Nonalcoholic fatty liver disease (NAFLD) patients can progress to cirrhosis. In these, there is a compensated stage in which esophageal varices can exist. However, no more than 20% of these patients have varices needing treatment (VNT).
ObjectiveEvaluate the accuracy of non-invasive models to predict esophageal varices, as well as their performance to avoid esophagogastroduodenoscopy (EGD) with a risk of missing VNT of less than 5%, in Brazilian patients with compensated advanced chronic liver disease (cACLD) secondary to NAFLD.
MethodsTwenty-one patients with biopsy-proven cACLD secondary to NAFLD were submitted to liver stiffness measurement (LSM) by transient elastography (TE), and data were collected to measure platelet count/spleen diameter ratio (PSR), LSM-spleen diameter to platelet ratio score (LSPS), varices risk score (VRS), Baveno VI, Expanded Baveno VI and NAFLD cirrhosis criteria.
ResultsThe mean age was 61 (±6.6) years, and 81% were female; 14% presented VNT. For detection of VNT, LSPS and VRS performed excellently, with an area under receiver operating characteristic (AUROC) of 0.961 for both. LSM presented an AUROC of 0.889 and a cutoff point of 21.8 kPa. LSPS and VRS enabled sparing 75–80% of EGDs for VNT, with no risk of missing varices. Expanded Baveno VI enabled sparing 71% of EGDs, with 4.8% risk of missing VNT.
ConclusionLSPS and VRS performed excellently in both predicting VNT and sparing EGD, and Expanded Baveno VI showed good performance in sparing EGDs, with acceptable risk of missing VNT. An LSM cutoff point was established and had good performance.
Chronic liver damage can progress to cirrhosis, leading to worse prognosis related to the development of portal hypertension [1]. During disease evolution, there is an asymptomatic phase in which clinically significant portal hypertension (CSPH) is already present. This phase is characteristically marked by imminent risk of decompensation [2,3].
Classically, measurement of hepatic venous pressure gradient (HVPG) is the gold-standard for the diagnosis of CSPH, as esophagogastroduodenoscopy (EGD) is for the diagnosis of esophageal varices. Once CSPH and esophageal varices are established, prophylatic measures can be initiated to prevent variceal bleeding, such as non-selective beta blockers or endoscopic band ligation [4]. However, both HVPG measurement and EGD are invasive and require more complex apparatus, especially the first one.
In the last decade, several non-invasive methods and models have been studied to identify CSPH or varices in compensated advanced chronic liver disease patients (cACLD). With the advent of transient elastography (TE) for non-invasive diagnosis of liver fibrosis, the paradigm has shifted to this method. Various studies have shown correlation between liver stiffness measurement (LSM) and the presence of CSPH or varices [5–12]. TE performs better at excluding varices than diagnosing them [13]. Besides, the prevalence of varices in cACLD, especially high-risk varices that need treatment, is quite low [14].
However, the majority of studies have addressed cACLD related to viral or alcoholic liver disease [5–8]. Currently, nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide [15]. It is also the most rapidly growing indication for liver transplantation in the United States [16]. The first study related to non-invasive evaluation of esophageal varices exclusively in NAFLD patients was published only recently. It was a multicentric study that included patients from North America, Europe and Hong Kong. There were no participants from Latin America [17]. In Brazil, as in other parts of the world, there is an obesity epidemic, and there are reports of a high prevalence of NAFLD [15,18]. Considering the different ethnic characteristics for intra-abdominal and hepatic adiposity, it is justifiable to study non-invasive methods/models to diagnose/exclude esophageal varices in Brazilian patients with cACLD due to NAFLD [19].
The objective of the present study was to evaluate the accuracy of non-invasive methods/models to predict any esophageal varices or varices needing treatment (VNT), as well as the proportion of EGDs that could be spared and the risk of not diagnosing any varices or VNT by these methods/models, in Brazilian patients with cACLD secondary to NAFLD.
