Viral hepatitis is a serious public health problem. The risk of progression to chronic hepatitis in hepatitis B virus (HBV) infection occurs in 5–10% of adults and is a leading cause of cirrhosis and hepatocellular carcinoma worldwide. Individuals infected with human immunodeficiency virus (HIV) may have coinfection with HBV. The existence of unvaccinated groups represents a significant risk not only individually but also at the community level. The aim of this study was to evaluate HBV vaccine response in adults with HIV infection.
Materials and methodsA retrospective, descriptive study of the cross-sectional type was carried out in an outpatient HIV referral center in southern Brazil. All medical records of adult HIV patients seen during January 2006 to December 2015 were selected. In statistical analysis, a significance level of 5% was used.
ResultsOf the 201 patients evaluated with a complete vaccination scheme, 55.72% were males, with a mean age of 43.86±12.68 years. Vaccine response occurred in 80.10% (161/201) of the patients, and it did not correlate with age, CD4+ cell count or viral load.
ConclusionHBV vaccine response in a HIV population was satisfactory, highlighting the importance of vaccination for prevention, cost reduction and better prognosis in preventing HBV/HIV coinfection.
Viral hepatitis represents a major global public health problem. It has an enormous impact on society and health care systems, accounting for 1.4 million deaths annually due to acute infection, cirrhosis and hepatocellular carcinoma [1–6].
Likewise, human immunodeficiency virus (HIV) infection is also a serious problem in public health, since the rate of new cases remains constant [7,8]. Global acquired immunodeficiency syndrome (AIDS) pandemic data up to 2016 recorded 36.7 million people infected [8,9]. The epidemiology of AIDS in Brazil is characterized by an affected population predominantly heterosexual, female, young, with low level of schooling and coming from the countryside [10–12].
People living with HIV may be co-infected with hepatitis B (HBV) or C (HCV) viruses, which may accelerate the course of liver disease [13,14]. HBV and HIV show the same route of transmission, mainly sexual and parenteral, facilitating the occurrence of HBV/HIV coinfection [15]. The prevalence of HBV surface antigen (HBsAg) in HIV patients is around 5%. The risk of progression to AIDS or death is almost double for those with HIV/HBV coinfection compared to individuals mono-infected with HIV [1,15–17].
Vaccines against HBV have good immunogenicity and are effective, but the efficacy decreases gradually after 40 years of age. Obesity, stress, smoking and alcohol abuse are factors also associated with lower vaccine efficacy. Immunogenicity is reduced in premature neonates, immunocompromised individuals, hemodialysis patients, and patients with heart disease, liver cirrhosis and chronic lung disease [18–22]. In our setting, when we evaluated vaccine response in patients with chronic hepatitis, it was lower than that of the controls [23].
Persons infected with HIV without evidence of HBV immunity should be guaranteed vaccine coverage. Ideally, vaccines should be administered soon after HIV diagnosis [19,24–26]. Some studies suggest that increased doses of the vaccine significantly improve immune responses in these patients [19,20–22].
A four-dose regimen of recombinant HBV vaccine is currently recommended in HIV-infected individuals, since it increases response rates and determines higher antibody titers, providing greater protection against HBV [3,22,27,28]. The response to vaccination against HBV has been associated with a better prognosis in this population of patients [28–32].
Proper vaccination should not be seen only as an individual benefit, but as an act of social responsibility and a commitment of the governments of each country to the health of its citizens. The aim of this study was to evaluate vaccine response against HBV in adults with HIV infection seen at a specialized outpatient clinic.
2Materials and methodsA retrospective cohort of HIV patients was evaluated. The patients were selected from the records of the specialized HIV outpatient clinic of the Health Department of Chapecó, a city in southern Brazil, from January 2006 to December 2015.
