1999-2017. The denominator for ‘proportion’ is total number of cirrhosis deaths, 1999-2017, from all causes
: Liver-related causes includes codes for varices, peritonitis, ascites, hepatic encephalopathy, hepatorenal syndrome, HCC, and sepsis
: Extrahepatic causes include codes for CVD, non-HCC malignancy, accidents, diabetes and influenza and pneumonia
: Cardiovascular causes includes codes for myocardial infarction, strokes, congestive heart failure, and hypertension, and excludes codes for esophageal and gastric varices
ASMR: age-standardized mortality rate
AAPC: average annual percent change
HRS: hepatorenal syndrome
HE: hepatic encephalopathy
HCC: hepatocellular carcinoma
CVD: Cardiovascular disease
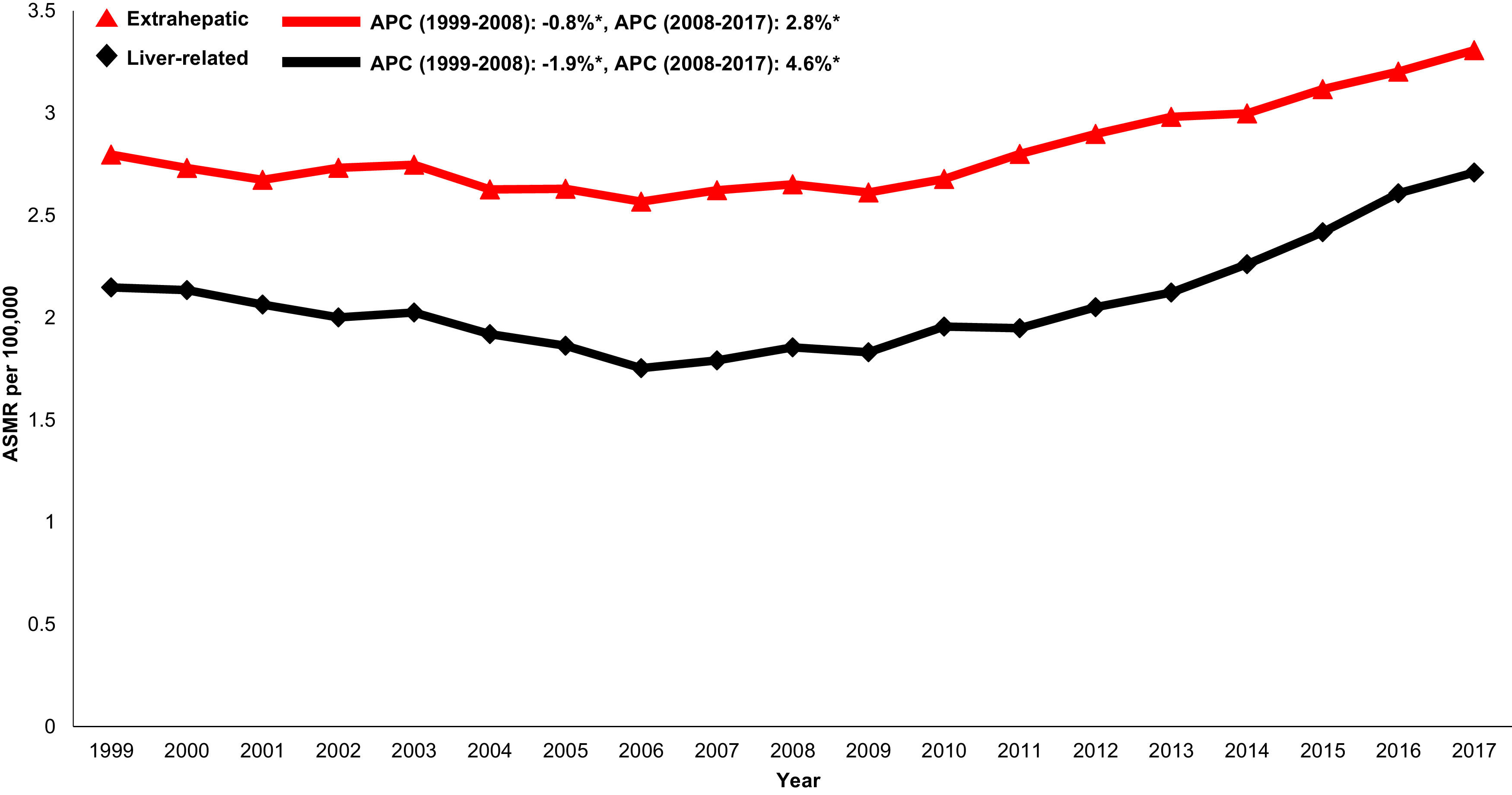
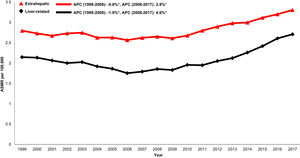
When examining trends over time, both liver-related (AAPC 1.3%, 95% CI 0.7-1.9) and extrahepatic (AAPC 1.0% 95%CI 0.7-1.2) mortality increased in the cirrhosis cohort from 1999-2017 (Table 1andFig. 1). Mortality related to CVD (AAPC 1.3%, 95% CI 1.1-1.5), influenza and pneumonia (AAPC 1.1%, 95% CI 0.3-1.8), diabetes (AAPC 0.9%, 95% CI 0.2-1.7) and accidents (AAPC 1.7%, 95% CI 0.3-3.2) rose. Mortality related to non-HCC malignancy declined in the cirrhosis cohort (AAPC -1.1%, 95% CI -1.1- 0.6).