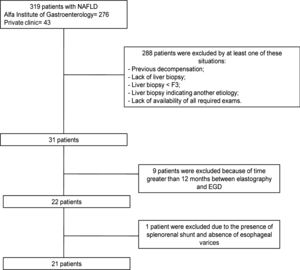
2Patients and methods2.1Patients and study designThis was a cross-sectional study based on a sample universe of 319 Brazilian patients, of which 276 patients were followed at the NAFLD Outpatient Clinic of the Alfa Institute of Gastroenterology of the Federal University of Minas Gerais and 43 patients followed at the private clinic of the main author in Belo Horizonte, Minas Gerais. Data were collected between October 2017 and March 2019. Patients of both sexes, aged 18 years and over, diagnosed with NAFLD and cACLD confirmed by the presence of F3 or F4 fibrosis on liver biopsy (according to Brunt et al. [20]) were included. The criteria adopted for the diagnosis of NAFLD were: presence of hyperechogenic liver on ultrasound; ethanol consumption less than 30 g/day for men and 20 g/day for women; hepatitis B and C negative serology; and absence of defining laboratory criteria for autoimmune liver diseases, hereditary hemochromatosis, Wilson's disease and alpha-1 antitrypsin deficiency.
Exclusion criteria were previous episode of decompensation (characterized by ascites, gastrointestinal bleeding, hepatic encephalopathy or jaundice); presence of portosystemic collateral circulation; and pregnancy. Fig. 1 depicts the sample universe of the study. The final number of patients studied was 21.
The study was approved by the Research Ethics Committee of the Federal University of Minas Gerais, CAAE 74559317.4.0000.5149.
2.2Clinical and propaedeutic assessmentThe following variables were included: age; sex; presence of F3 or F4 fibrosis on liver biopsy; any varices; VNT; body mass index; hyperglycemia (fasting glucose ≥ 100 mg/dL); dyslipidemia (HDL-cholesterol < 40 mg/dL for men and < 50 mg/dL for women; triglycerides ≥ 150 mg/dL); systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg; metabolic syndrome (International Diabetes Federation criteria); laboratory parameters [platelet count, alanine aminotransferase/reference value ratio; total bilirubin; serum albumin; international normalized ratio (INR)]; Model for End-Stage Liver Disease (MELD); spleen diameter; LSM by TE; platelet count/spleen diameter ratio (PSR); LSM-spleen diameter to platelet ratio score (LSPS); varices risk score (VRS); Baveno VI criteria; Expanded Baveno VI criteria; and NAFLD cirrhosis criteria (M probe). The time intervals between demographic and clinical data collection, transient elastography, abdominal ultrasound, and EGD could not exceed 12 months. Laboratory parameters were updated prior to elastography (platelet count, alanine aminotransferase, total bilirubin, fasting glucose, total and fractionated cholesterol, triglycerides, albumin, INR and creatinine).
2.3Transient elastographyTE was performed using Fibroscan® 502 (Echosens, Paris, France), M probe or, in the absence of valid measurements, XL probe. After fasting for at least 3 h, the patient was placed supine with the right arm in maximum abduction. The probe was positioned in an intercostal space over the right lobe of the liver, usually at the intersection between the middle axillary line and a transverse line, parallel to the costal edges, at the level of the xiphoid appendix. When triggered by the operator, the probe emitted a transient shear wave at a depth of 25–65 mm below the skin, penetrating the liver parenchyma. The liver stiffness measurement was obtained in about 10 s. Only exams that met the quality standards determined by the manufacturer were considered. The criteria for consideration were: ten valid measurements (i.e., adequate penetration of the liver parenchyma); success rate (ratio of number of valid measurements to total number of measurements) greater than or equal to 60%; and interquartile range, or IQR/M (dispersion index of ten measurements) less than 30%. The final result of the elastography was obtained by calculating the median stiffness values in the ten valid measurements.
All elastographies, except in two patients, were performed by the same examiner (the main author of this study), who has extensive experience with the method.