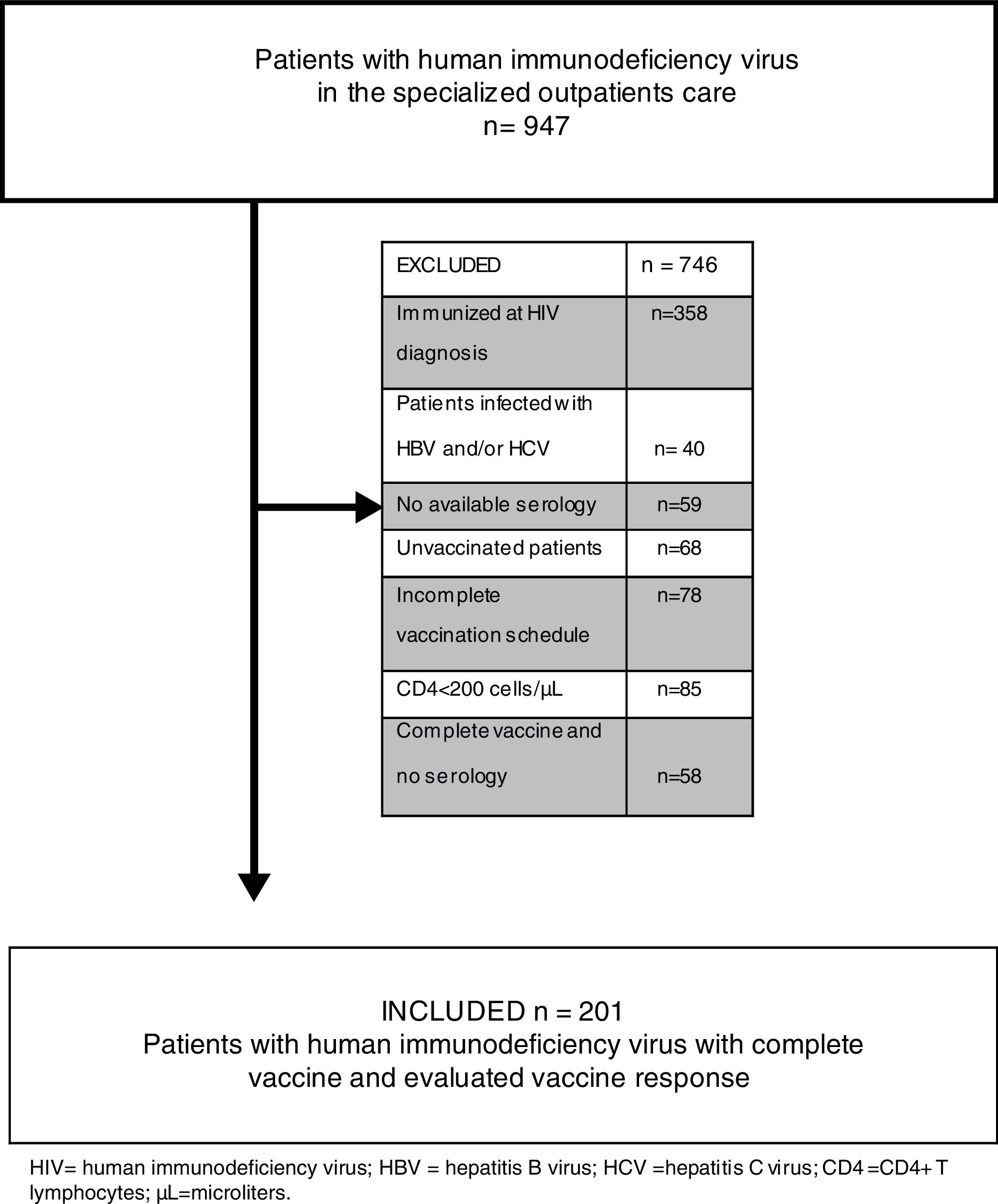
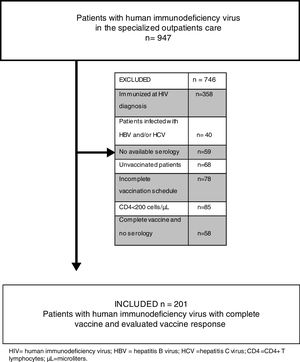
We evaluated all medical records of notified HIV patients, totaling 947 individuals. We excluded from the study 746 patients: those with previous immunization record [antibody against HBV surface antigen (anti-HBs) greater than 10IU/mL] at HIV diagnosis; those with HIV/HBV, HIV/HCV and HIV/HBV/HCV coinfection; those for whom no serological tests for viral hepatitis were performed; those who did not take the HBV vaccine; those who did not complete the vaccination scheme; those with CD4+ T lymphocytes less than 200cells/μL; those for whom no post-vaccination serological tests were performed; and those under 18 years of age. Finally, 201 patients were eligible for review (Fig. 1).