Annual changes in age-standardized mortality in cirrhosis cohort attributed to liver-related causes (varices, peritonitis, ascites, hepatic encephalopathy, hepatorenal syndrome, hepatocellular carcinoma, and sepsis), and extrahepatic causes (cardiovascular disease, non-hepatocellular carcinoma malignancy, accidents, diabetes, influenza and pneumonia). Asterix indicates statistically significant trend (p<.05)
ASMR: age-standardized mortality rate
APC: annual percent change
Within the cirrhosis cohort, changes in mortality in compensated cirrhosis were driven by extrahepatic causes of death, especially cardiovascular disease (AAPC 1.3%, 95% CI 1.1-1.5), accidents (AAPC 1.7%, 95% CI 0.3-3.2), and influenza and pneumonia (AAPC 1.1%, 95% CI 0.3-1.8). Non-HCC malignancy related mortality declined in this group (AAPC -1.1%, 95% CI -1.5- -0.6). By comparison, decedents with decompensated cirrhosis had stable changes in annual mortality related to CVD (AAPC -0.1%, 95% CI -0.3- 0.2), non-HCC malignancy (AAPC -0.1%, 95% CI -0.4- 0.3), diabetes (AAPC -0.2%, 95% -10.0- 0.5) and influenza pneumonia (AAPC 0.3%, 95% CI -0.3- 1.0) (Supplemental Table 4).
When examining etiology-based differences in cirrhosis mortality, a greater proportion of deaths were attributed to extrahepatic causes compared to liver-related causes across all etiologies of cirrhosis (Supplemental Table 5). Among decedents with HCV-related cirrhosis, both liver-related (AAPC 1.1%, 95% CI 0.6-1.6) and extrahepatic mortality increased (AAPC 0.8%, 95% CI 0.5- 1.2). Similar trends were observed in cirrhosis related to HBV (liver-related: AAPC 1.1%, 95% CI 0.6- 1.7; extrahepatic: AAPC 0.8%, 95% CI 0.6-1.1) and ALD (liver-related AAPC 1.0%, 95% CI 0.6-1.5; extrahepatic: AAPC 0.7%, 95% CI 0.4- 1.0). In cirrhosis related to NAFLD, both liver-related (AAPC 1.6%, 95% CI 0.7-2.6) and extrahepatic mortality (AAPC 1.3%, 95% CI 1.1-1.5) increased substantially.
3.3Cirrhosis, CHF and COPD related all-cause mortality, 2017As expected, ASMR in 2017 was higher in the CHF (n=80,480, 20.4/100,000) and COPD cohorts (n=155,621, 39.7/100,000) (Table 2). In the CHF cohort, ASMR was highest for CVD (1.3/100,000) followed by diabetes (1.1/100,000), influenza and pneumonia (0.9/100,000), malignancy (0.4/100,000) and accidents (0.2/100,000). In the COPD cohort, ASMR was highest for CVD (19.3/100,000), followed by diabetes (3.2/100,000), malignancy (1.8/100,000), accidents (0.5/100,000) and influenza and pneumonia (0.4/100,000).
Age-standardized mortality in cirrhosis, CHF and COPD.