2.4Non-invasive modelsNon-invasive models were obtained using their respective formulas:
- -
“PSR” (Giannini et al): platelet count/mm3 / spleen longitudinal diameter (mm) [21];
- -
“LSPS” (Kim et al): LSM (kPa) × spleen longitudinal diameter (cm) / platelet count (×109/L) [5];
- -
“VRS” (Berzigotti et al): −4.364 + 0.538 × spleen longitudinal diameter (cm) −0.049 × platelet count (x109/L) −0.044 × LSM (kPa) + 0.001 × (LSM × platelet count) [7];
- -
Baveno VI criteria: LSM < 20 kPa and platelet count > 150 × 103/mm3 [9];
- -
Expanded Baveno VI criteria: LSM < 25 kPa and platelet count > 110 × 103/mm3 [12];
- -
“NAFLD cirrhosis criteria” (M probe): LSM < 30 kPa and platelet count > 110 × 103/mm3 [17].
EGD was selected as the gold standard for diagnosis of esophageal varices. When present, they were classified as “any varices” and as VNT (medium-large caliber or small caliber with red spots on their walls) [9].
2.6Statistical analysisDemographic, clinical and exams data were stored in the Statistical Package for Social Sciences version 21.0 (SPSS Inc., Chicago, IL). Numerical variables were evaluated for normality (Shapiro-Wilk test) in order to select data presentation. The relationship between quantitative variables was established with Student's t test, or with a Mann-Whitney test when the distribution was not Gaussian. The Chi-square test was used to study the relationship between categorical variables and Fisher's exact test when there were caselles with values less than 5. P-values less than 0.05 were considered significant. Receiver operating characteristic (ROC) curves were done to establish cutoff values for LSM, PSR, LSPS and VRS in order to predict the presence of any varices and VNT. The sensitivity, specificity, positive predictive value, negative predictive value, positive likehood ratio (LR+) and negative likelihood ratio (LR–) of non-invasive models whose area under the ROC curve was greater than 0.5 were calculated. The percentage of EGDs that could be spared, expressed as the proportion of patients with values below the cutoff points obtained in the study for LSM, PSR, LSPS and VRS, and the proportion of patients within the Baveno VI, Expanded Baveno VI and NAFLD cirrhosis criteria (M probe), were also determined. The percentage of missing any varices and VNT was calculated using the ratio between the number of patients below the cutoff points or meeting each of the three criteria and presenting any varices or VNT at EGD and the total number of patients.
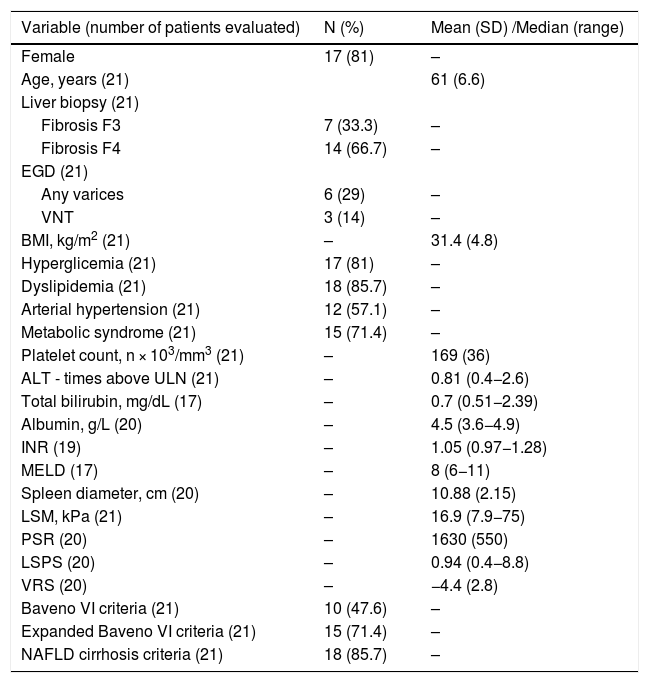
3ResultsTwenty-one patients were included in the study. Seventeen (81%) were female, and their mean age was 61 ± 6.6 years. Seven (33%) patients were F3 and 14 (67%) F4 on liver biopsy. Six patients (29%) had any varices and three (14%) had VNT. The main demographic, clinical, propedeutical and score data are described in Table 1.