The demographic, social and economic data were collected and analyzed, as well as information on HBV and HCV serology, CD4+ cell counts before vaccine, HIV viral load, HBV vaccine doses, living habits related to illicit drug and alcohol use, smoking and antiretroviral therapy (ART).
The vaccine regimen was performed in 4 double doses of recombinant HBV vaccine (Euvax B® – Sanofi Pasteur; 20μg HBsAg in 1.0mL) administered intramuscularly following the standard schedule (0, 1, 2 and 6 months).
Protocol exams for patients with HIV should be requested at the first visit, including anti-HBs evaluation (between 2006 and 2009 only qualitative method: electrochemiluminescence immunoassay analyzer (ECLIA), ARCHITECT I2000SR, reagent result ≥10IU/mL; since 2010, quantitative method: chemiluminescent magnetic microparticle immunoassay (CMIA), reagent result ≥10IU/mL). The anti-HBs was also performed 2 months after the end of the vaccination schedule in order to evaluate the vaccine response. A vaccine response was considered when the patients showed an anti-HBs level of >10IU/mL.
For statistical analysis, continuous variables were described as mean and standard deviation. Categorical variables were expressed as frequency and percentage. The categorical variables were compared by chi-square test or by Fisher's exact test when appropriate. The findings were considered significant when p<0.05.
The data were stored in Microsoft Office Excel 2010 and analyzed using Statistical Package for the Social Science version 22.0 (SPSS).
This project was submitted to and approved by the Ethics Committee of the Federal University of Health Sciences of Porto Alegre, Brazil, following the ethical precepts of the Declaration of Helsinki, revised in 2013 [33].
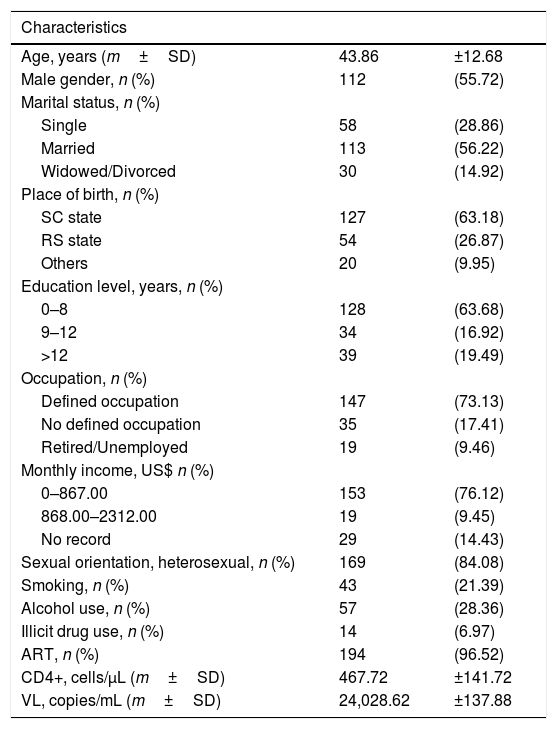
3ResultsOf the 201 patients analyzed (Table 1), the age ranged from 21 to 84 years, with a mean of 43.86 years±12.68; 112 (55.72%) were male; 167(83.08%) were white. Regarding marital status, 113 (56.22%) were married. The schooling level of 128 (63.68%) patients was elementary education (0–8 years of schooling). The majority of patients (n=147; 73.13%) had a defined occupation. The monthly income was up to U$ 867.00 in 153 (76.12%) patients. The sexual orientation of 169 (84.08%) patients was heterosexual. Forty-three (21.39%) patients were smokers, 57 (28.36%) reported alcohol abuse and 14 (6.97%) were users of illicit drugs. There were 194 (96.52%) patients who were on ART.
