Endovascular therapy represents a less invasive alternative to open surgery for reconstruction of the portal vein (PV) and the spleno-mesenteric venous confluence to treat Portal hypertension. The objective of this study is to determine if the Model for End-Stage Liver Disease (MELD) score is a useful method to evaluate the risk of morbidity and mortality during endovascular approaches.
Material and methodsPatients that underwent endovascular reconstruction of the PV or spleno-mesenteric confluence were identified retrospectively. Data were collected from November 2011 to August 2016. The MELD score was calculated using international normalized ratio, serum billirubin and creatinine. Patients were grouped into moderate (≤15) and high (> 15) MELD. Associations of the MELD score on the postprocedural morbidity, mortality and vessels patency were assessed by two-sided Fisher's exact test.
ResultsSeventeen patients were identified; MELD score distribution was: ≤ 15 in 10 patients (59%) and > 15 in 7 (41%). Even distribution of severe PV thrombosis was treated in both groups, performing predominately jugular access in the high MELD score group (OR 0.10; 95%; CI 0.014-0.89; p = 0.052) in contrast to a percutaneous transhepatic access in the moderate MELD score group. Analysis comparing moderate and high MELD scores was not able to demonstrate differences in mortality, morbidity or patency rates.
ConclusionMELD score did not prove to be a useful method to evaluate risk of morbidity and mortality; however a high score should not contraindicate endovascular approaches. In our experience a high technical success, good patency rates and low complication rates were observed.
Portal vein thrombosis (PVT) or its splenic and me-senteric branches may result in complete or partial oc-clusion.1 When chronically occluded, compensatory collateral network develops and is usually termed cav-ernoma.2 Thrombosis may be located either in the int-rahepatic or the extrahepatic portal system. General population prevalence is estimated in 1%,3 considerably increasing among patients with cirrhosis to 11-17%4,5 and 11-44% in those affected with hepatocellular carcinoma (HCC).6,7 Patients with cirrhosis and/or HCC are the most frequently affected; other predominant risk factors are the inherited or acquired pro-throm-botic states, accounting for approximately 60% if not affected by the latter etiologies described.8 Increased incidence of PVT has also been reported after ortho-topic liver transplantation with previous portosystemic shunt (38.9% vs. 13.8% in those without porto-systemic shunt).9
Sings of chronicity and possible etiology are established initially by noninvasive imaging as ultrasound followed by contrast enhanced computed tomography (CT) or magnetic resonance (MR) imaging confirm the diagnosis.10 In this clinical setting the liver function, blood cell count, fibrin, fibrinogen degradation products and D-dimer should be assessed.1 Tests to evaluate for pro-thrombotic conditions may be considered in special cases. PVT may worsen portal hypertension symptoms,4 affects post-transplantation survival while in waiting list11 and defines advanced malignant disease in HCC.12 Prevention of recurrent thrombosis and treatment of gastrointestinal bleeding and portal cholan-giopathy are the main therapeutic goals. Failure of primary prophylaxis with beta blockers or variceal ligation should prompt for shunting procedures.1 Even when anticoagulation appears to be effective to reduce thrombus propagation, in patients with cirrhosis and non-malignant PVT (40-80%);13,14 a chronic thrombus may reduce vessel patency.15 Portosystemic shunts thus may be considered in order to improve portal hypertension complications. The Model for End stage Liver Disease (MELD)was created to assess the 3-month survival of patients with liver failure undergoing transjugular intra-hepatic portosystemic shunt procedures (TIPS).16 Nowadays it is also a prognostic tool for a wide range of non transplant elective17-20 and emergent procedures.21 Value of this score for prehepatic portal hypertension has not been described. Shunting procedures as TIPS, have been reported to recanalize portal vein and prevent rethrombosis in some patients with cavernoma.22 Extensive thrombosis or inability to catheterize portal vein or collaterals forming portal cavernoma was considered to pose TIPS as an unfeasible option for treat-ment.23 Besides these reports, recent descriptions of portal vein recanalization have emerged as a promising as a promising option for chronic totally occluded PVT either by transjugular24 or transhepatic percutane-ous25,26 access.
Previous experience with iatrogenic non cirrhotic non-malignant stenosis has been reported by our team.27 The experience gained in endovascular PVT recanaliza-tion procedures due to its minimal invasiveness and high success rates28 is reported.