| Deaths (proportion) † | ASMR 2017(95% CI) | AAPC 1999-2017(95%CI) | Trend Segments | |
|---|---|---|---|---|
| Cirrhosis Mortality | ||||
| All causes | 504,192 | 9.3 (9.2 to 9.4) | 1.0%* (0.7 to 1.4) | 1999-2009: 0.3%*2009-2015: 3.5%*2015-2017: 0.6% |
| Liver-related‡ | 130,954 (26.0%)§ | 2.7 (2.7 to 2.8) | 1.3%* (0.7-1.9) | 1999-2007: -2.5%*2007-2012: 2.7%*2012-2017: 6.1%* |
| Cardiovascular disease ¶ | 129,377 (25.7%) | 2.5 (2.4 to 2.5) | 1.3%*(1.1 to 1.5) | 1999-2008: -0.7%*2008-2017: 3.3%* |
| Non-HCC Malignancy | 11,124 (2.2%) | 0.2 (0.16 to 0.18) | -1.1%* (-1.5 to -0.6) | |
| Accidents (unintentional injuries) | 5,363 (1.1%) | 0.1 (0.10 to 0.12) | 1.7%* (0.3 to 3.2) | |
| Diabetes | 33,821 (6.7%) | 0.6 (0.56 to 0.6) | 0.9%* (0.2 to 1.7) | 1999-2003: 2.4%2003-2010: -1.0%2010-2017: 2.0%* |
| Influenza and pneumonia | 26,684 (5.3%) | 0.51 (0.49 to 0.54) | 1.1%* (0.3 to 1.8) | 1999-2010: -0.9%2010- 2017: 4.2%* |
| Sepsis | 46,759 (9.3%) | 1.1 (1.0 to 1.1) | 3.6%* (3.2 to 4.1) | 1999-2009: 1.4%*2009-2017: 6.4%* |
| CHF Mortality | ||||
| All causes | 1,171,928 | 20.4 (20.3 to 20.6) | 0.1% (-0.5 to 0.8) | 2005-2011: -2.6%*2011-2017: 3.9%* |
| Cardiovascular disease | 92,476 (7.9%) | 1.3 (1.2 to 1.3) | -2.0% (-5.3 to 1.3) | 1999-2003: -5.2%2003-2006: 10.3%2006-2017: -4.0%* |
| Malignancy | 28,123 (2.4%) | 0.4 (0.35 to 0.39) | -3.3%* (-4.8 to -1.8) | 1999-2014: -5.0%*2014-2017: 5.4%* |
| Accidents (unintentional injuries | 11,396 (1.0%) | 0.2 (0.14 to 0.16) | -2.9%* (-4.6 to -1.2) | 1999-2012: -4.7%*2012-2017: 1.8% |
| Diabetes | 69,330 (5.9%) | 1.1 (1.05 to 1.12) | -0.7% (-1.7 to 0.3) | 2006-2009: -7.4%*2009-2014: -1.22014-2017: 3.5%* |
