Background. Improving estimation of long-term survival of patients with end-stage liver disease after orthotopic liver transplantation (OLT) would optimize decisions on eligibility for transplant. We aimed to externally validate previously derived Charlson Comorbity Index for OLT (CCI-OLT); subsequently, we developed a new model to predict 5-year mortality after transplant.
Material and methods. This single center retrospective cohort study included 524 consecutive adult cirrhotic patients who underwent OLT in 2002-2012. External validation of CCI-OLT used Kaplan-Meier method. Derivation of the new predictive model used Cox proportional hazards regression.
Results. One-, 3-, and 5-year cumulative survival after OLT was 89%, 80%, and 73%, respectively. CCI-OLT was not associated with 5-year mortality after transplant (P = 0.34). We derived and internally validated a new predictive model of 5-year mortality after OLT based on six pre-transplant characteristics of patients: age, body mass index, hepatitis C, hepatic encephalopathy, intensive care unit stay at transplant, and live donor (C-index = 0.64). We further developed a nomogram to estimate individual probability of 1-, 3-, and 5-year survival after OLT.
Conclusions. In our cohort, CCI-OLT was not associated with survival following transplant. The new predictive model discriminative capacity was only modest, suggesting that pre-transplant characteristics are of limited value in predicting post-transplant outcomes in thoroughly selected patients.
Orthotopic liver transplantation (OLT) is the definitive treatment for a wide range of liver diseases.1 Survival and quality of life has been improving as a result of advances in organ preservation and allocation, surgical techniques, immunosuppression regimens, and overall medical management.2,3
Despite improving post-transplant outcomes, many patients still succumb to the complications of end-stage liver disease while awaiting OLT due to organ scarcity. The Model for End-Stage Liver Disease (MELD) accurately predicts mortality prior to OLT,4 and has been widely used to prioritize candidates for organ allocation. This widespread use of the MELD has led to a reduction in waitlist new registrations, waiting time, and mortality, and an increase in transplant procedures, without significantly altering overall survival after OLT.5 In fact, while accurate at predicting survival in the absence of transplant, the MELD has not been shown to accurately predict outcomes after OLT.6
Nevertheless, survival benefit from OLT needs to account for a minimum expected predicted post-transplant survival,7 which has been quantified as greater than 50% at 5 years after transplant.8 In this regard, the development of predictive rules for long-term survival after OLT based on pre-trans-plant characteristics of potential liver recipients would provide fundamental information to clinicians to better advise their patients on potential survival, prioritize organ use, and compare outcomes between different transplant centers.9
The Charlson Comorbidity Index for OLT (CCI-OLT), an adaptation of the classical Charlson Comorbidity Index, was recently developed to predict 5-year mortality after OLT for patients with end-stage liver disease.10–12 It is a composite index based on the presence of five comorbidities before transplant: coronary artery disease; diabetes mellitus; chronic pulmonary obstructive disease; connective tissue disease; and kidney injury.12 However, after its development, there has not been an appropriate external validation of this index.
Accordingly, our first objective was to validate the CCI-OLT in our cohort of cirrhotic patients undergoing OLT. As CCI-OLT did not predict post-transplant mortality in our cohort (see Results), our second objective was to develop a new predictive rule for 5-year mortality after OLT based on an alternative set of pre-transplant characteristics of potential liver recipients.
Material and MethodsThe reporting of this study followed the STROBE statement.13 The local Health Research Ethics Board approved this study and the requirement for individual informed consent was waived.
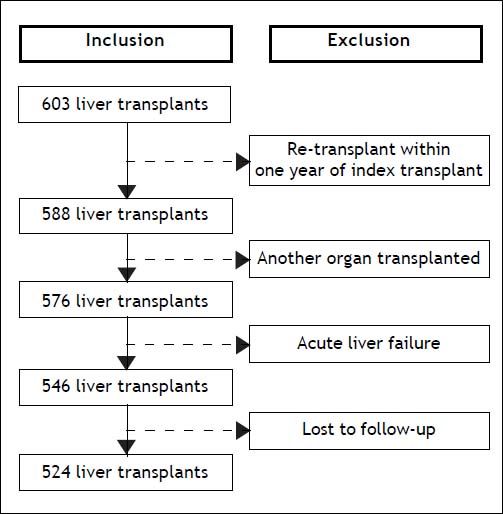
Design, setting, and participantsThis was a single center retrospective cohort study, which included consecutive adult (≥ 18 years of age) cirrhotic patients who underwent OLT in a Canadian transplant center (University of Alberta Hospital, Edmonton, Alberta) between January 1, 2002 and June 30, 2012. Patients were excluded if:
- a)
They had a re-transplant within one year of the initial transplant.