The aim of this study is to determine if high MELD score (> 15 points) has an impact in mortality and patency rates when endovascular portal or spleno-mesenteric confluence reconstruction is performed.
Material and MethodsRetrospective review of 69 interventional procedures reported as TIPS at our institution and further stratified as portal or spleno-mesenteric reconstruction according to angiographic findings. These procedures were performed between November 2011 and August 2016 in a single center in Mexico City by a single operator. Medical records were carefully reviewed; demographic, perioperative and procedural details from hospital records and clinic visits were entailed for population description. The study was approved by the Institutional Ethics Committee and informed consent was given. Relevant comorbidities, potential causes and risk factors for PVT, suitability and transplantation status were also collected. Portal vein patency was assessed either by splenoportography, enhanced CT or ultrasound. Complications and mortality were recorded. Portal vein thrombosis was graded as previously described by Yerdel MA, et al.8 -grade 1, < 50% PVT with or without minimal occlusion of the SMV; grade 2, > 50% PVT with or with- out minimal occlusion of the superior mesenteric vein (SMV); grade 3, complete PV and proximal SMV thrombosis; and grade 4, complete PV and entire SMV thrombosis. Portal hypertension manifestations along with associated hepatic, splenic and cava vein thrombosis were described. Acute PVT was considered if symptoms developed less than 60 days before presentation and patients demonstrated no evidence of portal hypertension nor collateral circulation. Chronic PVT was suggested with complete vessel obliteration or no apparent remnant, calcification within wall of vessel or thrombus, splenomegaly, and portal hypertension.2 Indications for PVT recanalization were discussed in a multidisciplinary committee mainly related to refractory portal hypertension symptoms and/or suitability for transplantation. Procedure details such as anesthesia type, delivered stent, access type and cannu-lation methods were evaluated. Most procedures were performed by a standard technique. Long 11Fr sheath and Rosh Uschinda needle were utilized to cannulate the supra hepatic and portal vein for jugular access. Per-cutaneously, Chiba Needle (Reli®) puncture along with Cope Mandrill Wire guide followed by Neff access set (Cook Medical®) were required. Hydrophilic 0.035 guide wires were used for navigation and stiff 0.035 wires for pig tail allocation. Portal gradients were measured only when stenosis was not evident. Different size non-compliant balloons were used for portosystemic communication when needed (except in the postrans-plantation group) over angiographic stiff guide wires. Systematic sheath advance while deflating the balloon was done to reduce bleeding complications. Stent selection relied on availability and length of stenosis. Gel-foam sponge (Pfizer®) provided hemostasis for percutaneous access. Gore Viatorr TIPS and Viabahn® (W.L. Gore and Associates, Flagstaff, Arizona) endopro-thesis were considered as covered stents, even when the former is composed of a 2 cm segment of bare metal stent as well (Figure 1A-B and Figure 2A-C).
Evaluated end points were technical success, death, graft patency and postoperative complications. Technical success was defined by portal flow reconstitution and/or collateral absence by splenoportography. Regarding patency; thrombosis and dysfunction were investigated. Dysfunction was considered when more than one intervention was needed to achieve success. Primary patency was considered when neither thrombosis nor dysfunction was recorded during follow up. Adjuvant procedures to regain patency after the first procedure classified patients in the secondary permeability group. Days until compromised patency events and through to the last follow up imaging study were considered for permeability documentation. MELD score was calculated with INR, serum bilirubin and creatinine values. The mathematical formula for determining MELD score is 0.957 log (creat-inine) þ 0.378 log (total bilirubin) þ 1.120 log (INR) þ 0.6431 (16). MELD score stratification was done according to Hanje and Patel publication.29 For purposes of this study, patients were classified according to this score independently of their transplantation status into high (> 15 points) and moderate (≤ 15 points) risk for further evaluation. Demographics, medical history and intervention details were described using proportions of means and standard deviation. Comparison was made between MELD score cohorts (high and medium risk) using two sided Fisher's exact test. Statistical significance was considered when p < 0.05.
ResultsSeventeen single operator extra-hepatic portal and spleno-mesenteric venous confluence recanalization were identified. All patients were Mexicans and had preoperative INR and serum bilirubin, and creatinine available for evaluation. Distribution was 10 (59%) patients in the moderate risk MELD score group and 7 (41%) in the high risk MELD score group. Similar age groups were found between both evaluated groups. Moderate risk patients were slightly predominant female gender. Comorbidities were equally distributed (Table 1).