Demographic, clinical, propaedeutical and score data.
| Variable (number of patients evaluated) | N (%) | Mean (SD) /Median (range) |
|---|---|---|
| Female | 17 (81) | – |
| Age, years (21) | 61 (6.6) | |
| Liver biopsy (21) | ||
| Fibrosis F3 | 7 (33.3) | – |
| Fibrosis F4 | 14 (66.7) | – |
| EGD (21) | ||
| Any varices | 6 (29) | – |
| VNT | 3 (14) | – |
| BMI, kg/m2 (21) | – | 31.4 (4.8) |
| Hyperglicemia (21) | 17 (81) | – |
| Dyslipidemia (21) | 18 (85.7) | – |
| Arterial hypertension (21) | 12 (57.1) | – |
| Metabolic syndrome (21) | 15 (71.4) | – |
| Platelet count, n × 103/mm3 (21) | – | 169 (36) |
| ALT - times above ULN (21) | – | 0.81 (0.4−2.6) |
| Total bilirubin, mg/dL (17) | – | 0.7 (0.51−2.39) |
| Albumin, g/L (20) | – | 4.5 (3.6−4.9) |
| INR (19) | – | 1.05 (0.97−1.28) |
| MELD (17) | – | 8 (6−11) |
| Spleen diameter, cm (20) | – | 10.88 (2.15) |
| LSM, kPa (21) | – | 16.9 (7.9−75) |
| PSR (20) | – | 1630 (550) |
| LSPS (20) | – | 0.94 (0.4−8.8) |
| VRS (20) | – | −4.4 (2.8) |
| Baveno VI criteria (21) | 10 (47.6) | – |
| Expanded Baveno VI criteria (21) | 15 (71.4) | – |
| NAFLD cirrhosis criteria (21) | 18 (85.7) | – |
EGD, esophagogastroduodenoscopy; VNT, varices needing treatment; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine aminotransferase; ULN, upper limit of normal; INR, international normalized ratio; MELD, model for end-stage liver disease; LSM, liver stiffness measurement; PSR, platelet count/spleen diameter ratio; LSPS, LSM-spleen diameter to platelet ratio score; VRS, varices risk score; NAFLD, nonalcoholic fatty liver disease.
For Baveno VI criteria, Expanded Baveno VI criteria and NAFLD cirrhosis criteria, it is described the number of patients within each criteria.
Univariate analysis was performed to assess the possible association between the variables studied and presence of any varices and VNT. Regarding the presence of any varices, the variables that were significant were spleen diameter (p = 0.017), LSM (p = 0.011), LSPS (p = 0.003), VRS (p = 0.004) and Expanded Baveno VI criteria (p = 0.031). Serum albumin showed statistical trend (p = 0.062). In relation to VNT, the following variables were significant: albumin (p = 0.012), LSM (p = 0.035), PSR (p = 0.033), LSPS (p = 0.007), and VRS (p = 0.003). The p-values of spleen diameter, platelet count and INR were 0.05, 0.059 and 0.085, respectively.