- b)
They were concomitantly transplanted with another organ (kidney or pancreas).
- c)
Their primary diagnosis was acute liver failure.
- d)
They were lost to follow-up.
Cirrhosis was defined as bridging fibrosis on previous liver biopsy or a composite of clinical signs and findings provided by laboratory tests, endoscopy, and radiologic imaging.14 Complications of cirrhosis considered were infection, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome. The definition of each of these complications is provided in Supplementary Methods.
Comorbidities included in the CCI-OLT were defined based on the study that developed this index to facilitate comparisons.12 Coronary artery disease was defined as documented history of myocardial infarction or coronary disease on angiography. Diabetes mellitus was defined as chronic hyperglycemia (fasting plasma glucose ≥ 7mmol/l (126 mg/dL) or glycated hemoglobin ≥ 6.5%), requiring outpatient medications at any time during the month preceding transplant.15 Chronic obstructive pulmonary disease was defined as documented chronic pulmonary obstruction (based on pulmonary function tests) with frequent requirement for medications (e.g. bronchodilators, corticosteroids). Connective tissue disease included diagnostics of systemic lupus, rheumatoid arthritis, scleroderma, or seronegative spondyloarthropathy. Kidney injury was defined by a serum creatinine equal or greater than 133 µmol/l (1.5 mg/dL) on the most recent pre-transplant assessment.16 CCI-OLT is therefore a composite index calculated by adding the individual scores earned by each of the five comorbidities (total score out of 10).12 The individual scores are assigned as follows: 2 for coronary artery disease, 1 for diabetes mellitus, 3 for chronic obstructive pulmonary disease, 2 for connective tissue disease, and 2 for kidney injury.12 Only patients with inactive or controlled comorbidities were included.
The University of Alberta Hospital liver transplant program indications and contra-indications for OLT and immunosuppression regimen in use are specified in Supplementary Methods.
Primary exposure and outcomePrimary exposure was the liver transplant. Primary outcome was long-term mortality after OLT, which was defined as 5-year mortality.
Data collectionThe University of Alberta Hospital liver transplant program started in 1989 and since 1995 has maintained a dedicated computerized database using the Organ Transplant Tracking Record (OTTR, HKS Medical Information Systems, Omaha, Nebraska, USA). Patients were initially identified using OTTR. Data on patients’ age, sex, race, body mass index, indications for OLT, complications of cirrhosis, comorbidities (CCI-OLT), serum parameters, severity of liver disease indexes (Child-Turcotte-Pugh and MELD), the need for intensive care unit (ICU) admission just prior to OLT, Sequential Organ Failure Assessment (SOFA), time between listing and transplant, live donor transplant, follow-up, and survival status were retrieved from OTTR and patients’ medical records.
Statistical analysisCategorical variables were presented as proportions and continuous variables as mean and standard deviation (SD), if normally distributed, or median and inter-quartile range (IQR), if non-normally distributed.
Univariable comparisons were performed taking into account time-to-event (Kaplan-Meier method for categorical variables and univariable Cox proportional hazards regression for continuous variables). External validation of CCI-OLT was done using the Kaplan Meier method. Survival was then modeled with Cox proportional hazards regression. We first specified a full model of ten variables based on previously reported clinically relevant knowledge, the number of events in our sample, and data inspection. The rate of missing values for these variables was lower than 3%. Single imputation was performed in order to use the complete set of patients. Due to obvious non-linear relationship with the primary outcome, body mass index was modeled with restricted cubic splines (3 knots). Further selection of variables in the model was done with Lasso shrinkage of regression coefficients. This approach provides less biased and unstable models than methods relying on the significance of the variables (automatic stepwise selection methods).17 Variables with regression coefficients shrunk to zero were dropped from the model. This selection method yielded a final model with six variables, which was subsequently internally validated with bootstrapping (two-hundred replications). Regression coefficients were corrected for optimism also with bootstrapping. Discriminative capacity of the final model was assessed by the C-index.18 Based on the final model, a nomogram to predict 1-, 3-, and 5-year survival after OLT was derived as previously described.19 Statistical analysis was performed with IBM SPSS Statistics, Version 20 (IBM Corp., North Castle, New York, USA) and R software (R Foundation for Statistical Computing, Vienna, Austria), with the aid of rms and penalized packages.
