Introduction. The present study aimed to elucidate the potential antifibrotic effects of pinocembrin (PIN), a flavanone found abundantly in honey and propolis, by studying its effect on different oxidative stress, inflammatory and fibrosis markers in an experimental model of CCl4-induced liver fibrosis.
Material and methods. PIN (20 mg/kg) was given orally 3 times/week for 6 consecutive weeks alternating with CCl4 (0.5 mL/kg, 1:1 mixture with corn oil, i. p.) twice weekly. Different hepatotoxicity indices, oxidative stress, inflammatory and liver fibrosis markers were assessed.
Results. PIN significantly restored liver transaminases and total cholesterol to normal levels. Also, PIN ameliorated oxidative stress injury evoked by CCl4 as evidenced by inhibition of reduced glutathione depletion and lipid peroxidation as well as elevation of antioxidant enzyme superoxide dismutase (SOD). Further, PIN up-regulated the nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2), thereby inducing the expression and activity of the cytoprotective enzyme hemeoxygenase-1 (HO-1). Moreover, PIN alleviated pro-inflammatory cytokines such as TNF-α via inhibiting nuclear factor-KB (nF-kB) activation. As markers of fibrosis, collagen and α-SMA expression increased markedly in the CCl4 group and PIN prevented these alterations. In addition, PIN down-regulated TGFβ1 and p-Smad2/3, thereby inhibiting TGFβ1/Smad signaling pathway.
Conclusion. These results suggest that PIN possess potent antifibrotic effects that can be explained on its antioxidant properties. It ameliorates oxidative stress and inflammation during induction of fibrogenesis via its ability to augment cellular antioxidant defenses, activating Nrf2-mediated HO-1 expression and modulating NF-KB and TGF-β1/Smad signaling pathway.
Liver fibrosis is a prevalent result of different ceaseless liver damages, which results from inordinate accumulation of extracellular matrix (ECM), because of inequality between ECM synthesis and deterioration.1 Hepatic stellate cells (HSCs) undergo activation due to many factors,2,3 converting to vitamin A-losing and α-smooth muscle actin (α-SMA) expressing myobroblasts.4 Also, activated HSCs produce fibrillar collagens leading to deposition of fibrotic matrix.5
Although growth factors, inflammatory factors and free radicals may participate in HSCs activation, data evoke a strong link with the transforming growth factor-β (TGF-β ) signaling pathway.6 Indeed, Wynn reported that modulation of TGF-β/Smad signaling by down-regulation of TGF-β expression may be efficient in inhibiting tissue fibrosis.1 After binding of TGF-β to the type II receptor, kinases phosphorylate type I receptor, which further phosphorylates Smad2 and Smad3. Phosphorylated Smad2 and Smad3 then form a heteromeric complex with Smad4 that translocates to the nucleus and binds to the promoter of TGF-β-responsive genes to regulate their expression.7 TGF-β1 also stimulates Smad3-dependent transcription of fibril collagens, which inhibit degradation of ECM.8 Therefore, TGF-β1/Smad signaling pathway is considered a target to inhibit the activation of HSCs and ameliorate liver fibrosis.
In addition, oxidative stress promotes HSCs activation and collagen production and plays an important role in the pathogenesis of liver fibrosis.9 Nuclear erythroid 2-related factor 2 (Nrf2) is an oxidative stress-mediated transcription factor with a variety of downstream targets aimed at cytoprotection. Activating Nrf2, promotes the expression of its target genes and increases the antioxidant enzymes activity.10 Furthurmore, activation of Nrf2 may be a safe and effective strategy for anticipation of liver fibrosis and that Nrf2 activators may have great capability versus liver fibrosis.11
Pinocembrin (5, 7-dihydroxyflavanone, PIN) is a fla-vanone found plentifully in honey and propolis.12 Many studies have established that PIN possesses multiple activities, of which; neuroprotective,13,14 anti-inflammatory,15 anti-oxidant,16,17 hepatoprotective18 and anti-proliferative effects.19 PIN regulated the production of TNF-α via inhibiting NF-κB, ERK1/2, JNK and p38MAPK in lipopolysaccharide-induced inflammatory responses15 and induced nuclear translocation of Nrf2 and expression of HO-β1 in neuroblastoma SH-SY5Y cells.20 Propolis also prevented the effects of TGF-β1-induced Smad2 activation pathway in fibrotic lung diseases.21 Furthermore, Dodonaea viscosa ethanol extract, in which PIN was present, was examined in vivo against CCl4-induced liver fibrosis in rats22 and managed to decrease liver fibrosis severity, hepatic enzymes and oxidative stress markers and normalized hepatic architecture. In addition, PIN inhibited thioacetamide-induced tissue Transglutaminase mRNA expression in Hep 3B carcinoma cell line, resulting in inhibition of hepatic fibrosis and cirrhosis.18 These findings indicated that PIN has protective effects on fibrosis but the mechanism by which PIN antagonizes liver fibrosis has not been fully examined. In the present study, we aimed at predicting the ability of PIN to attenuate the progression of chronic liver fibrosis induced by long-term administration of carbon tetrachloride (CCl4) through reducing oxidative stress, cellular inflammation and downregulating the expression of pro-fibrogenic markers by modulating TGF-β/Smad and Nrf-2 pathways.