| Influenza and pneumonia | 83,742 (7.1%) | 0.93 (0.90 to 0.96) | -4.9%* (-6.1 to -3.7) | 1999-2005: -4.8%*2005-2009: -10.4%*2009-2018: -2.2%* |
| Sepsis | 32,255 (2.8%) | 0.60 (0.58 to 0.62) | 0.6% (-0.5 to 1.7) | 1999-2013: -1.1%2013- 2017: 6.8%* |
| COPD Mortality | ||||
| All cause | 2,504,845 | 39.7 (39.46-39.97) | -0.4* (-0.6 to - 0.2) | |
| Cardiovascular disease | 1,160,462(46.3%) | 19.3 (19.2 to 19.5) | 0.1% (0.2 to 0.4) | |
| Malignancy | 12,233 (0.5%) | 1.8 (1.7 to 1.8) | -2.1%* (-2.3 to -1.9) | |
| Accidents (unintentional injuries) | 37,865 (1.5%) | 0.5 (0.5 to 0.55) | -1.8%* (-1.8 to -0.2) | |
| Diabetes | 182,628 (7.3%) | 3.2 (3.1 to 3.3) | 1.9%* (1.4 to 2.4) | 1999-2008: 4.6%*2007-2017: 1.8%* |
| Influenza and pneumonia | 240,474 (9.6%) | 0.4 (0.36 to 0.4) | -15.9%* (-19.0 to -12.7) | 1999-2007: -3.6%*2007-2010: -63.3%*2010-2017: 2.7% |
| Sepsis | 84,941 (3.4%) | 1.5 (1.46 to 1.5) | 0.8%* (0.5 to 1.2) | |
Asterix (*) indicates significant (p<.05) trend
No significant joinpoint trends were identified in deaths related to malignancy or accidents in cirrhosis and all-cause mortality, cardiovascular disease, malignancy, or accidents in COPD
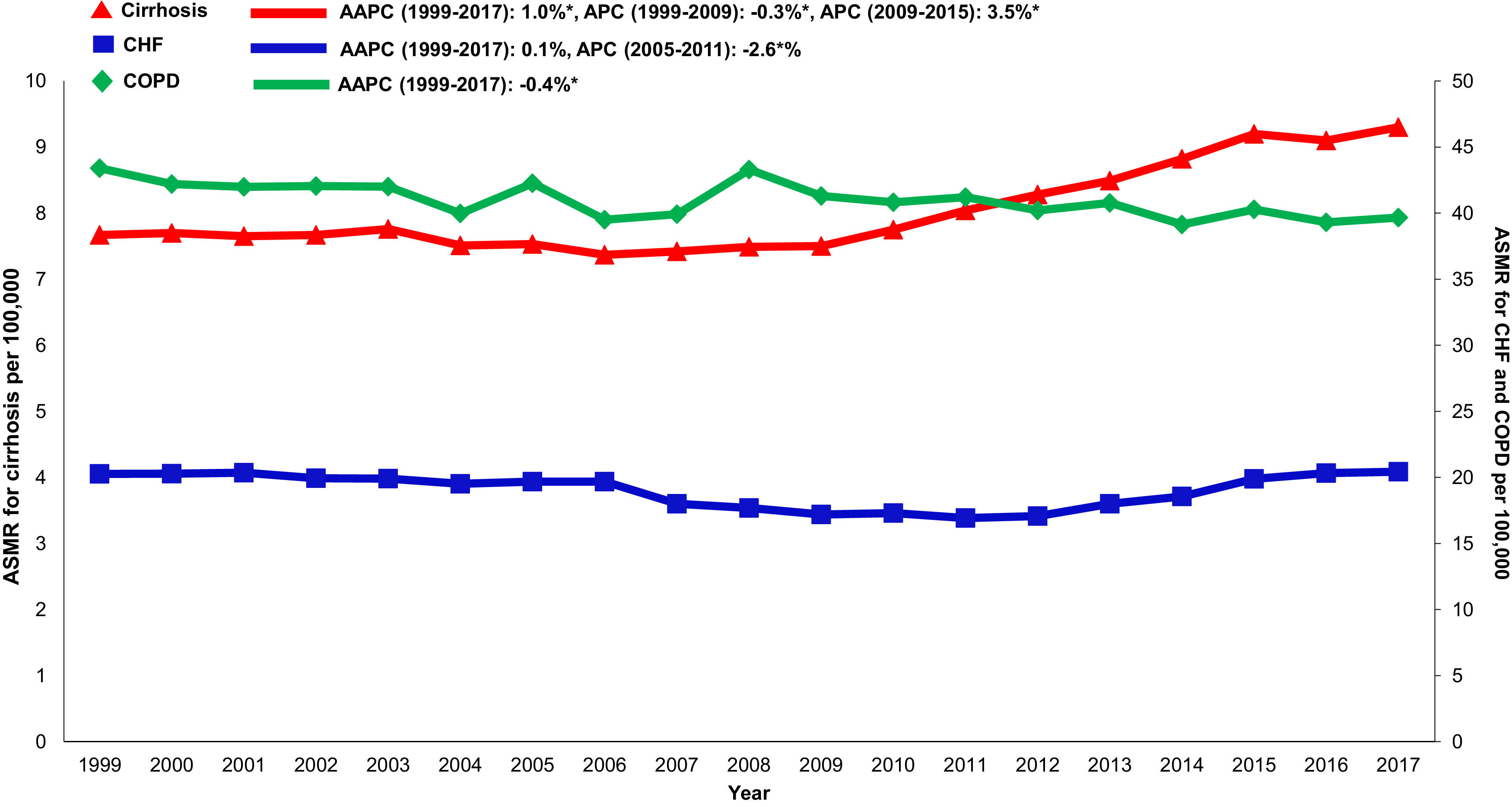
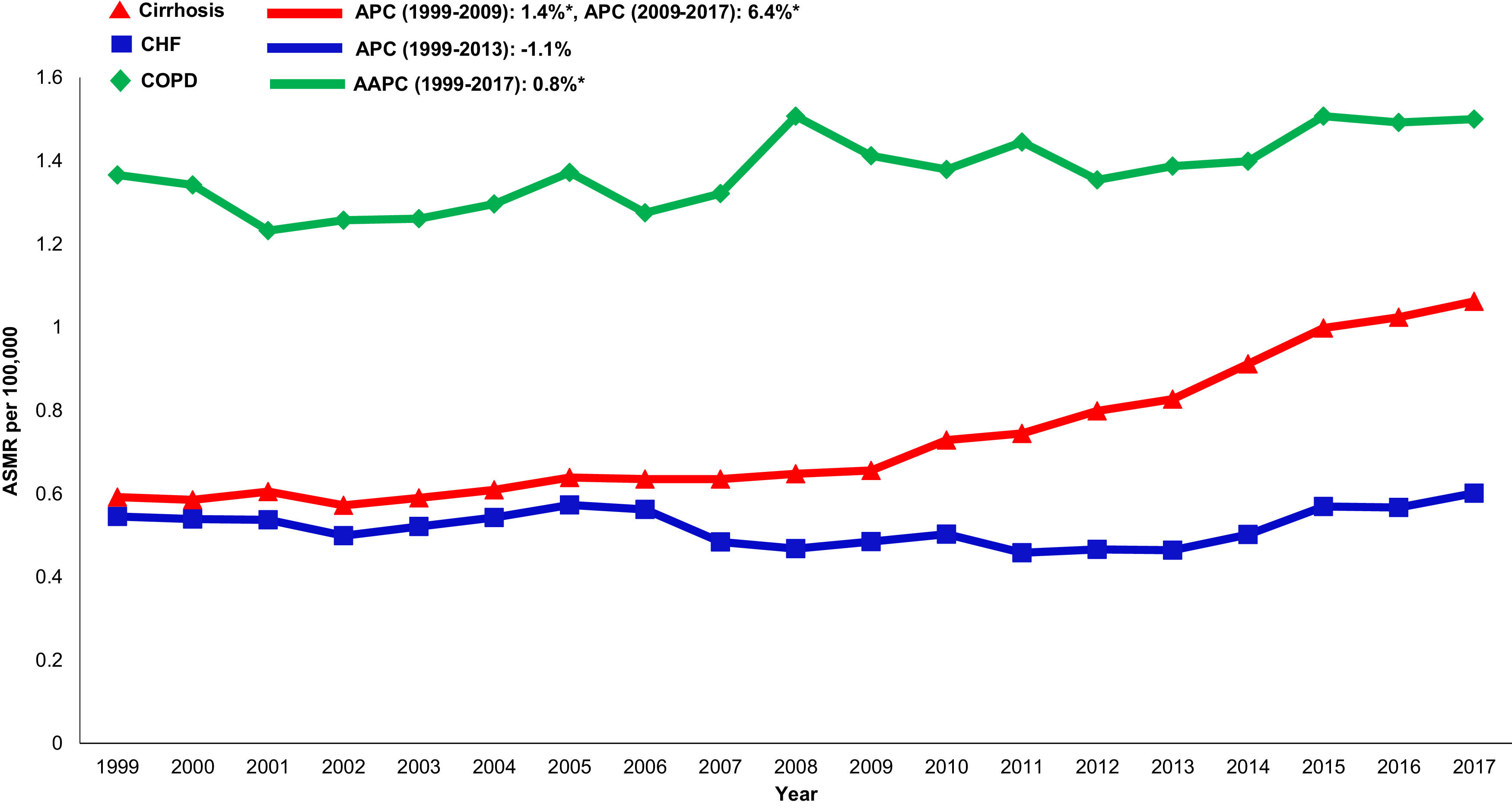
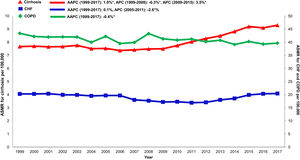
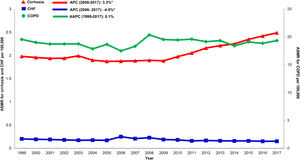
Annual trends in all-cause mortality were higher in the cirrhosis cohort (AAPC 1.0%, 95% CI 0.7-1.4) than in CHF (AAPC 0.1%, 95% CI -0.5-0.8) or COPD (AAPC -0.4%, -0.6- -0.2) cohorts (Table 2,Figs. 2,3). In recent years, cirrhosis-related mortality increased (2008-2017: APC 2.4%, 95% CI 1.8-3.0) and exceeded annual changes in CHF (2008-2017: APC 1.6%, 95% CI 0.9-2.4) and COPD (2008-2017: APC -0.4%, 95% CI -0.6- -0.2) related mortality.