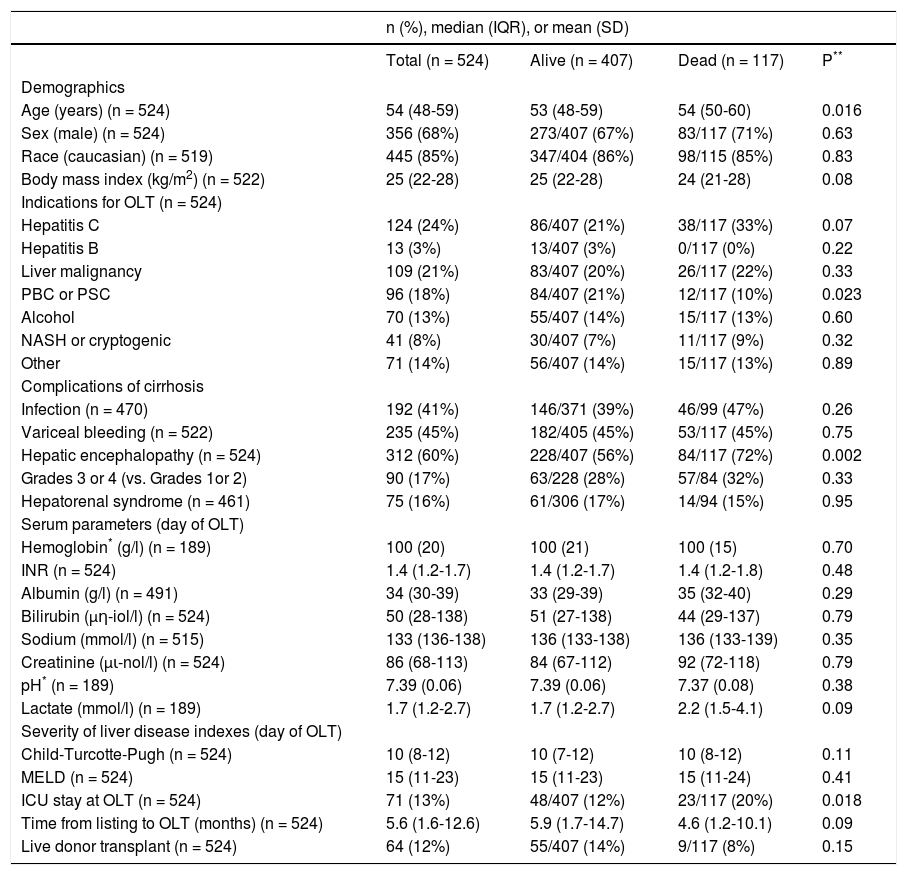
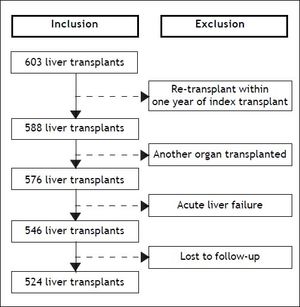
ResultsPre-transplant characteristics of patientsOf the 603 OLT procedures performed at the University of Alberta Hospital during the study period, 524 met the eligibility criteria (Figure 1). Median age was 54 years (IQR, 48-59) and 68% were men. Median body mass index was 25 kg/m2 (IQR, 22-28). The most common indications for OLT were hepatitis C (24%), liver malignancy (21%), and primary biliary cirrhosis or primary sclerosing cholangitis (18%). On transplant waitlist, hepatic encephalopathy was the most frequent complication of cirrhosis (60%). On the day of OLT, median biochemical MELD score was 15 (IQR, 11-23). ICU admission just prior to OLT was required for 13% of patients. Organ support was necessary for the majority of patients in ICU: mechanical ventilation in 76%, vasopressors in 71%, and renal replacement therapy in 69%. On the day of OLT, patients in the ICU had a mean SOFA score of 16 (SD, 4). A liver from a live donor was used in 12% of patients. Pretransplant characteristics of patients are summarized in table 1.