Characteristics of patients undergoing endovascular recanalization of the porto-mesenteric system organized by MELD score.
| Characteristic | Overall (n = 17) | MELD ≤ 15 (n = 10) n(%) | MELD > 15 (n = 7) |
|---|---|---|---|
| Age | 48.6 ± 15.8 | 48.2 ± 15.9 | 49.2 ± 16.9 |
| Female gender | 9 (52.9) | 6 (60) | 3 (42.8) |
| Acquired risk factor | 15 (88.2) | 8 (80) | 7 (100) |
| Cirrhosis | 3 (20) | 1 (12.5) | 2 (28.5) |
| Malignancy | 4 (26.6) | 2 (25) | 2 (28.5) |
| Inflammatory | 4 (26.6) | 1 (12.5) | 3 (42.8) |
| OLT | 4 (26.6) | 4 (50) | 0 (0) |
| Type of malignancy | |||
| Hepato-pancreato-biliary | 6 (35.3) | 3 (30) | 2 (28.5) |
| Other | 2 (11.7) | 1 (10) | 1 (14.2) |
| Inflammatory factor | |||
| Surgery | 8 (47.0) | 7 (70) | 1 (14.2)* |
| CUCI | 1 (5.88) | 0 (0) | 1 (14.2) |
| Duodenal ulcer | 1 (5.88) | 0 (0) | 1 (14.2) |
| Type of Surgery | |||
| Gastrectomy | 2 (11.7) | 1 (10) | 1 (14.2) |
| Cholecystectomy | 2 (11.7) | 1 (10) | 1 (14.2) |
| OLT | 4 (23.5) | 4 (40) | 0 (0) |
| Pancreatectomy | 1 (5.88) | 1 (10) | 0 (0) |
| Comorbidities | |||
| Hypertension | 3 (17.6) | 2 (20) | 1 (14.2) |
| Inflammatory bowel syndrome | 1 (5.88) | 0 (0) | 1 (14.2) |
| Chronic renal insufficiency | 1 (5.88) | 0 (0) | 1 (14.2) |
| Dyslipidemia | 1 (5.88) | 1 (10) | 0 (0) |
| Family history | 0 (0) | 0 (0) | 0 (0) |
| Previous treatment | |||
| Pharmacological | 10 (58.8) | 6 (60) | 4 (57.1) |
| Endoscopic | 10 (58.8) | 6 (60) | 4 (57.1) |
| Surgical | 4 (23.5) | 3 (30) | 1 (14.2) |
MELD: Model for End Stage Liver Disease score. OLT: Orthotopic liver transplantation. CUCI: Colitis Ulcerative Chronic Idiopathic. All p values were non significant, except from * 0.0364 OR 14 (1.31-178.5 95% CI).
Overall acquired risk factors were identified. One prothrombotic state (methylenetetrahydrofolate reductase C677T mutation) and one idiopathic portal fibrosis accounted for the congenital factors. No evidence of familiar PVT was found.
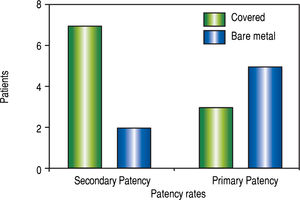
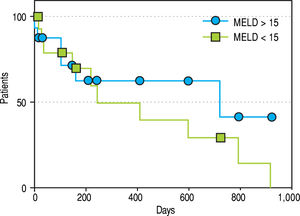
Among the acquired risk factors detected, cirrhosis, malignancy, inflammatory and ortothopic liver transplantation (OLT) were the leading causes. Equal distribution between groups is described (Table 1). Some patients had history of malignancy previous to PVT such as Wilms tumour and gastric GIST. The others were identified as a causal factor, especially when hepatopancreato-biliary malignancy diagnosed. Of these, one small HCC was found after hepatectomy for liver transplantation. Surprisingly, no myeloproliferative disorder was identified (Table 1). Among the inflammatory factors, surgery significantly increased the propensity for PVT in the medium risk group of patients (p = 0.036 -OR 14 (1.31-178.5 95%CI-). Subanalysis demonstrated that OLT leaded this group but no difference among other surgeries was found (Table 1). Medical, endoscopic and surgical treatments were homogeneous in both groups. Therapy was instituted in most of the cases with non selective beta blockers and variceal ligature. Two Sugiura and one Warren procedures were also done previous to PV reconstruction (Table 1). Despite equal proportion between groups, chronic onset PVT was treated more frequently in contrast to acute onset. Though not frequent, porto systemic shunt was evenly distributed (Table 2) Five porto-systemic shunts were found: 3 spleno-renal, 1 meso-renal and 1 spleno-es-ophagic. No increase in thrombosis was found in patients with portosystemic shunt (p = 0.20 OR, 0-2.08 95%CI). The majority of PVT reconstructions were distributed between Yerdel grade III and IV severity score. No post-sinusoidal hypertension was treated but pre-sinusoidal and sinusoidal portal hypertension were equally