Materials And MethodsDrugs and chemicalsPinocembrin (purity > 99.7%) was purchased from Sichuan Research Center of Traditional Chinese Medicine (Chengdu, China), 2-hydroxypropyl-β-cyclodextrin (HPβ CD) from Roquette (France-Europe) and Carbon Tetrachloride (CCl4), bovine serum albumin, reduced glutathione (GSH), Ellman’s reagent [3,3’-dithiobis (6-nitrobenzoic acid)] and thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). n-Butanol and formaldehyde 37% were purchased from ElNasr Chemical Co. (Egypt). All other chemicals and solvents were of highest grade commercially available.
AnimalsMale Wistar rats (180–220 g) were obtained from Nile Co. for Pharmaceutical and Chemical Industries, Egypt. Rats were housed in an air-conditioned atmosphere; at a temperature of 25 °C. Animals were acclimated for 2 weeks before experimentation. They were kept on a standard diet and water ad libitum. Standard diet pellets (El-Nasr, Abu Zaabal, Egypt) contained not less than 20% protein, 5% fiber, 3.5% fat, 6.5% ash and 1% vitamin mixture. The study protocol was approved by the Ethical Committee, Faculty of Pharmacy, Ain Shams University, Egypt. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the National Institutes of Health guidelines, U.S.A.
Experimental designRats were randomly assigned into four groups (fifteen animals in each group) and treated for six weeks as follows; Group (A) served as control group and was given 1 mL/kg body weight from 20% HPβCD (the vehicle of PIN) through oral gavage three times per week and corn oil 0.5 mL/kg body weight i.p. twice per week alternating with HPβCD. Group (B) was given PIN 20mg/kg body weight dissolved in 20% HP CD through oral gavage three times per week. Group(C) was given CCl4 (0.5 mL/kg body weight, 1:1 mixture with corn oil, i.p.), twice weekly to induce liver fibrosis and group (D) was given PIN 20 mg/kg body weight dissolved in 20% HPβCD through oral gavage three times per week alternating with CCl4 (0.5 mL/kg body weight, 1:1 mixture with corn oil, i.p.), twice weekly.
Twenty-four hours after the last oral gavage, blood samples were collected from the retro-orbital plexus and allowed to clot. Serum was separated by centrifugation at 5,000 rpm for 10 min and used for the assessment of liver functions. Rats were sacrificed and liver tissues were dissected out and washed with ice-cold saline. Part of liver was homogenized in saline then the homogenate was used for assessment of oxidative stress and anti-inflammatory markers while other specimens from liver were fixed in 10% buffered formaldehyde for histopathological and immunohistochemical investigation.
Assessment of hepatotoxicity indicesSerum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total cholesterol (TC) were estimated using available commercial kits (Biodiagnostic, Giza, Egypt). Liver index was calculated according to the formula: Liver index was = (liver weight/body weight) x 100
Reduced glutathione (GSH), lipid peroxides (malondialdehyde, MDA) and superoxide dismutase (SOD) were determined in liver homogenate using colorimetric kits (Biodiagnostics, Cairo, Egypt).
Quantitative ELISA immunoassayTNF-α level in liver homogenate was assessed as an inflammatory marker. Determination of TNF-α was performed using commercial ELISA kit (R&D Systems, MN, USA) according to the manufacturer’s instructions. The quantities of rat TNF-α were expressed as pg/mg protein and were determined according to method of Olsson.23
Real-time RT-PCR analysisNF-kB as an inflammatory marker and collagen I as a liver fibrosis marker were determined using RT-PCR. Total RNA was extracted from liver tissue using SV Total RNA Isolation system as recommended by the manufacturer (Promega, Madison, WI, USA). Then, reverse-transcribed using reverse transcriptional polymerase chain reaction (RT-PCR) kit (Stratagene, USA) and quantitative real-time PCR was performed using SYBR Green. β-actin was used as standard housekeeping control. Levels of NF-κB and collagen I were calculated based on the method of Pfaffl.24 The primers were as follows: NF-kB1; forward: 5’-TACTCTGGCGCAGAAATTAGGTC-3’, reverse: 5’-CTGTCTCGGAGCTCGTCTATTTG-3’ and collagen I; Forward: 5-TGGAGAGAGAGGTGAACAAGG-3 and Reverse: 5-CATCACCCTTAGCACCATCG-3.