Pre-transplant characteristics for the entire cohort and stratified by survival status at 5 years after transplant.
| n (%), median (IQR), or mean (SD) | ||||
|---|---|---|---|---|
| Total (n = 524) | Alive (n = 407) | Dead (n = 117) | P** | |
| Demographics | ||||
| Age (years) (n = 524) | 54 (48-59) | 53 (48-59) | 54 (50-60) | 0.016 |
| Sex (male) (n = 524) | 356 (68%) | 273/407 (67%) | 83/117 (71%) | 0.63 |
| Race (caucasian) (n = 519) | 445 (85%) | 347/404 (86%) | 98/115 (85%) | 0.83 |
| Body mass index (kg/m2) (n = 522) | 25 (22-28) | 25 (22-28) | 24 (21-28) | 0.08 |
| Indications for OLT (n = 524) | ||||
| Hepatitis C | 124 (24%) | 86/407 (21%) | 38/117 (33%) | 0.07 |
| Hepatitis B | 13 (3%) | 13/407 (3%) | 0/117 (0%) | 0.22 |
| Liver malignancy | 109 (21%) | 83/407 (20%) | 26/117 (22%) | 0.33 |
| PBC or PSC | 96 (18%) | 84/407 (21%) | 12/117 (10%) | 0.023 |
| Alcohol | 70 (13%) | 55/407 (14%) | 15/117 (13%) | 0.60 |
| NASH or cryptogenic | 41 (8%) | 30/407 (7%) | 11/117 (9%) | 0.32 |
| Other | 71 (14%) | 56/407 (14%) | 15/117 (13%) | 0.89 |
| Complications of cirrhosis | ||||
| Infection (n = 470) | 192 (41%) | 146/371 (39%) | 46/99 (47%) | 0.26 |
| Variceal bleeding (n = 522) | 235 (45%) | 182/405 (45%) | 53/117 (45%) | 0.75 |
| Hepatic encephalopathy (n = 524) | 312 (60%) | 228/407 (56%) | 84/117 (72%) | 0.002 |
| Grades 3 or 4 (vs. Grades 1or 2) | 90 (17%) | 63/228 (28%) | 57/84 (32%) | 0.33 |
| Hepatorenal syndrome (n = 461) | 75 (16%) | 61/306 (17%) | 14/94 (15%) | 0.95 |
| Serum parameters (day of OLT) | ||||
| Hemoglobin* (g/l) (n = 189) | 100 (20) | 100 (21) | 100 (15) | 0.70 |
| INR (n = 524) | 1.4 (1.2-1.7) | 1.4 (1.2-1.7) | 1.4 (1.2-1.8) | 0.48 |
| Albumin (g/l) (n = 491) | 34 (30-39) | 33 (29-39) | 35 (32-40) | 0.29 |
| Bilirubin (μη-iol/l) (n = 524) | 50 (28-138) | 51 (27-138) | 44 (29-137) | 0.79 |
| Sodium (mmol/l) (n = 515) | 133 (136-138) | 136 (133-138) | 136 (133-139) | 0.35 |
| Creatinine (μι-nol/l) (n = 524) | 86 (68-113) | 84 (67-112) | 92 (72-118) | 0.79 |
| pH* (n = 189) | 7.39 (0.06) | 7.39 (0.06) | 7.37 (0.08) | 0.38 |
| Lactate (mmol/l) (n = 189) | 1.7 (1.2-2.7) | 1.7 (1.2-2.7) | 2.2 (1.5-4.1) | 0.09 |
| Severity of liver disease indexes (day of OLT) | ||||
| Child-Turcotte-Pugh (n = 524) | 10 (8-12) | 10 (7-12) | 10 (8-12) | 0.11 |
| MELD (n = 524) | 15 (11-23) | 15 (11-23) | 15 (11-24) | 0.41 |
| ICU stay at OLT (n = 524) | 71 (13%) | 48/407 (12%) | 23/117 (20%) | 0.018 |
| Time from listing to OLT (months) (n = 524) | 5.6 (1.6-12.6) | 5.9 (1.7-14.7) | 4.6 (1.2-10.1) | 0.09 |
| Live donor transplant (n = 524) | 64 (12%) | 55/407 (14%) | 9/117 (8%) | 0.15 |
n: frequency. IQR: inter-quartile range. SD: standard deviation. OLT: orthotopic liver transplantation. PBC: primary biliary cirrhosis. PSC: primary sclerosing cholangitis. NASH: nonalcoholic steatohepatitis. INR: international normalized ratio. MELD: Model for End-Stage Liver Disease. ICU: intensive care unit.
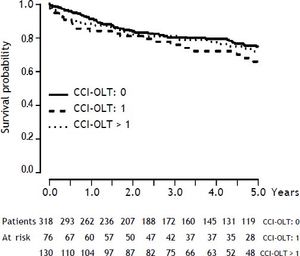
Overall, the median follow-up time after OLT for the entire cohort was 3.5 years (IQR, 1.4-6.4). For the survival analysis, follow-up time was capped at 5 years after OLT. A total of 117 deaths and 23 retransplants occurred during this selected time period. The 1-, 3-, and 5-year cumulative patient survival after OLT was 89%, 80%, and 73%, respectively. Causes of death were sepsis in 26%, malignancy (both recurrent and de novo) in 24%, recurrence of non-malignant liver disease in 21%, cardiovascular event in 15%, chronic rejection in 4%, and other in 10%.