Demographic characteristics of patients with HIV.
| Characteristics | ||
|---|---|---|
| Age, years (m±SD) | 43.86 | ±12.68 |
| Male gender, n (%) | 112 | (55.72) |
| Marital status, n (%) | ||
| Single | 58 | (28.86) |
| Married | 113 | (56.22) |
| Widowed/Divorced | 30 | (14.92) |
| Place of birth, n (%) | ||
| SC state | 127 | (63.18) |
| RS state | 54 | (26.87) |
| Others | 20 | (9.95) |
| Education level, years, n (%) | ||
| 0–8 | 128 | (63.68) |
| 9–12 | 34 | (16.92) |
| >12 | 39 | (19.49) |
| Occupation, n (%) | ||
| Defined occupation | 147 | (73.13) |
| No defined occupation | 35 | (17.41) |
| Retired/Unemployed | 19 | (9.46) |
| Monthly income, US$ n (%) | ||
| 0–867.00 | 153 | (76.12) |
| 868.00–2312.00 | 19 | (9.45) |
| No record | 29 | (14.43) |
| Sexual orientation, heterosexual, n (%) | 169 | (84.08) |
| Smoking, n (%) | 43 | (21.39) |
| Alcohol use, n (%) | 57 | (28.36) |
| Illicit drug use, n (%) | 14 | (6.97) |
| ART, n (%) | 194 | (96.52) |
| CD4+, cells/μL (m±SD) | 467.72 | ±141.72 |
| VL, copies/mL (m±SD) | 24,028.62 | ±137.88 |
HIV=human immunodeficiency virus; m=mean; SD=standard deviation; n=number; %=percentage; SC=Santa Catarina, Brazil; RS=Rio Grande do Sul, Brazil; US$=American dollars; ART=antiretroviral therapy; CD4+=CD4+ T lymphocytes; μ/L=microliters; VL=viral load.
The mean CD4+ T lymphocyte count was 467.72±141.72cells/μL, and HIV viral load was 24,028.62±137.88copies/mL.
Regarding vaccine response, 161 (80.10%) patients responded to the HBV vaccine.
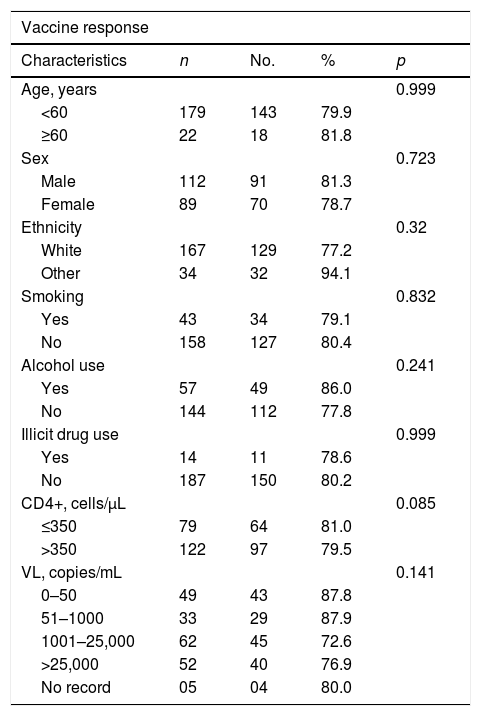
Several characteristics of the patients were evaluated in relation to the vaccine response. Of the evaluated characteristics we emphasized age. When considering the patients under 60 years of age (179), 143 (79.90%) were responders to the vaccine, and among the patients aged 60 years or older [22], 18 (81.80%) were responders (p=0.999) (Table 2).