Western blot analysisSome markers for oxidative stress and liver fibrosis were assessed using western blot analysis including Nrf-2, HO-1 and p-Smad2/3. Total proteins were extracted from liver using RIPA buffer (Sigma). The protein concentration of lysate was quantified using a BCA Protein Assay kit according to Bradford technique.25 Proteins were separated according to their molecular weight, transferred to nitrocellulose membrane [Protran BA 83 Cellulosenitrat (E), Whatman]. Primary antibodies of p-Smad2/3, Nrf-2 and HO-1 (Santa Cruz, CA, USA) were used. β-actin monoclonal antibody (Santa Cruz, CA, USA) then was added as an internal control.
ImmunohistochemistryAs indices for liver fibrosis, TGF-β1 and α-SMA were assessed using immunohistochemical detection. Paraffin embedded tissue sections of 3 μm thickness were rehydrated first in xylene and then in graded ethanol solutions. The slides were then blocked with 5% bovine serum albumin (BSA) in tris buffered saline (TBS) for 2 h. The sections were then immunostained with one of the following primary antibodies; mouse monoclonal to rat α-SMA (Santacruz Biotech, Cat No. sc-53142) and rabbit polyclonal to rat TGF-β1 (Santacruz Biotech, Cat No. sc-146) antibodies at a concentration of 1 μg/mL containing 5% BSA in TBS and incubated overnight at 4 °C. After washing the slides with TBS, the sections were incubated with goat anti-rabbit secondary antibody. Sections were then washed with TBS and incubated for 5–10 min in a solution of 0.02% diaminobenzidine containing 0.01% H2O2. Counter staining was performed using hematoxylin, and the slides were visualized under a light microscope.
Histopathological examinationFor light microscopy, liver specimens were taken from the right lobe and fixed in 10% formalin and processed for paraffin sections of 4 μm thickness. Sections were stained with hematoxylin and eosin for routine histopathological examination.
Statistical analysisData are presented as mean ± SD. Multiple comparisons were performed using one-way ANOVA followed by Tukey-Kramer as a post hoc test. The 0.05 level of probability was used as the criterion for significance. All statistical analyses and graphs sketching were performed using GraphPad Prism (ISI1 software, USA) version 5 software.
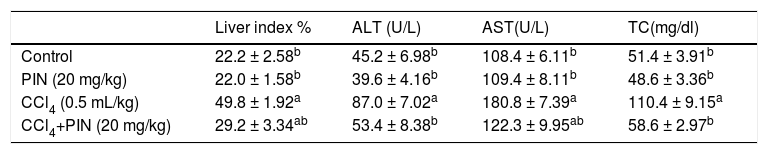
ResultsHepatotoxicity indicesEstimation of liver enzymes and TC revealed that CCl4 induced a significant increase in serum ALT, AST and TC levels by 92, 67 and 114%, respectively as compared to the control group. Concurrent treatment of intoxicated group with PIN significantly reduced ALT and TC levels as well as relative liver weight similar to that of the control group and significantly reduced AST level but still significantly higher than the control group. PIN alone did not modify liver function (Table 1).
Liver indices for rats treated with CCl4 and/or PIN.
| Liver index % | ALT (U/L) | AST(U/L) | TC(mg/dl) | |
|---|---|---|---|---|
| Control | 22.2 ± 2.58b | 45.2 ± 6.98b | 108.4 ± 6.11b | 51.4 ± 3.91b |
| PIN (20 mg/kg) | 22.0 ± 1.58b | 39.6 ± 4.16b | 109.4 ± 8.11b | 48.6 ± 3.36b |
| CCl4 (0.5 mL/kg) | 49.8 ± 1.92a | 87.0 ± 7.02a | 180.8 ± 7.39a | 110.4 ± 9.15a |
| CCl4+PIN (20 mg/kg) | 29.2 ± 3.34ab | 53.4 ± 8.38b | 122.3 ± 9.95ab | 58.6 ± 2.97b |
Data are the mean ± S.D. (n = 8). a or b: Significantly different from the control or CCl4 group respectively at P < 0.05 using ANOVA followed by Tukey-Kramer as a post hoc test.