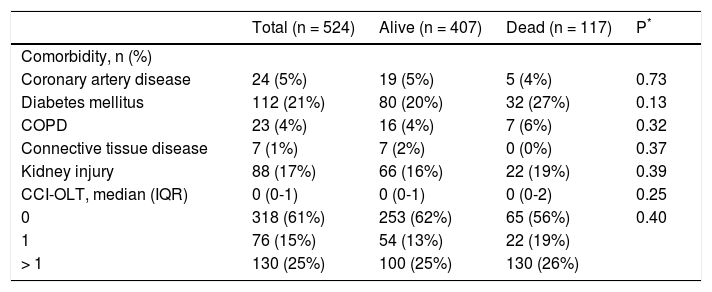
Prior to transplant, 39% of patients had at least one comorbidity, the most common being diabetes mellitus (21%) and kidney injury (17%), as shown in Table 2. While diabetes mellitus showed a nonsignificant trend towards higher likelihood of 5-year mortality after OLT (20% vs. 27%; P = 0.13), none of the other comorbidities were associated with 5-year mortality after transplant (P > 0.30 for all comparisons).
Pre-transplant comorbities and Charlson Comorbidity Index for Orthotopic Liver Transplantation (CCI-OLT) for the entire cohort and stratified by survival status at 5 years after transplant.
| Total (n = 524) | Alive (n = 407) | Dead (n = 117) | P* | |
|---|---|---|---|---|
| Comorbidity, n (%) | ||||
| Coronary artery disease | 24 (5%) | 19 (5%) | 5 (4%) | 0.73 |
| Diabetes mellitus | 112 (21%) | 80 (20%) | 32 (27%) | 0.13 |
| COPD | 23 (4%) | 16 (4%) | 7 (6%) | 0.32 |
| Connective tissue disease | 7 (1%) | 7 (2%) | 0 (0%) | 0.37 |
| Kidney injury | 88 (17%) | 66 (16%) | 22 (19%) | 0.39 |
| CCI-OLT, median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-2) | 0.25 |
| 0 | 318 (61%) | 253 (62%) | 65 (56%) | 0.40 |
| 1 | 76 (15%) | 54 (13%) | 22 (19%) | |
| > 1 | 130 (25%) | 100 (25%) | 130 (26%) |
n: frequency. COPD: chronic obstructive pulmonary disease. CCI-OLT: Charlson Comorbidity Index for Orthotopic Liver Transplantation. IQR: inter-quartile range.
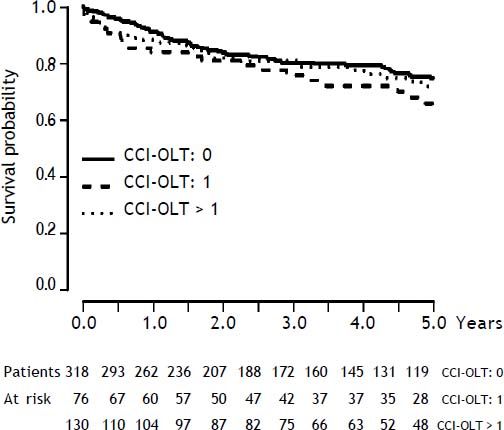
As presented also in Table 2, CCI-OLT was not associated with 5-year mortality after OLT, both as a continuous (median, 0 vs. 0; P = 0.25) and a categorical variable (P = 0.40 for the comparison between the three categories). Kaplan-Meier cumulative survival after transplant stratified by CCI-OLT categories 0, 1, and > 1 was not significantly different (Figure 2: Log-Rank test P = 0.34).
New predictive model of 5-year mortality after OLTAs per univariable comparisons shown in Table 1, age (median, 53 vs. 54; P = 0.016), hepatic encephalopathy (any degree by West Haven criteria; 56% vs. 72%; P = 0.002), and ICU stay at OLT (12% vs. 20%; P = 0.018) were significantly associated with 5-year mortality after OLT. Hepatitis C (21% vs. 33%; P = 0.07) showed a non-significant trend towards higher likelihood of 5-year mortality after OLT. On the contrary, body mass index (median, 25 vs. 24; P = 0.08) and live donor transplant (14% vs. 8%; P = 0.15) revealed a non-significant trend towards a lower likelihood of 5-year mortality after OLT.