distributed in both groups (Table 2). Thrombosis included main portal vein in all non-transplanted cases, extension to other branches was documented in 84.6% of the imaging studies (53.8% SMV alone and 30.7% to both splenic and SMV). Prominent collaterals was the most frequent concomitant imaging finding in all chronic occlusions including one umbilical vein re-canalization. Also hepatic artery compensatory dilatation (> 5 mm) was found in 15% of the patients, caudate lobe hypertrophy in 52.9%, left lobe atrophy in 35% and generalized atrophy in 23.5% of patients. All procedures were performed under general anesthesia. Jugular access was more frequently chosen in the high risk group; whereas percutaneous approach was routinely performed in the moderate risk group. This could be achieved either by transhepatic or trans-splenic access. Hybrid procedures were done in combination of jugular and percutaneous approach. Systemic and portal can-nulation were performed according to accessibility ease (Table 2). When transjugular access was selected, middle supra hepatic vein was cannulated in 41.6%, followed by the right supra hepatic vein (33.3%). Left supra hepatic vein and superior cava vein were selected once. Right portal vein was cannulated to complete procedure in 70.5% of the interventions. Only 2 procedures required more than one attempt (2 attempts) to achieve recanalization. First attempt technical success was documented in 88.2%, and 100% when a second attempt was performed. Overall complication occurrence was as frequent as 76% (Table 2), comprising 29% for septic shock, 29% for bleeding and other minor complications like encephalopathy and contrast nephropathy. Seventy five percent of the complications were treated with medical management alone. Minor bleeding cases received transfusion in one case and manual compression due to inguinal hematoma, in the other. Embolization was done for 3 major bleeding cases. Two graft infections were documented and treated with antibiotics. Complication rates were similar between groups as well as mortality (Table 2). Only one mortality in the high risk group was related to the procedure due to major bleeding. The rest of the mortalities happened as expected survival for malignancy and secondary to pulmonary septic complications non related to the procedure. Bare metal stents were deployed in 41%. Covered stents such as Viatorr or Viabahn were used in 23% respectively and 2 combined Viatorr with either bare metal stent or Viabahn. Most of the procedures were successfully completed with one stent, only four required 2 stents and one procedure merited 4 stents. Differences in patency rates are shown in figure 3 (p = 0.1, OR 5.8 0.75-38 95%CI). No overall patency differences could be demonstrated in this series, neither for primary nor for secondary patency. Figure 4 shows a Kaplan-Meier analysis of the patency rates of those patients with MELD > 15 and those ≤ 15. Thrombosis and dysfunction were evenly distributed in both groups (Table 3). Every subject in the pretransplantation group achieved transplantation with an end to end anastomosis after portal vein reconstruction was performed. Also, PVT reconstructions performed in the postrasplanta-tion group, improved liver function and remained patent. Concomitant biliary decompression secondary to portal biliopathy was done in 17.6% by means of endo-scopic or percutaneous catheters. Biliary drainage did not correspond with an increased incidence of infectious events (p = 0.19 OR 7.33, 0.58-113.6 95%IC) Anti-coagulation was instituted in the postoperative period in 17% of cases and antiplatelet therapy in 58%. No specific treatment was initiated for those with active bleeding conditions.