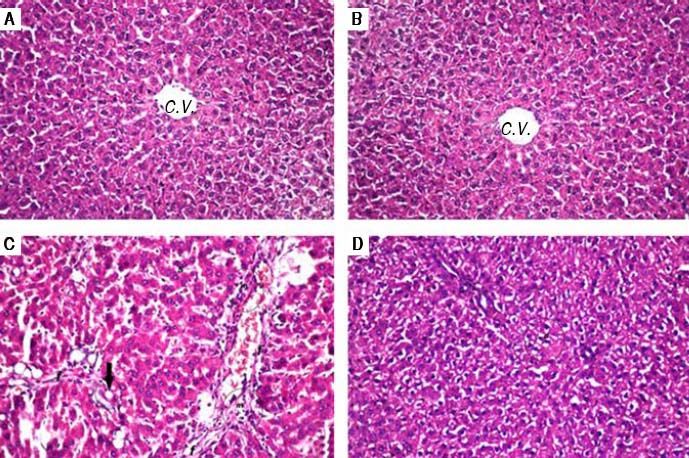
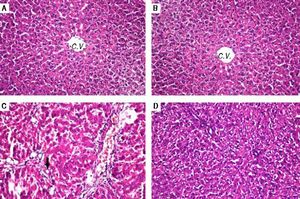
Histopathological examination (Figure 1) of liver tissue revealed that control group showed normal architecture of the central veins and surrounding hepatocytes in the hepatic parenchyma and no histopathological alterations were recorded (Figure 1A). Group received PIN alone showed normal histology of central vein and surrounding hepatocytes (Figure 1B). CCl4-intoxicated group showed focal fibrosis (f) with inflammatory cells infiltration (m) in the portal area with extension to the adjacent parenchyma and congestion in the hepatic sinusoids (s), along with fatty change (arrow) in some individual hepatocytes (Figure 1C). Concurrent treatment of intoxicated group with PIN illustrated minimal degeneration (d) in hepatocytes (Figure 1D).
Photomicrographs of liver sections stained by H & E (x 40). A. Control group showing normal histological structure of the central veins and surrounding hepatocytes in the hepatic parenchyma. B. Group received PIN alone showed norma histology of centra vein and surrounding hepatocytes. C. CCl4-intoxicated group showing focal fibrosis (f) with inflammatory cells infiltration (m) in the portal area with extension to the adjacent parenchyma and congestion in the hepatic sinusoids (s) along with fat deposition (arrow). D. CCl4-intoxicated group treated with PIN illustrated minimal degeneration (d) in hepatocytes.
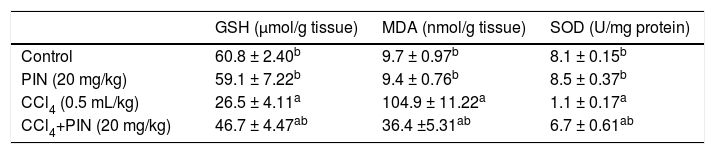
Table 2 shows the effect of PIN treatment on CCl4-induced changes in protein thiols and lipid peroxides measured as GSH and MDA levels, respectively as well as liver antioxidant enzyme SOD. CCl4-intoxicated group showed a significant depletion in hepatic GSH content by 56% and reduction in SOD by 86% in addition to a significant increase in MDA by 10 folds as compared to the control group. Nonetheless, pretreatment with 20 mg/kg PIN significantly restored the depleted GSH content and SOD activity and caused a significant decrease in lipid peroxides when compared to CCl4-intoxicated group. Rats treated with PIN only did not show any significant changes in all studied to the control group.
Oxidative stress markers of rats treated with PIN and/or CCl4.
| GSH (μmol/g tissue) | MDA (nmol/g tissue) | SOD (U/mg protein) | |
|---|---|---|---|
| Control | 60.8 ± 2.40b | 9.7 ± 0.97b | 8.1 ± 0.15b |
| PIN (20 mg/kg) | 59.1 ± 7.22b | 9.4 ± 0.76b | 8.5 ± 0.37b |
| CCl4 (0.5 mL/kg) | 26.5 ± 4.11a | 104.9 ± 11.22a | 1.1 ± 0.17a |
| CCl4+PIN (20 mg/kg) | 46.7 ± 4.47ab | 36.4 ±5.31ab | 6.7 ± 0.61ab |
Data are the mean ± S.D. (n = 8). a or b: Significantly different from thhe control or CCl4 group respectively at P < 0.05 using ANOVA followed by Tukey-Kramer as a post hoc test
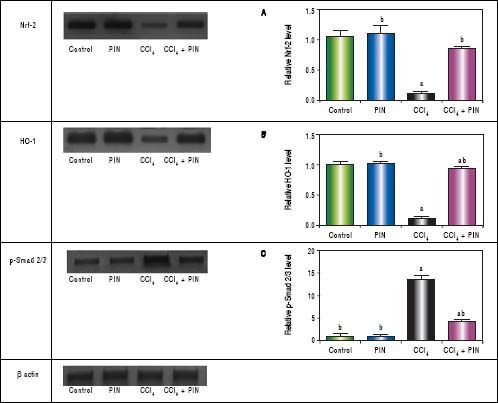
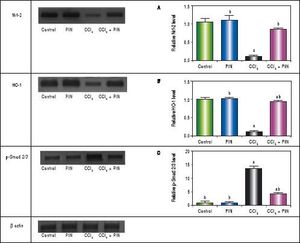
In addition, western blot analysis revealed significant decreases in Nrf-2 and HO-1 in CCl4-intoxicated group by 89 and 86%, respectively as compared to control group. Treatment with PIN 20 mg/kg significantly increased Nrf-2 and HO-1 levels by 72 and 78% respectively in comparison with CCl4-intoxicated group. No change was observed in Nrf-2 and HO-1 levels in animals treated with PIN only (Figures 2A and B).