Hepatitis B virus vaccine response rates according to characteristics of HIV-infected patients.
| Vaccine response | ||||
|---|---|---|---|---|
| Characteristics | n | No. | % | p |
| Age, years | 0.999 | |||
| <60 | 179 | 143 | 79.9 | |
| ≥60 | 22 | 18 | 81.8 | |
| Sex | 0.723 | |||
| Male | 112 | 91 | 81.3 | |
| Female | 89 | 70 | 78.7 | |
| Ethnicity | 0.32 | |||
| White | 167 | 129 | 77.2 | |
| Other | 34 | 32 | 94.1 | |
| Smoking | 0.832 | |||
| Yes | 43 | 34 | 79.1 | |
| No | 158 | 127 | 80.4 | |
| Alcohol use | 0.241 | |||
| Yes | 57 | 49 | 86.0 | |
| No | 144 | 112 | 77.8 | |
| Illicit drug use | 0.999 | |||
| Yes | 14 | 11 | 78.6 | |
| No | 187 | 150 | 80.2 | |
| CD4+, cells/μL | 0.085 | |||
| ≤350 | 79 | 64 | 81.0 | |
| >350 | 122 | 97 | 79.5 | |
| VL, copies/mL | 0.141 | |||
| 0–50 | 49 | 43 | 87.8 | |
| 51–1000 | 33 | 29 | 87.9 | |
| 1001–25,000 | 62 | 45 | 72.6 | |
| >25,000 | 52 | 40 | 76.9 | |
| No record | 05 | 04 | 80.0 | |
HIV=human immunodeficiency virus; n=total; no.=number with vaccine response; CD4+=CD4+ T lymphocytes; μ/L=microliters; VL=viral load; mL=milliliters.
When the CD4+ T lymphocyte count was evaluated, there was also no difference in response to the vaccine. Of the 122 patients with a count greater than 350cells/μL, 79.50% showed response to the vaccine, and of the 79 with a count of 350 or less, response was 81.00% (p=0.085) (Table 2).
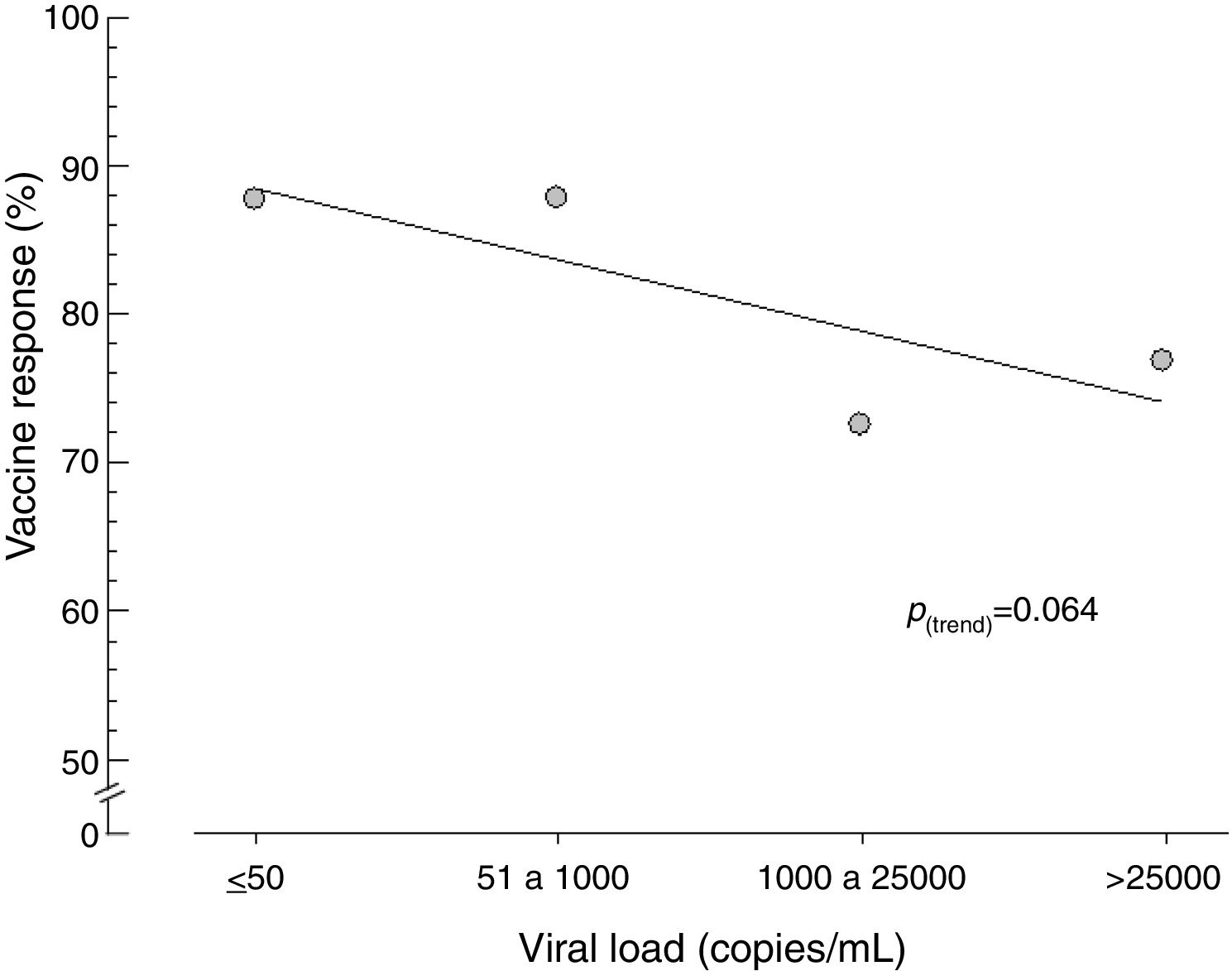
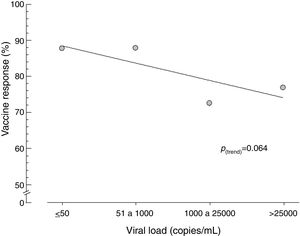