Portal hypertension and procedure characteristics.
| Characteristic | Overall (n = 17) | MELD ≤ 15 (n = 10) | MELD > 15 (n = 7) |
|---|---|---|---|
| Family history | 0 (0) | 0 (0) | 0 (0) |
| Chronic PVT | 12 (70.5) | 7 (70) | 5 (71.4) |
| Porto-systemic shunt | 5 (29.4) | 4 (40) | 1 (14.2) |
| Obstruction severity | |||
| Yerdel type 1 | 0 (0) | 0 (0) | 0 (0) |
| Yerdel type 2 | 2 (11.7) | 1 (10) | 1 (14.2) |
| Yerdel type 3 | 11 (64.7) | 8 (80) | 3 (42.8) |
| Yerdel type 4 | 4 (23.5) | 1 (10) | 3 (42.8) |
| Portal hypertension | |||
| Pre-sinusoidal | 10 (58.8) | 6 (60) | 4 (57.1) |
| Sinusoidal | 7 (41.1) | 4 (40) | 3 (42.8) |
| Post-sinusoidal | 0 (0) | 0 (0) | 0 (0) |
| Access type | |||
| Jugular | 7 (41.1) | 2 (20) | 5 (71.4)* |
| Percutaneous | 5 (29.4) | 5 (50) | 0 (0)** |
| Hybrid | 5 (29.4) | 3 (30) | 2 (28.5) |
| Systemic cannulation | |||
| Right SH | 4 (23.5) | 1 (10) | 3 (42.8) |
| Middle SH | 5 (29.4) | 2 (20) | 3 (42.8) |
| Left SH | 1 (5.88) | 1 (10) | 0 (0) |
| None (percutaneous) | 4 (23.5) | 4 (40) | 1 (14.2) |
| Cava vein | 2 (11.7) | 2 (20) | 0 (0) |
| Portal cannulation | |||
| Right PV | 12 (70.5) | 6 (60) | 6 (85.7) |
| Left PV | 2 (11.7) | 2 (20) | 0 (0) |
| PV confluence | 2 (11.7) | 2 (20) | 0 (0) |
| Colateral | 1 (5.88) | 0 (0) | 1 (14.2) |
| Overall Technical success | 17 (100) | 10 (100) | 7 (100) |
| Mortality Complications | 5 (29.4) 13 (76.4) | 2 (20) 7 (70) | 3 (42.8) 6 (85.7) |
PVT: Porto-mesenteric vein thrombosis. MELD: Model for End Stage Liver Disease Analysis demonstrated *0.052 OR 0.1 (0.014-0.89 95%CI); ** 0.040 OR Infinity and no statistically significant differences in the rest of the variables studied.
Patency according to MELD
| Characteristic | Overall (n = 17) | MELD ≤ 15 (n = 10) | MELD > 15 (n = 7) |
|---|---|---|---|
| Thrombosis | 4 (23.5) | 1 (10) | 3 (42.8) |
| Dysfunction | 4 (23.5) | 3 (30) | 1 (14.2) |
| Primary Patency | 9 (52.9) | 6 (60) | 3 (42.8) |
| Rates Secondary | 6 (75) | 4 (100) | 2 (50) |
| Patency Rates Overall Patency | 15 (88.2) | 10 (100) | 5 (71.4) |
MELD: Model of end stage liver disease. No statistical difference was found between groups.
Many strategies have been described for PVT reca-nalization. Anticoagulation, thrombolysis and mechanical thrombectomy are considered for the acute onset. When chronic occlusion setting is to be treated, TIPS has been posed as an option to reanalyze PVT and even prevent rethrombosis30,31 by restoring portal flow through a low resistance shunt.22 Feasibility of TIPS is clearly related to the extent of thrombosis and the experience of each centre. The majority of TIPS candidates have advanced cirrhosis with a high MELD score. TIPS are generally contraindicated in high MELD scores due to further deterioration in the liver function and consequent mortality risk.32
In this retrospective review, no differences in PVT associated risk factors could be demonstrated in our population. Cirrhosis and hepatobiliary malignancies were the leading causes. Low incidence of prothrombotic syndromes corresponded with other population prevalence descriptions.3 Inflammatory factors accordingly highlight surgery, especially when OLT was performed.9
As MELD score highly correlates with Child Tur-cotte Pugh Score and utilizes objective and accessible laboratory parameters, its calculation is an attractive tool for the surgeon to assess patient risk undergoing surgical procedures under general anesthesia.21 Though MELD score is intended to be predictive for liver disease, no predictive score has been developed to date for patients with prehepatic portal hypertension undergoing interventional nor open procedures. MELD score was used as means of categorizing this group of patients.