Effect of treatment with 20 mg/kg PIN on Nrf-2, HO-1 and p-Smad2/3 released during CCl4-finduced hepatotoxicity in rats assessed using Western Blot analysis. Values are given as mean + S.D. for groups of 15 rats for each. (a or b) significantly different from the control or CCi4 group respectively at P < 0.05 using ANOVA followed by Tukey-Kramer as a post hoc test.
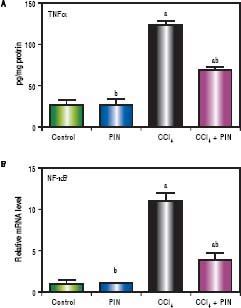
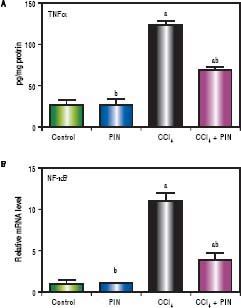
CCl4-intoxicated group showed proinflammatory response as evidenced by significant increase in both total TNF-a and NF-kB in the liver tissue by 4 and 11 folds respectively, as compared to the control group (Figures 3A & B). Treatment with 20 mg/kg PIN exhibited anti-inflammatory effects by significantly reducing liver TNF-α and NF-kB contents by 2 and 7 folds respectively, as com pared to CCl4-intoxicated group. On the other hand, there was no significant change in liver TNF-α and NF-kB content in animals treated with PIN alone.
A.Effect of treatment with 20 mg/kg PIN on TNF-α released during CCl4-induced hepatotoxicity in rats using ELISA. Values are given as mean + S.D. for groups of 15 rats for each. B. Effect of treatment with 20 mg/kg PIN on NF-kB released during CCl4-induced hepatotoxcity in rats using Real-time PCR. Values are given as mean + S.D. for groups of 15 rats for each (a or b) significantly different from the control or CCl4 group respectively at P < 0.05 using ANOVA followed by Tukey-Kramer as a post hoc test.
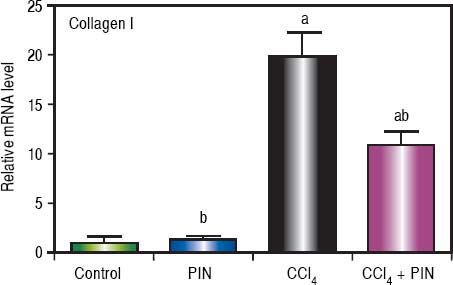
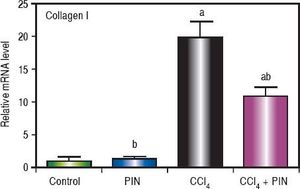
Analysis of collagen I levels by RT-PCR showed an increase by 20 folds in CCl4-intoxicated group as compared to control group. Treatment with PIN 20 mg/kg decreased collagen I level by 9 folds as compared to CCl4-intoxicated group. PIN only treated group did not show any significant changes in collagen I levels from control group (Figure 4).
Effect of treatment with 20 mg/kg PIN on Collagen I released during CCl4-induced hepatotoxicity in rats assessed using Real-time PCR. Values are given as mean + S.D. for groups of 15 rats for each (a or b) significantly different from the control or CCl4 group respectively at P < 0.05 using ANOVA followed by Tukey-Kramer as a post hoc test.