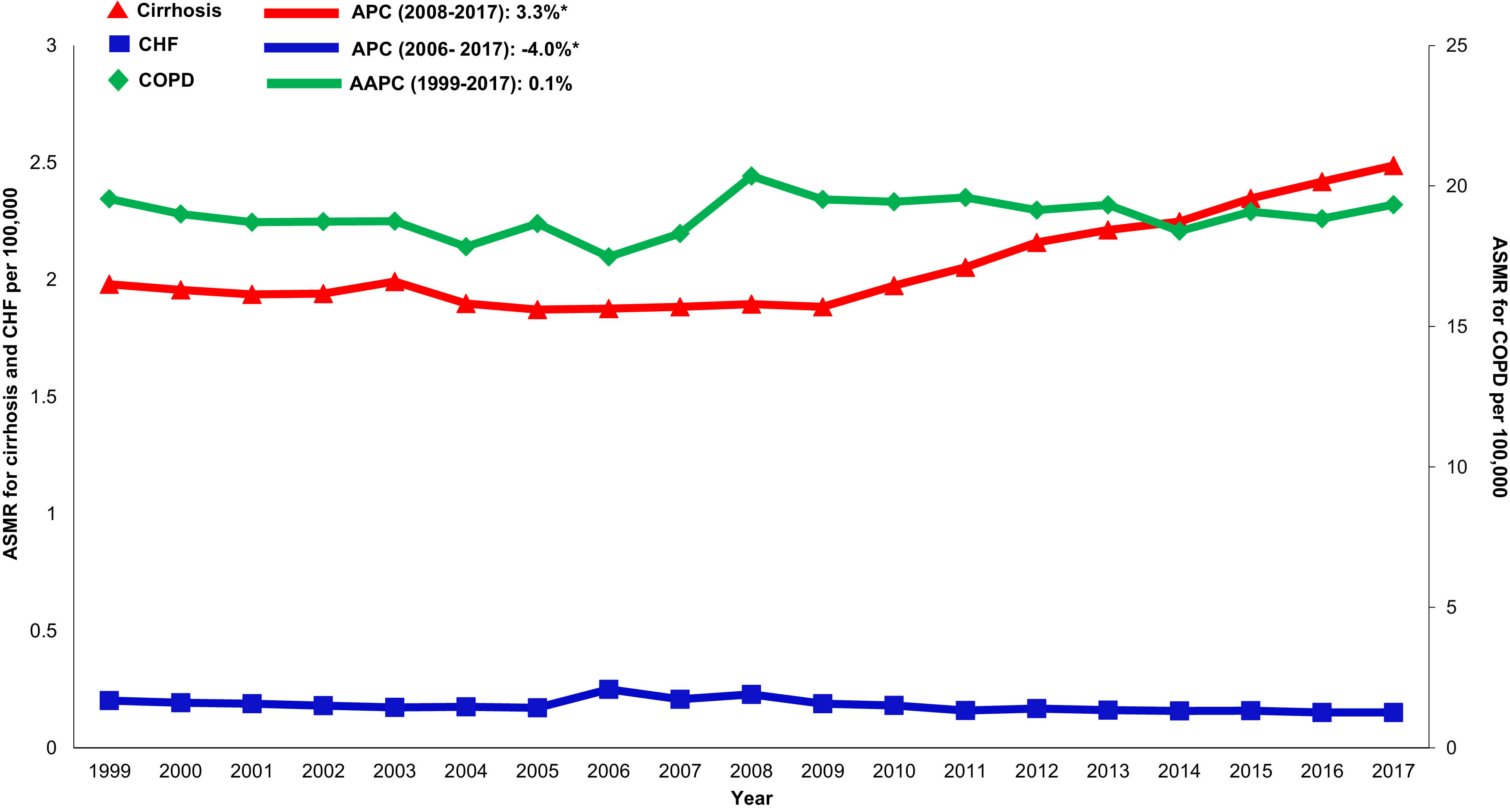
Cardiovascular: Cardiovascular-related mortality increased in the cirrhosis cohort (AAPC 1.3%, 95% CI 1.1- 1.5) (Table 2,Fig. 4). Mortality declined in CHF (AAPC -2.0%, 95% CI -5.3- 1.3) and was unchanged in COPD (AAPC 0.1%, 95% CI -0.2- 0.4). Among decedents > 65 years within the cirrhosis cohort (Supplemental Table 6), cardiovascular mortality increased (AAPC 0.8%, 95% CI 0.5- 1.1). In contrast, mortality declined in CHF (AAPC -1.1%, 95% CI -1.1- -1.7) and was unchanged COPD (AAPC 0.0%, 95% CI -0.3- 0.3). Differences were more pronounced among women (Supplemental Table 7): Cardiovascular mortality increased among women in the cirrhosis cohort (AAPC 1.2%, 95%CI 0.8- 1.7), declined in CHF (AAPC -1.3%, 95% CI -2.4- -0.3) and increased minimally in COPD (AAPC 0.6%, 95% CI 0.3- 0.9). Cardiovascular mortality increased markedly in rural populations with cirrhosis (AAPC 2.6%, 95% CI 1.4- 3.9) (Supplemental Table 8). In contrast, mortality declined among rural populations within the CHF cohort (AAPC -0.7%, 95% CI -2.8- 1.5) and increased minimally in the COPD cohort (AAPC 1.6%, 95% CI 1.2- 2.0).
Influenza & pneumonia: Influenza and pneumonia-related mortality increased in the cirrhosis cohort (AAPC 1.1%, 95% CI 0.3- 1.8) while declining in CHF (AAPC -4.9%, 95% CI -6.1- -3.7) and COPD (AAPC -15.9%, 95% CI -19.0- -12.7) cohorts. Among decedents >65 years, influenza and pneumonia-related mortality remained stable in the cirrhosis cohort (AAPC -0.2% 95% CI -0.7-1.1) with significant rise in recent years (APC 2009-2017 3.5%). By comparison, mortality declined in both CHF (AAPC -5.1%, 95% CI -6.3- -4.0) and COPD (AAPC -16.4%, 95% CI -19.7- -12.9) cohorts. Influenza and pneumonia-related mortality increased among women in the cirrhosis cohort (AAPC 1.9%, 95% CI 1.0- 2.9) compared to declining rates among women in CHF (AAPC -5.2%, 95% CI -6.6- -3.8) and COPD (AAPC -15.2% 95% CI -18.8- 11.5) cohorts respectively. Influenza and pneumonia-related mortality increased among rural populations in the cirrhosis cohort (AAPC 1.9%, 95% CI 0.9- 2.8) while declining in CHF (AAPC -5.2%, 95% CI -5.8- -4.5) or COPD (AAPC -14.7%, 95% CI -20.7- -8.3) cohorts.
Accidents (unintentional injuries): Accident-related mortality increased in the cirrhosis cohort (AAPC 1.7%, 95% CI 0.3- 3.2) while declining in CHF (AAPC -2.9%, 95% CI -4.6- -1.2) and COPD (AAPC -1.8%, 95% CI -1.8- -0.2) cohorts.
Malignancy: Non-HCC malignancy-related mortality declined in the cirrhosis cohort (AAPC -1.1%, 95% CI -1.5- -0.6). Steeper declines were observed in CHF (AAPC -3.3%, 95% CI -4.8- -1.8) and COPD (AAPC -2.1%, 95% CI -2.3- -1.9) cohorts.
Diabetes: Diabetes-related mortality increased in the cirrhosis cohort (AAPC 0.9%, 95% CI 0.2- 1.7) while declining in CHF (AAPC -0.7%, 95% CI -1.7- 0.3) and increasing in COPD (AAPC 1.9%, 95% CI 1.4- 2.4) cohort.
Further details of trends among women, decedents >65 years and rural populations are provided in Supplemental Table 6-8.