Some reports have proposed novel methods for percutaneous portal vein recanalization with angioplasty and/or stent placement33,34 as an option for portal hypertension symptoms treatment. Our study demonstrated no utility for MELD score as a predictive tool for PVT recanalization. Even when thrombus had progressed to complete occlusion or a fibrotic cord and thus difficulty in portal vein recanalization increases, it is a feasible procedure in either high or intermediate MELD risk scores. This contrasts with initial reports where TIPS post procedure mortality was increased specially if MELD is > 1832 or with more recent reports that suggest higher mortality and complications when abdominal procedures other than TIPS28 or peripheral vascular procedures25 are performed with scores > 15. This contrast may be explained by the etiology diversity (different from liver disease) and due to type 2 error related to the small number of patients analysed. Importantly, mortality was not related to the procedure in none but one case. This correlates with other recent publications where balloon assisted TIPS has been performed in order to recanalyze PV.26 Mortality was related to comor-bidities and malignancy. Also fatal complications rarely occurred.30,36 Access site is not related with procedural success, main determinant of choice is the ease cannula-tion to partially patent vessels that suggests procedure feasibility. Combination of transhepatic, trans-splenic and/or transjugular approaches may facilitate the proce-dure30,37 independently of the MELD score. Tendency to select percutaneous approach in the intermediate risk MELD score is explained by the predominance of postrasplanted patients in this group and no requisite for systemic shunt. Contrasting tendency is apparent in the high risk group where jugular approach was more importantly performed due to the portal hypertensive shunting necessity. Improvements in medical treatment may explain the absence of infectious complications in the group where concomitant biliary drainage was performed, which has been reported as a frequent known complication when done in the preoperative setting.38 Technical success is probably better determined by operator skills, learning curve and patient clinical and anatomical characteristics instead of MELD score. Our results are similar to those achieved by experienced hands described as 67-100% for TIPS39,40 and 98-100% for direct PVT recanalization.41 Technical success persisted in both groups regardless of portal occlusion severity (grade III and IV). Surprisingly, no difference in patenty, thrombosis or dysfunction was found in either MELD score risk group. Dysfunction and/or thrombosis rates were reported in this study from 10-42% similar to other reported series ranging from 21-38%.32,39,41 No increase in thrombosis even after por-tosystemic shunt was recorded seems contrasting with other reports.9 This behavior is explained by concomitant plug closure of the latter.
Despite no statistical significance established on primary patency rates according to stent characteristics, covered stents tend to have better outcomes as reported by Luca, et al. where dysfunction at 12 and 24 months was 38% and 85% in the bare stent group compared to the covered stent group (21% and 29%, respectively).40 Complications were evenly distributed in both groups independently of MELD score and resolved only with medical treatment in the majority of cases. PVT is an important prognostic factor for cirrhosis and also bears significance in individuals undergoing liver transplantation. Although PVT is no longer an absolute contraindication for liver transplantation, patients with extensive thrombosis are amenable to require non-anatomical means of reconstruction of portal flow.42 Fortunately all patients with PVT recanalization in this series achieved transplantation with an end to end anastomosis avoiding the higher morbidity and short term mortality related to cavo-portal or reno-portal reconstructions. Retrospective analysis of this series is a well-known frailty. Also, procedure novelty limits sample increase in order to reach enough statistical power to support our hypothesis. Our results justify prospective cohort study initiation in order to avoid selection and information bias and to better asses MELD score associated peripro-cedural risks for PVR. Other described limitations include the centre experience and the resource availability. This series was performed by a single expert operator, making reproducibility difficult. Stents and guide wires can be expensive in addition to angiog-raphy suites which are not always available in all Latin America healthcare systems. On the other hand, divers etiology inclusion made selection heterogeneous even when this scenario may be closer to real life practice experiences. Nevertheless, since different groups were enrolled for endovascular PVR analysis (pre-transplan-tation, post-transplantation, malignant and idiopathic groups), further detailed investigation should be conducted in each group to determine differences between groups once sample is increased. This may also prompt future research related to the etiology involved. In spite previous shortcomings endovascular PV reconstruction procedures demonstrated a high success and patency rates and acceptable complication incidence.
In conclusion, MELD score did not prove useful to predict morbidity nor mortality when endovascular portal vein or spleno-mesenteric reconstruction was performed in this case series. High technical success and patency rates were described along with a low complication incidence. This minimally invasive option also improves candidacy for transplantation and liver function after OLT; thus it should be considered in order to lower morbidity and mortality related to open portal vein reconstructions.
Conflict of InterestThe authors do not have conflict of interest.