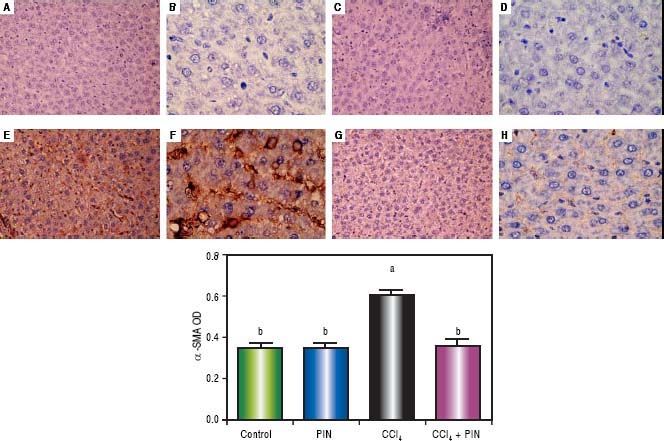
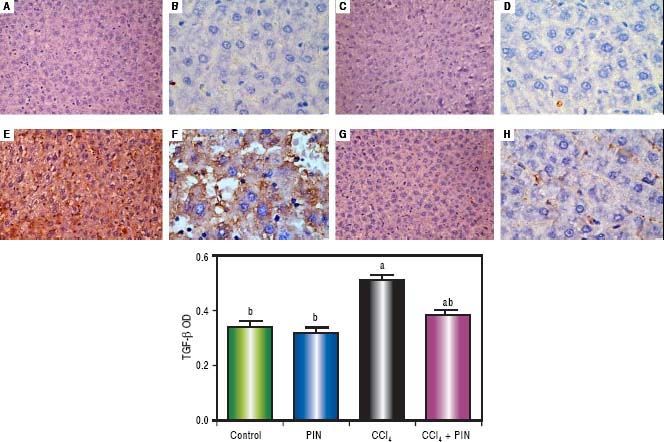
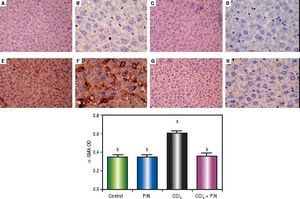
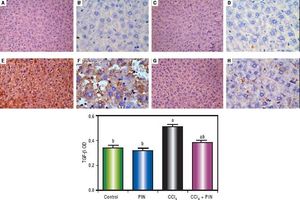
In addition, immunostaining of fibrogenic markers αSMA and TGF-β (Figures 5 and 6, respectively) revealed that CCl4 caused significant increase of α-SMA and TGF-β expression by 75 and 48% (Figures 5E, 5F and 6E, 6F), respectively which were evident from intense brown staining. Treatment of rats with 20 mg/kg PIN significantly inhibited the expression of α-SMA and TGF-β by 69 and 35% (Figures 5G, 5H and 6G, 6H), respectively when compared to CCl4-intoxicated group. No change was observed in α-SMA and TGF-β expression in PIN only treated group when compared to control group (Figures 5C, 5D and 6C, 6D). Figures 5I and 6I represent OD quantification results of the stained regions for α-SMA and TGF-β, respectively. Further, western blot analysis detected significant increase in the p-Smad 2/3 levels by 10 folds in CCl4-intoxicated group as compared to control group and treatment with PIN 20 mg/kg managed to decrease p-Smad 2/3 level by 8 folds as compared to CCl4-intoxicated group. PIN only treated group did not show any significant changes in p-Smad 2/3 levels from control group (Figure 2C).
Expression of α-Smooth Muscle Actin (α-SMA) by immunohistochemical staining (A, C, E, G with magnification 40x and B, D, F, H with magnification 100x). Photomicrographs of histologics sections of liver depictng (A,B) control group, (C,D) PIN only treated group, (E,F) CCl4 (0.5 mL/kg) intoxicated group, (G,H) CCl4 (0.5 mL/kg) + PIN (20 mg/kg) treated group, (I) quantitative image analysis for immunohistochemical staining expressed as optical densities (OD) across 10 different fields for each rat section. Values are given as mean±S.D. For groups of 15 rats for each. (a) P < 0.05 statistically significant from control group and (b) P < 0.05 statistically significant from CCl4 group using one-way analysis of variance (ANOVA) followed by Tukey-Kramer as a post hoc test. For immunohistochemical analyses, brown color (positive) indicates specific immunostaining of α-SMA and light blue color (negative) indicates hematoxylin staining. CCl4 intoxication significantly increased α-SMA expression as indicated by intense brown staining compared to the control group. PIN treatment significantly reduced α-SMA expressions compared to CCl4 group. However, there was no significant difference in α-SMA expression in PIN only treated group as compared to the control group.
Expression of Transforming Growth Factor-β1 (TGF - β1) by immunohistochemical staining (A, C, E, G with magnification 40x and B, D, F, H with magnification 100x). Photomicrographs of histological sections of liver depicting (A,B) control group, (C,D) PIN only treated group, (E,F) CCl4 (0.5 mL/kg) intoxicated group, (G,H) CCl4 (0.5 mL/kg) + PIN (20 mg/kg) treated group, (I) quantitative image analysis for immunohistochemical staining expressed as optical densities (OD) across 10 different fields for each rat section. Values are given as mean±S.D. For groups of 15 rats for each. (a) P < 0.05 statistically significant from control group and (b) P < 0.05 statistically significant from CCl4 group using one-way analysis of variance (ANOVA) followed by Tukey-Kramer as a post hoc test. For immunohistochemical analyses, brown color (positive) indicates specific immunostaining of TGF -β1 and light blue color (negative) indicates hematoxylin staining. CCl4 intoxication significantly increased TGF -β1 expression as indicated by intense brown staining compared to the control group. PIN treatment significantly reduced TGF -β1 expressions compared to CCl4 group. However, there was no significant difference in TGF -β1 expression in PIN only treated group as compared to the control group.