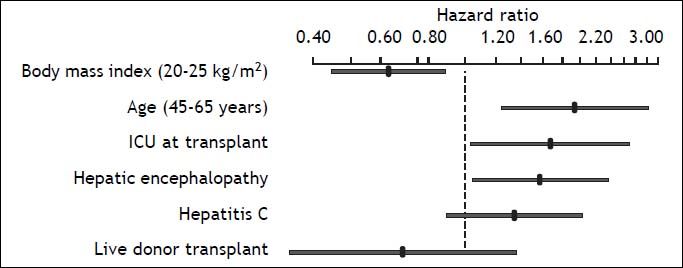
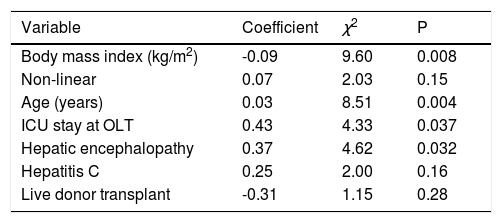
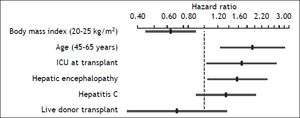
After variable selection procedures (see Material and Methods section), these six variables were deemed to be the best to derive the new predictive model of 5-year mortality after OLT. As presented in Table 3, age (P = 0.004), body mass index (P = 0.008), hepatic encephalopathy (P = 0.032), and ICU stay at OLT (P = 0.037) were the most influential predictive factors of 5-year mortality after transplant. Hazard ratios of the variables in the new predictive model are displayed in Figure 3. The C-index of the predictive model was 0.64.
Cox proportional hazards regression new predictive model of 5-year mortality after transplant.
| Variable | Coefficient | χ2 | P |
|---|---|---|---|
| Body mass index (kg/m2) | -0.09 | 9.60 | 0.008 |
| Non-linear | 0.07 | 2.03 | 0.15 |
| Age (years) | 0.03 | 8.51 | 0.004 |
| ICU stay at OLT | 0.43 | 4.33 | 0.037 |
| Hepatic encephalopathy | 0.37 | 4.62 | 0.032 |
| Hepatitis C | 0.25 | 2.00 | 0.16 |
| Live donor transplant | -0.31 | 1.15 | 0.28 |
Model measures (n = 524): χ2, 27.77 (Df, 7). P < 0.001. C-index, 0.64. ICU: intensive care unit. OLT: orthotopic liver transplantation.
Cox regression hazard ratios for the six variables in the new predictive model of 5-year mortality after liver transplant. The hazard ratio for body mass index reflects the relative change in risk of a patient with a body mass index of 25 as compared with a patient with a body mass index of 20. The hazard ratio of age reflects the relative change in risk comparing a 65-year-old patient with 45-year old one.
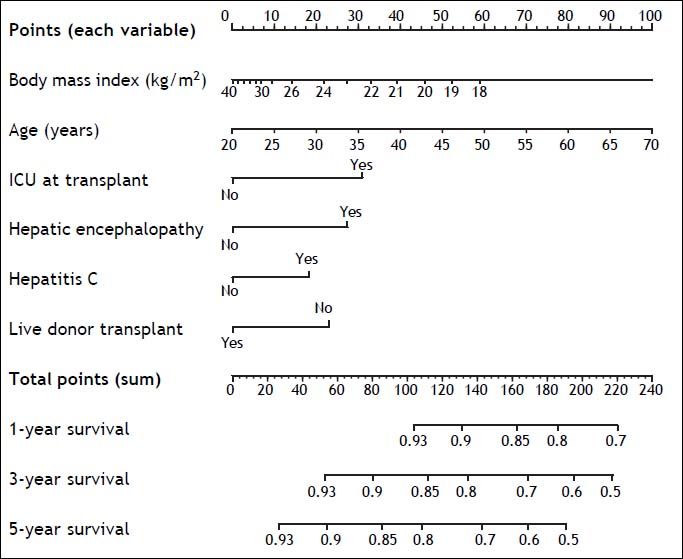
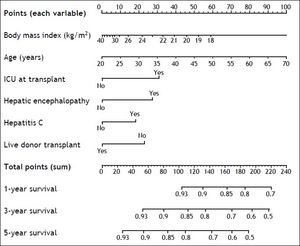
Based on the six variables included in the new predictive model of 5-year mortality after OLT, a nomogram to estimate individual predicted probability of 1-, 3-, and 5-year survival after transplant was derived (Figure 4). Total points are calculated by adding points earned by each of the six predictive variables.