Similarly, when viral load was stratified, no statistical difference was observed in vaccine response, although those who had a viral load of 1000copies/mL or less had a tendency toward a better response to vaccination (Fig. 2) and Table 2).
4DiscussionViral hepatitis and HIV are a serious health problem, and it is essential to prevent hepatitis from HBV to provide the HIV-infected patient a better prognosis.
The present study demonstrated that 205 (21.65%) HIV-positive patients, although evaluated in a referral center, did not have HBV and HCV serology (n=59) or a vaccination scheme (n=146) (Fig. 1). This finding serves as an alert to other reference centers, since it means the loss of the opportunity to identify the serological status of this population, as well as the chance to propose vaccination, reducing the known risks of HBV/HIV coinfection. Current evidence suggests that HIV has an adverse effect on HBV-related liver disease progression, increased risk of cirrhosis, liver-related mortality, and hepatocellular carcinoma, especially at lower CD4+ cell counts. Besides that, HBV coinfection has a significant impact on HIV outcomes – when compared with HBV negative patients, those with HBV at the time of HIV diagnosis had a distinguished risk of AIDS or death event [9,14,16,17].
Our study showed a population of patients with a predominance of white men with an age of around 40 years, where the majority were on ART and had a CD4+ cell count greater than 350cells/μL, making it possible to compare results with the literature, due to the similarities in the demographic data of these populations studied [16,21,31,34–39].