The present study was designed to examine the potential antifibrotic effect of PIN in an experimental model of liver fibrosis via studying its effects on different oxidative stress, inflammatory and fibrosis markers. The CCl4-induced hepatotoxicity model is a commonly used model for the screening of the hepatoprotective activities of drugs. It is well known that prolonged administration of CCl4 leads to liver fibrosis, cirrhosis, and hepatocellular carcinoma.
In the present study, chronic exposure of rats to CCl4 caused a significant increase in serum ALT and AST, which resulted from the hepatocyte damage and inflammation.26 Relative liver weight and serum TC were also significantly increased that confirming the induction of liver hypertrophy according to previous study.27 Histopathological examination, the “gold” tool for the diagnosis of hepatic fibrosis, revealed the presence of fibrosis with several histopathological changes that are in accordance with previous study.28 Treatment of animals with PIN (20 mg/kg) significantly decreased ALT, AST and TC and ameliorated the histopathologic changes induced by CCl4 confirming that PIN effectively opposes the progression of CCl4-induced liver injury. These results are in agreement with Shalaby, Abd-Alla22 study which confirmed that an extract of D. viscosa extracts containing PIN as one of its prominent constituents managed to protect against hepatic fibrosis through down-regulation of liver enzymes and normalization of hepatic architecture.
Previous literature elucidated that CCl4-induced oxidative stress is the cornerstone underlying its hepatotoxicity and it is due to reductive dehalogenation of CCl4 by the cytochrome P450 enzyme system to the highly reactive trichloromethyl radical in the presence of oxygen. These free radicals damage the hepatocellular membrane via lipid peroxidation, followed by release of inflammatory mediators from activated inflammatory cells which are thought to potentiate CCl4-induced hepatic injury.29 Oxidative stress occur when there is an inequality between production of reactive oxygen species (ROS) and cellular antioxidant activity.30 Cellular antioxidant capacity depends on the presence of GSH and antioxidant enzymes such as SOD. A critical regulator of cellular defense against oxidative stress is Nrf2.31 It is responsible for regulating the antioxidant response element (ARE)-driven expression of genes encoding the majority of antioxidant and phase II detoxification enzymes such as HO-1.32 Several antioxidants have been reported to increase Nrf2 activation, mediated by HO-1 expression, thereby attenuating oxidative stress in vitro and in vivo.33,34 Thus, pharmacological activation of Nrf2 has been suggested to offer a novel therapeutic approach to the prevention and treatment of liver injury and fibrosis.35 In the present study, CCl4 found to induce an increase in the oxidative stress profile as indicated by significant rise in lipid peroxidation, GSH depletion and significant reduction in antioxidant enzyme (SOD). The decline in SOD with concomitant MDA elevation, strongly indicate oxidative damage.36 These results are in agreement with previous studies.22,37 Also, western blot analysis revealed that CCl4 significantly down-regulated the expression of Nrf2 and HO-1 which is consistent with prior observation.37 Pretreatment with PIN significantly guarded against the oxidative stress effects by decreasing MDA and increasing GSH and SOD, which was previously proven by Shalaby, Abd-Alla,22 as well as restoring Nrf2 and HO-1 to normal level. Induction of HO-1 expression under regulation of Nrf2-ARE is consistent with previous studies38,39 who reported that up-regulation of HO-1 expression inhibited oxidative stress in association with increased Nrf2-ARE-binding activity in hepatocytes and protected liver cells from apoptosis. Also, Jin, Liu20 research had proved that PIN has cytoprotective role in dopaminergic cell culture systems by increasing Nrf2 and the subsequent HO-1 levels. These findings imply that PIN effectively attenuated the progression of CCl4-induced oxidative stress by restoring liver antioxidant capacity.
Beside the oxidative stress, accumulating evidences have revealed that excessive ROS induced by CCl4 can stimulate circulating monocytes and tissue macrophages, which lead to the synthesis and release of a variety of proinflammatory cytokines.40,41 In particular, TNF-α plays a key role in the development and maintenance of inflammation and that its elevation is associated with many liver diseases.41,42 Furthermore, NF-kB is a nuclear transcription factor that regulates the expression of a large number of genes that are critical for the regulation of apoptosis, viral replication, tumorigenesis, inflammation, and various autoimmune diseases.43 In addition, HSCs activation is associated with elevation of NF-kB activity.44 In the present study, CCl4 significantly up-regulated the expression of TNF-α and NF-kB in rat liver and, treatment with PIN significantly reduced their expression. These results suggested that PIN could alleviate liver injury caused by CCl4 through suppressing inflammatory response.