DiscussionExternal validation of CCI-OLTIn a large cohort of cirrhotic patients undergoing OLT, we found that the previously derived CCI-OLT was not associated with 5-year mortality after transplant.12
In their study, Volk, et al.12 reported proportions of pre-transplant comorbidities (e.g. diabetes mellitus, 23%; and kidney injury, 16%) and CCI-OLT categories (0, 62%; 1, 16%; and > 1, 22%) similar to the ones we found in our study. However, they reported a 5-year survival after OLT (68%) lower than the one we found in our cohort. This difference in long-term survival after transplant may be explained by the older character of their cohort (1994-2005), as there is mounting evidence that cirrhotic patients outcomes after OLT have been improving throughout time due to several advances in the overall management of those patients before and after transplant.20,21 An additional explanation to that dissimilarity in long term survival after OLT may be the overall severity of liver disease in the two cohorts. Despite the median biochemical MELD score in their cohort16 being similar to the one found in our study, they did not report the prevalence of additional markers of severity of liver disease, such as the complications of cirrhosis, which may not be entirely reflected by the MELD, but are still most relevant to the prognosis of these patients.22,23
In summary, CCI-OLT cannot be used as a predictive rule for 5-year mortality after transplant in our center and possibly in other centers whose populations have similar characteristics to ours.
New predictive model of 5-year mortality after OLTDue to the lack of external validity of CCI-OLT, we developed a new predictive model of 5-year mortality after OLT for adult cirrhotic patients. After a variable selection process described in Material and Methods section, the final model was based on the following pre-transplant characteristics: age, body mass index, hepatitis C etiology, hepatic encephalopathy, the need for ICU stay just prior to OLT, and enrollment for live donor transplant. To allow for a potential translation into clinical practice, we went further to create an easy-to-use nomogram that estimates the individual probability of 1-, 3-, and 5-year survival after OLT.
The potential strengths of the new predictive model reside in its simplicity, as it relies only on six pieces of information easily available to clinicians, and its internal validity, due to the thorough statistical approach used for its derivation. Predicted 5-year mortality after transplant was uniformly under 50%, which complies with the widely accepted selection criterion for OLT, a minimum expected predicted 5-year survival after transplant greater than 50%.8 In this regard, an example to show the ultimate utility of the new predictive model would be to calculate the predicted 5-year survival after OLT for a patient with end-stage liver disease, 60 years of age, a body mass index of 20 kg/m2, hepatitis C, hepatic encephalopathy, admitted to the ICU, and not a candidate to receive a liver from a live donor. Using the derived nomogram, this patient would have an estimated predicted 5-year survival after OLT lower than 50% (total points > 190), which could factor in the decision for listing for OLT.
In the new predictive model of 5-year mortality after OLT, age, body mass index, hepatic encephalopathy, and the need for ICU stay just prior to OLT are the most influential predictive factors, which is in part consistent to what has been previously described.
In their study, Thuluvath, et al.9 developed a model to predict 5-year survival after OLT based on the following seven categorized pre-transplant characteristics of patients: age, body mass index, United Network for Organ Sharing (UNOS) status,24 etiology of liver disease, serum bilirubin, serum creatinine, and race. However, the reported 5-year survival for this cohort (61%) was lower than the one we found in our study. As neither overall MELD score nor the prevalence of complications of cirrhosis were specified in their study, the overall severity of liver disease may be sparingly reflected by the proportion of patients classified as UNOS status 1 (17%), given the fact that they did not exclude patients transplanted for acute liver failure. Therefore, the difference in long-term survival between the two cohorts may be explained by the older character of their cohort (1987-2001).20,21 Additionally, even with a fairly larger cohort of patients (n = 9,100), discrimination of their predictive model (C-index 0.63) was similar to ours. The fact that the new predictive model explains a low proportion of variability in 5-year mortality after OLT is not unexpected, as patients undergoing OLT need to be thoroughly selected25 and other factors cannot be accounted for prior to transplant, namely donor, peri-operative, and postoperative related factors.26–28
In the regression model (Table 3), the age coefficient was calculated as “per year increase”, which makes its absolute value low. Nevertheless, as shown in the hazard ratios plot (Figure 3), the probability of survival at 5 years after transplant between patients with 45 and 65 years old almost doubles, which favors the relevance of age for LT recipients prognosis. In the nomogram, age is presented until 70 years because selected patients at this age may still be eligible for OLT. In fact, age restrictions for transplant vary by center, but ‘physiologic’ rather than chronologic age seems to be most important.29,30
Also in the nomogram, due to the non-linear behavior of BMI, individual risk variations for scores between 18 and 30 kg/m2 were clearly more important than those for scores between 30 and 40 kg/m2. By considering BMI a possible surrogate for patients’ nutritional status, the risk of transplanting a less well nourished patient, with all its physiological implications, is probably greater.