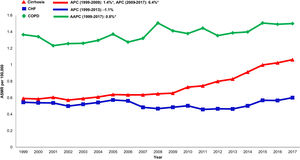
3.6Annual trends in sepsis related mortality in cirrhosis, CHF and COPD, 1999-2017In the cirrhosis cohort, sepsis related mortality rose markedly throughout the study period (AAPC 3.6%, 95% CI 3.2-4.1) and was especially high in recent years (APC 2009-2017: 6.4%) (Table 1). Sepsis was a major driver of liver-related mortality. Liver related mortality was markedly increased (AAPC 1.3%, 95% CI 0.7-1.9) with sepsis considered as a liver-related cause of death. On sensitivity analysis, when sepsis was considered as an extrahepatic cause, annual increases in liver-related mortality were not as high (AAPC 0.7% -0.2- 1.5) (Supplemental Table 3). When compared to other chronic diseases, sepsis-related mortality increased more rapidly in the cirrhosis cohort (AAPC 3.6%, 95% CI 3.2- 4.1) while rates were unchanged in the CHF cohort (AAPC 0.6%, 95% CI -0.5- 1.7) and increased minimally in the COPD cohort (AAPC 0.8%, 95% CI 0.5- 1.2) (Table 2,Fig. 5).
4DiscussionFrom 1999-2017, annual changes in cirrhosis mortality attributed to extrahepatic causes (namely CVD and extrahepatic infections) rose in the cirrhosis cohort. The proportion of extrahepatic deaths exceeded liver-related deaths in all etiologies of cirrhosis. Annual increases in extrahepatic mortality were larger in cirrhosis compared to other chronic diseases. Additionally, women, decedents > 65 years, and rural populations with cirrhosis had disproportionately higher rates of extrahepatic mortality.
Multiple studies have demonstrated increases in all-cause mortality in cirrhosis over the last decade [1-6,8,9]. However, our study highlights the need to shift focus towards also considering extrahepatic causes. The rising impact of extrahepatic causes of death on cirrhosis mortality may reflect (1) an aging liver population with increasing comorbidities, (2) improved care of PH- related complications, or (3) certain manifestations (e.g. infection) being the penultimate expression of decompensated cirrhosis [19].
Overall, extrahepatic causes of death were significant drivers of mortality in compensated cirrhosis when compared to decompensated cirrhosis in our study. Additionally, liver-related mortality rose among all etiologies of cirrhosis. In the last 10 years, liver-related mortality was highest among decedents with ALD and NAFLD. Rising incidence of hepatic encephalopathy may be a contributing factor [20,21]. Alcohol-related cirrhosis may be associated with higher risk of developing HE when compared to NAFLD cirrhosis [22]. We also observed that HCC-related mortality was an important contributor to liver-related mortality in our study. Growing evidence suggests that alcohol consumption and related cirrhosis, NAFLD, and NASH may directly contribute to the development of HCC and are becoming increasingly common causes of HCC-related mortality at a national level [23]. Lastly, sepsis was a predominant driver of liver related mortality in our study among all etiologies of sepsis. Rise in multidrug resistant bacteria likely contributes to the increased rates of mortality related to sepsis, peritonitis, and ascites. However, while liver-related mortality remains high because of these entities, a greater proportion of cirrhosis deaths in our study were attributed to extrahepatic causes of death. This further lends support to the fact that equal attention needs to be paid towards extrahepatic causes of death.
Extrahepatic mortality in cirrhosis compared to other chronic diseases: The absolute number of deaths attributed to CHF and COPD were expectedly higher than deaths attributed to cirrhosis in our study. However, overall trends in mortality remained stable or declined in CHF and COPD while increasing in cirrhosis. CVD remains a major contributor to mortality across all chronic diseases. However, there has been an overall decline in cardiovascular mortality due to nationwide initiatives aimed at improving public awareness about reducing cardiometabolic risk factors [12,24]. For example, primary CHF admissions have declined over the years and deaths are more likely to be related to non-CHF causes (e.g. atrial fibrillation) or extra-cardiac causes (e.g. malignancy, diabetes, COPD, or kidney disease) [14].
Sepsis related mortality was higher in cirrhosis than in CHF or COPD in our study. In the cirrhosis cohort, increase in liver-related mortality, especially within the last decade, was predominantly driven by sepsis. Peritonitis and ascites-related mortality did not fully account for the robust increase in liver-related mortality in the cirrhosis population and mortality in the cirrhosis population was strongly driven by other infections including influenza and pneumonia. Therefore, increased vigilance for non-liver sources of sepsis including respiratory, urinary, or viral causes is needed.
Cirrhosis mortality in demographic subgroups: Extrahepatic causes differentially impacted key demographic subgroups with cirrhosis. For example, rural populations with cirrhosis had higher rates of CVD and infection-related mortality compared to CHF and COPD populations in our study. One explanation may be that the cirrhosis population consists of fewer insured patients and Medicare beneficiaries compared to CHF or COPD populations [8]. Rates of cardiovascular-related mortality were also higher among women with cirrhosis while rates declined in women with CHF and minimally increased in women with COPD. Interestingly, diabetes-related mortality did not increase alongside cardiovascular mortality among women with cirrhosis suggesting that there may be non-traditional risk factors for CVD in cirrhosis beyond metabolic syndrome. While female gender may be protective in multiple cardiovascular diseases resulting in longer survival compared to males, drivers may be different in cirrhosis and merits further investigation [25].