To further explore the mechanism underlying the anti-fibrotic effect of PIN, several fibrogenic markers were assessed. It is believed that HSCs are a major type of fibrogenic liver cells found during liver injury and are responsible for the progression of hepatic fibrosis.45 Activation of HSCs towards myofibroblast-like cells is characterized by several key phenotypic features. Those features include proliferation, liver content of α-SMA; a microfilament protein that has been explored as a marker for activated HSCs,46,47 and finally accumulation of ECM mainly collagen, which is stimulated by the multifactorial growth factor TGF-β 48 In this study, the levels of collagen I and α-SMA in liver tissue were significantly increased in CCl4 intoxicated rats which is consistent with another study,49 whereas PIN treatment markedly decreased the levels of these fibrogenic parameters. These data indicate that PIN can prevent collagen and α-SMA accumulation caused by the chronic liver disease.
Furthermore, TGF-β1 is stored as an inactivated protein bound to a latency-associated peptide. Once activated, TGF-β1 binds its cognate receptors and functions in auto-crine and paracrine manners to exert its biological and pathological activities via Smad-dependent and -independent signaling pathways. Smads are intracellular signal transductive molecules of the TGF-β super family. Smad signal transduction pathways are thought to mediate TGF-β 1-induced collagen synthesis and to play a crucial role in the process of liver damage and recovery, as well as in the development of liver fibrosis.50,51 Studies have demonstrated that TGF-β1/Smad signaling pathway might play a prominent role in the activation of HSCs and the regulation of the production, degradation, and accumulation of ECM proteins.50 Therefore, the inhibition of the TGF-β/ Smad signaling pathway is an attractive therapeutic target for the prevention of liver fibrosis.52,53 In the present study, PIN treatment attenuated the increase in level of the prominent profibrogenic cytokine TGF-β1 induced by CCl4, a finding indicates the inhibitory effect of PIN to the proliferative activity of HSCs. Moreover, CCl4-induced liver fibrosis was associated with a marked activation of Smad2/3 and PIN treatment was able to inhibit the phosphorylation of Smad2/3 and reversed the effect of CCl4. Therefore, the decreased fibrosis level after PIN treatment may be mediated via inhibition of TGF-β1/ Smad signaling pathway.
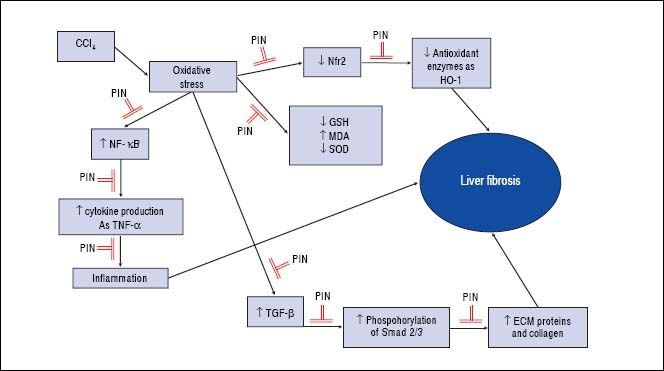
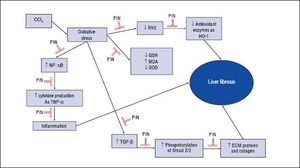
In summary, this study demonstrates that PIN has promising anti-fibrotic effects by inhibiting the TGF-β1/ Smad and activating Nrf2/HO-1 signaling pathways, as well as decreasing oxidative stress and proinflammatory cytokines production (Figure 7).
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
CCl4: carbon tetrachloride.
- •
GSH: reduced glutathione.
- •
HO-1: heme oxygenase-1.
- •
HPβCD: 2-hydroxypropyl-β-cyclodextrin.
- •
MDA: malondialdehyde.
- •
NF-kB: nuclear factor-KB.
- •
Nrf2: nuclear factor erythroid 2 (NF-E2)-related factor 2.
- •
PIN: pinocembrin.
- •
ROS: reactive oxygen species.
- •
SOD: superoxide dismutase.
- •
TC: total cholesterol.
- •
TGF-β: transforming growth factor-β.
- •
TNF -α: tumor necrosis factor-a.
- •
α-SMA: α-smooth muscle actin.
The authors gratefully thank Roquette (France-Europe) for the free supplement of 2-hydroxypropyl-β-cyclodextrin (HPβCD).