Neither Volk, et al.12 nor Thuluvath, et al.9 reported the prevalence of complications of cirrhosis in their cohorts, so our description of pre-transplant hepatic encephalopathy as a predictor of long-term mortality after OLT may only be compared with a recent study from Ahmed, et al.;31 they too showed that pre-transplant hepatic encephalopathy was associated with increased post-OLT mortality. While pre-transplant hepatic encephalopathy has been reported to be associated with post-transplant neurocognitive changes,32,33 the mechanistic explanation for its influence on post-OLT outcomes needs further clarification.
Prognosis of critically-ill cirrhotic patients requiring ICU admission depends on the number of organ failures.14 In our cohort, organ support in the ICU may have been an additional surrogate for disease severity with impact on post-OLT outcomes, as suggested previously.34
Hepatitis C showed a tendency to be associated with worse outcomes after OLT, as it has been described.35 New treatment regimens for hepatitis C after transplant may help change this course.36,37 Nevertheless, there may be a relevant time lag before sicker patients, those with acute on chronic liver failure, actually see the presumable benefit of those new treatment regimens.
The fact that our study included patients receiving a liver from a live donor may reinforce comparability of our results, as this practice is becoming more frequent and most recent publications report reasonable post-transplant outcomes for this particular group of liver recipients.38,39
In summary, even with a large cohort of patients and up-to-date robust statistical approach, prognostication of long-term survival following OLT based only on pre-transplant patients’ characteristics may be difficult, as patients undergo such a thorough selection process before inclusion in the waitlist for transplant.
Limitations of the studyThe results of our study need to be considered in the context of the following limitations. First, despite the largest referral base for OLT in western Canada, this was a retrospective analysis of prospectively collected data from a single transplant center and may have been prone to selection bias. Second, even though our cohort of patients represents a very recent period of time (2002-2012), thus being likely to reflect a reliable “realworld” setting, with outcomes closer to day-to-day clinical practice, and despite modeling with state-of-the-art statistical methodologies to reduce bias (shrinkage and bootstrapping), generalization of our results would require external validation. In this regard, as individual transplant centers, especially from different countries, have diverse groups of patients and practices regarding listing for transplant, organ allocation, and medical and surgical management strategies, such generalization of results may be difficult. While a more site-specific application of a predictive model such as ours may be worthwhile, in the future, larger multicenter studies may help to clarify which factors should we consider most for predicting post-OLT outcomes and thus optimize selection of patients for OLT.
ConclusionsIn our cohort of adult cirrhotic patients, CCI-OLT was not associated with survival after transplant. Based on six pre-transplant characteristics of these patients, we derived a new predictive model of 5-year mortality after OLT. The relatively modest discriminative capacity of this model suggests that pre-transplant characteristics may be of limited value in predicting post-transplant outcomes in such highly selected patients.
Abbreviations- •
CCI-OLT: Charlson Comorbity Index for Orthotopic Liver Transplantation.
- •
ICU: Intensive Care Unit.
- •
IQR: inter-quartile range.
- •
MELD: Model for End-Stage Liver Disease.
- •
OLT: orthotopic liver transplantation.
- •
OTTR: Organ Transplant Tracking Record.
- •
SD: standard deviation.
- •
SOFA: Sequential Organ Failure Assessment.
- •
UNOS: United Network for Organ Sharing.
F.S.C. Conceived the idea of the study, wrote and extensively revised the manuscript, compiled the final database, and performed statistical and data analyses.
S.M.B. Provided content expertise, significant guidance on analysis and interpretation of data, and assisted extensively with manuscript revision.
J.G.A. Provided content expertise, performed significant statistical analysis (bootstrapping, multivariable modeling), significant guidance on analysis and interpretation of data, and assisted extensively with manuscript revision.
N.M.K. Provided content expertise and significantly revised the final manuscript
G.M. Helped compile the database and revised the final manuscript.
P.F. Significantly revised the final manuscript.
C.J.K. Conceived the idea for the study, provided content expertise, significant guidance on compilation of the database, analysis and interpretation of data, and assisted extensively with manuscript revision.
Funding SourcesDr. Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology and Clinical Investigator Award from Alberta Innovates - Health Solutions. Drs. Cardoso and Fidalgo are supported by an unrestricted educational grant donated by Gambro Inc.