Clinical and policy implications: Steps to improve cirrhosis mortality should begin with a shift in focus towards early intervention and reduction of modifiable risk factors. This includes frequent monitoring of lipid profiles, hemoglobin A1C, and administration of age-specific vaccinations, cancer screening, and mood screening. Healthcare models such as the patient-centered medical home may be useful in coordinating care across a multidisciplinary team and multiple health care systems [26]. Additionally, women, individuals age >65 years, and rural populations with cirrhosis require targeted resource allocation and outreach programs to reduce mortality.
Exploring risk reduction strategies utilized in patients with HIV may be informative. Deaths in this population are increasingly CVD-related not only due to traditional cardiometabolic risk factors (e.g age, sex, smoking status, total cholesterol level), but also HIV-related factors such chronic inflammation mediated by viral infection and side effects of antiretrovirals, specifically protease inhibitors [27]. As a result, commonly used risk prediction models, such as the atherosclerotic CVD (ASCVD) score, have shown to underestimate CVD risk in HIV-infected individuals and potentially exclude them from receiving lipid lowering therapies or additional screening (e.g., coronary artery calcium score) [28]. Differences in practice patterns of physicians treating HIV infected individuals are also a contributory factor; one analysis [29] noted that physicians were less likely to prescribe guideline-recommended aspirin and statins to HIV-infected compared to HIV-uninfected patients. This may reflect an uncertainty of statin efficacy in the HIV population, concern for interactions with antiretroviral therapies, or side effects with long term use. To address these issues, large scale trials investigating long term safety and efficacy of statin therapy in HIV- infected patients are currently underway [28]. Similar analysis of CVD risk models and statin use in cirrhosis populations may be useful.
CVD risk reduction is also the focus in patients with rheumatoid arthritis (RA), where CVD accounts for nearly 40% of deaths [30]. Emerging data on benefits of physical activity in RA also warrant attention. In one study, six months of individualized aerobic and resistance training three times per week led to marked improved aerobic capacity (measured by VO2 max), blood pressure, lipids levels and 10-year CVD event probability when compared to a group receiving education on exercise only [31]. These data merit consideration for use in the cirrhosis population.
Our study has several strengths. We examined a national database for cause-specific mortality in cirrhosis to capture recent trends compared to other major chronic conditions. We used validated ICD-10 coding to define chronic diseases and completed sensitivity analysis using various coding algorithms to ensure consistency of results ([1,2,6,7,10]). Additionally, while changes in coding practices over time may result in increased physician use of cirrhosis codes with ICD-10 compared to previous ICD versions, [10] our analysis of death records begins in 1999, which ensures that death certificate data was coded with a single (ICD-10) coding system.
Several limitations exist. First, death certificate data alone is insufficient to estimate the national disease burden [10]. Healthcare data from other sources (e.g hospital and VA databases) used in combination with death certificate data may provide additional information. Second, causes of death are subject misclassification among physicians leading to under or overestimation of mortality [32]. Misclassification of death data may occur between 13-30% of death certificates in published studies ([33,34]). Physicians may be more likely to code for a liver-related cause of death in a decedent known to have cirrhosis compared to a decedent without cirrhosis [35]. Additionally, increasing prevalence of cirrhosis in the US population may contribute to increased coding on death certificates because of rising awareness. [6] While absolute number of deaths may be impacted by these limitations, the overall rate of change in annual mortality, which was the focus of this study, may be less affected. Third, liver-related, extrahepatic, and disease-specific causes of death in cirrhosis, CHF and COPD did not account for all deaths occurring between 1999-2017. While 60% of deaths in the cirrhosis population were attributed to extrahepatic and liver-related causes, causes of death in the remaining population were not evaluated. This is because we did not explore deaths attributed to other top 15 causes of national mortality (such as renal diseases, Alzheimer's, Parkinson's, or aspiration pneumonitis) and deaths occurring outside of the top 15 causes were also not assessed. However, the intent of our study was to assess the leading causes of extrahepatic deaths across various chronic conditions. Fourth, when determining the impact of cardiovascular related mortality, we did not account for differences in baseline cardiovascular risk factors among each chronic disease cohort. Risk factors are not generally captured on death certificates as underlying or additional causes of death. Future investigations using prospective data can allow for a better understanding and interpretation of these mortality trends.
In summary, annual mortality in cirrhosis is increasingly driven by extrahepatic causes of death such as cardiovascular disease and extrahepatic infections. The impact on mortality is larger in cirrhosis as compared to other chronic diseases. Mitigating these trends may require a shift from how we currently define drivers of mortality in cirrhosis and further lends support to the need for an integrated model of cirrhosis care.