HBV vaccine response was 80.10%. However, other studies have found various response rates. In Brazil, Martins et al. [31] performed a study in the southern region with 300 HIV patients who showed a 57.4% vaccine response. Pinto et al. [40] performed a study in the southeastern region of Brazil, obtaining a vaccine response of 56.7%. On the other hand, Potsch et al. [39] analyzed 167 infected patients, also in the southeastern region of Brazil, and found a vaccine response of 91%. Rey et al. [29] compared two groups of patients vaccinated with a standard dose and double dose, and found that 55 and 87.5% exhibited a vaccine response, respectively. Bailey et al. [41] studied 356 vaccinated patients and found a 47% vaccine response. Mena et al. [42] in a review study, analyzed three casuistics: the first one performed in France in 2011, with vaccination coverage of 61.9%; the second one in the United Kingdom in 2009, with 58.2% vaccine response; and the third was carried out in Brazil in 2013, with 57.4% seroconversion. In other studies, the seroconversion rate ranged from 52.6 to 91% [21,24,28,30,37,43,44]. As we know, vaccine response is quite variable, depending very much on the methods of vaccination and the population evaluated. A possible explanation for our good vaccine response rates is the vaccine scheme used, with 4 double doses of recombinant HBV vaccine. Other studies, with much more modest rates, used single doses or a reduced number of applications, or even had more than one possible vaccine scheme [29,30,31,37,40–42].
Comparing the vaccine responses of patients with HIV vaccine responders in relation to age, we found that there was no statistically significant difference between patients aged less than 60 years and those aged 60 years or older. Martins et al. [31] and Bailey et al. [41] did not find a relation between vaccine response and age. The response rates of these patients were 79.90 and 81.80%, respectively. However, some studies have reported older age as an important risk factor in low vaccine response [20,27,36,45–47]. In the present study, there was also no statistically significant difference in vaccine response with regard to CD4+ cell count: 79.50% responded when CD4+ was higher than 350cells/μL and 81.00% when counts were equal to or less than 350cells/μL. Pinto et al. [40] and Bailey et al. [41] also did not correlate vaccine response with CD4+ cell count. However, studies of HIV patients with a mean age of 40 years on ART with double dose HBV vaccine obtained better vaccine responses when the CD4+ cell count was greater than 350cells/μL [18,21,27–30,34,38,41,45]. Landrum et al. [46] reported that the increased risk of HIV-positive responders to HBV vaccine depends on the availability of the vaccine, the guarantee of completion of the vaccination scheme, and the effective use of ART at the time of vaccination. Individuals coinfected with HIV and HBV, especially those with low CD4+ cell counts, are at a higher risk of cirrhosis and mortality from liver disease, emphasizing the importance of comprehensive prevention, vaccination, identification and management of HBV in HIV-infected persons. The results observed here did not show a statistically significant difference in vaccine response according to HIV viral load. It should be emphasized, however, that patients with lower viral load had a higher vaccine response. It is possible that a sample with a greater number of patients could uphold this trend. Several studies have demonstrated that undetectable or low viral load is the most important variable for the best vaccine response against HBV [18,21,27,28,30,32,37,41,43,45,46]. Bailey et al. [41] observed in multivariate analysis that only viral load was predictive of an immune response. O’Bryan et al. [32] followed 186 HIV patients for 5 years and found that the persistence of HBV vaccine response was longer when these patients had undetectable or low viral loads [18].
In view of the good results obtained in the present study with the current vaccination scheme for HIV patients (80%) and the aim to increase the quality of the services provided to this population (we emphasize that 21% of the initial cohort did not have an HBV and HCV serological evaluation or vaccination), it is necessary that clinical protocols be strictly followed and that health care professionals receive guidance and training so that public policies are fully implemented. It is important that the HBV vaccine be offered to all HIV-seropositive patients, reinforcing protection, prevention and reduction of costs and damages caused with regard to HBV/HIV coinfection.
AbbreviationsHIV human immunodeficiency virus acquired immunodeficiency; syndrome hepatitis B virus hepatitis B surface antigen antibody against hepatitis B virus surface antigen hepatitis C virus antiretroviral therapy
The author Arlete F. Rech-Medeiros performed the data collection. All authors performed literature review and compiled data and assisted in writing the final manuscript equally.
Conflict of interestsNothing to declare.
Source of financial supportNothing to declare.
We would like to express our sincere gratitude to the statistician who assisted us in this study, Dr. Mário Bernardes Wagner, postdoctoral fellow in Statistics of Data Analysis in Clinical Research at the former King's College School of Medicine and Dentistry, University of London.