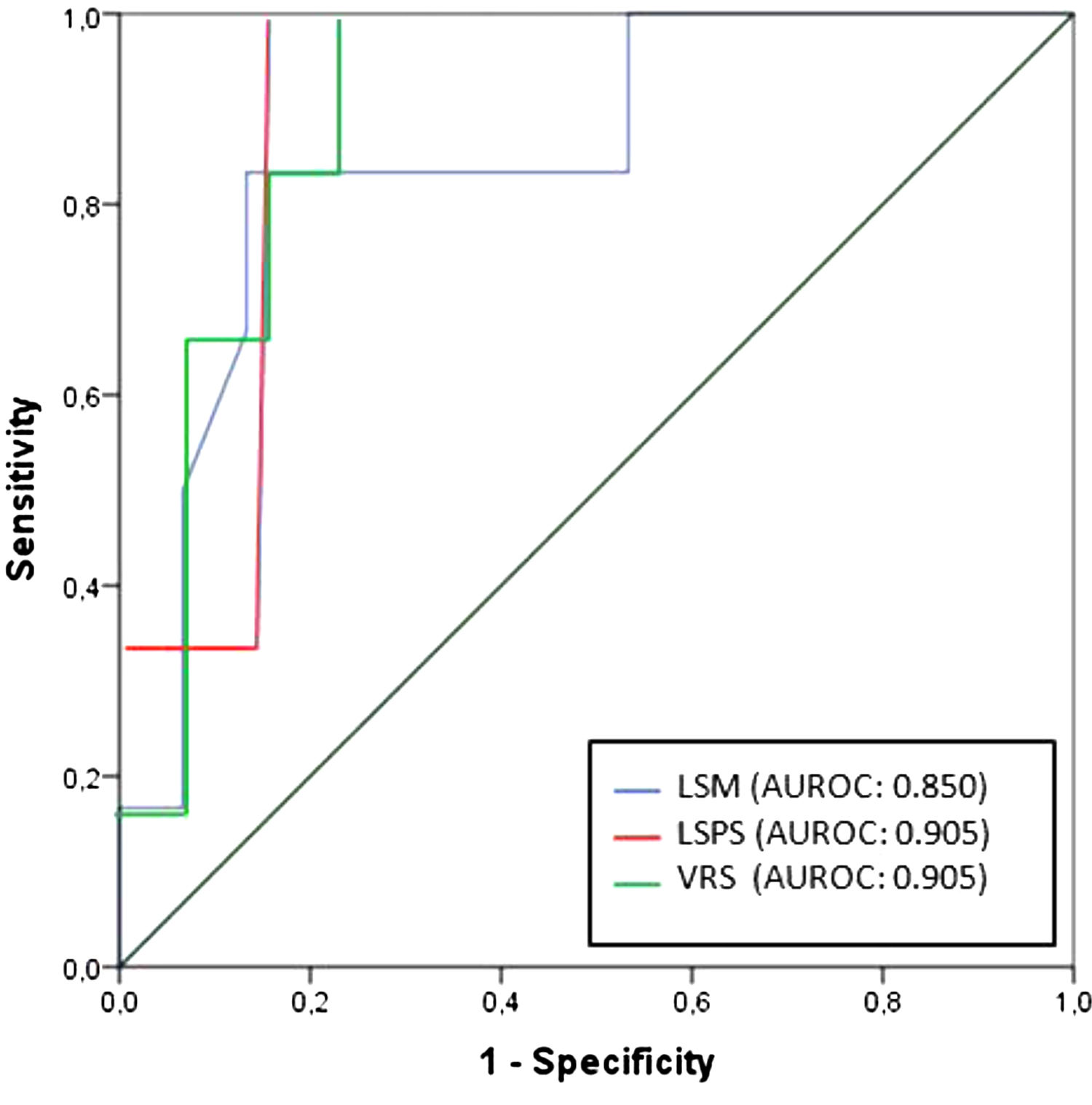
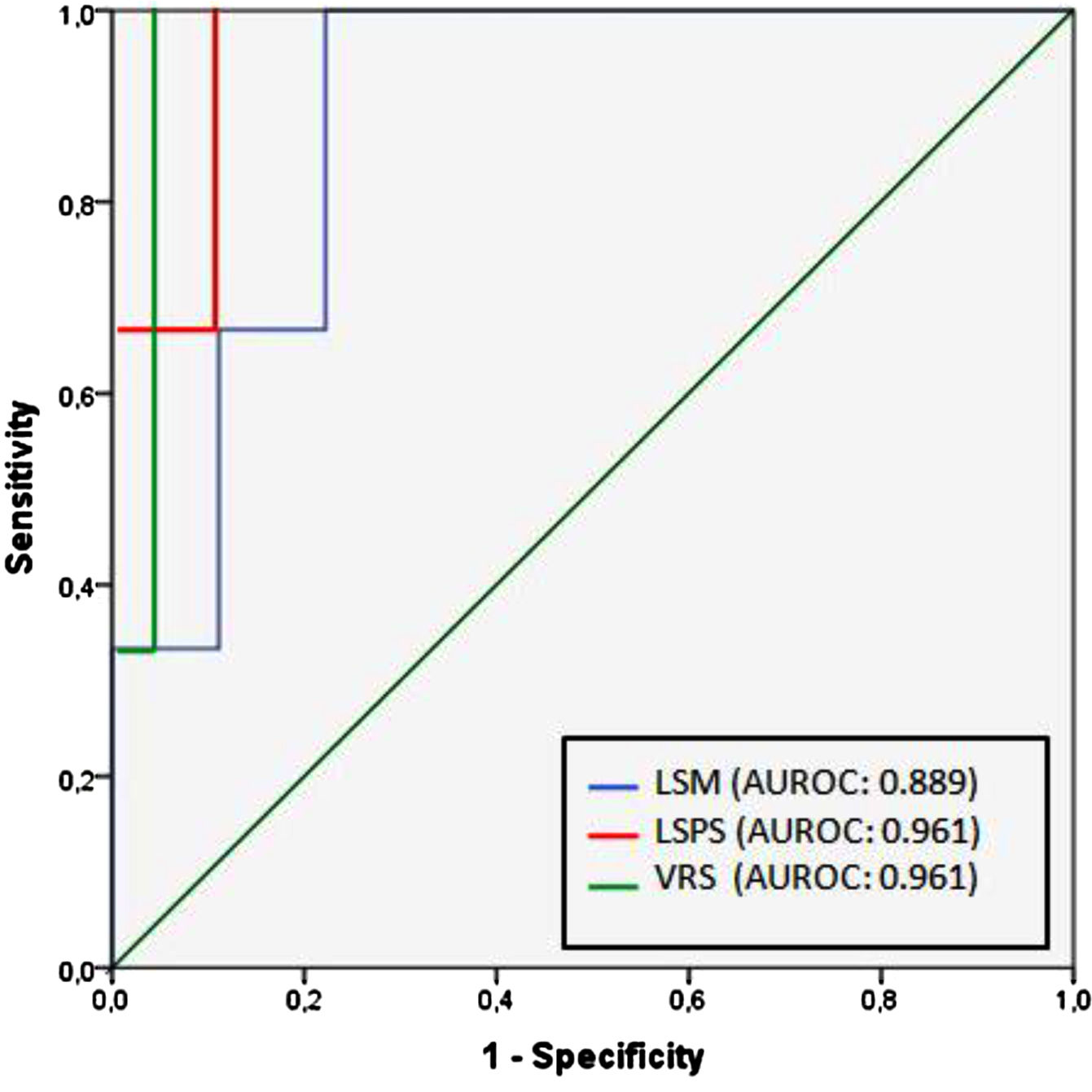
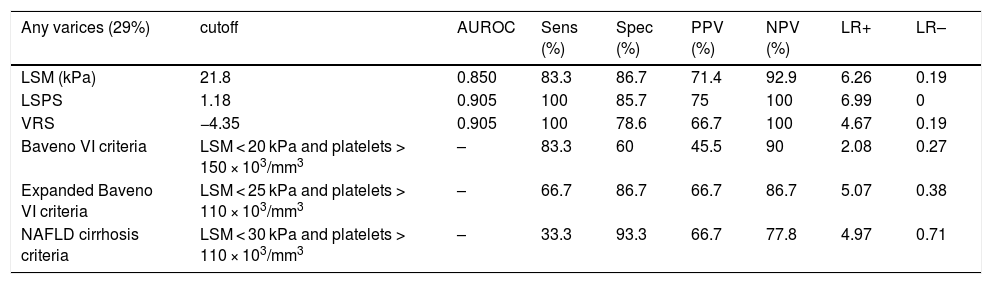
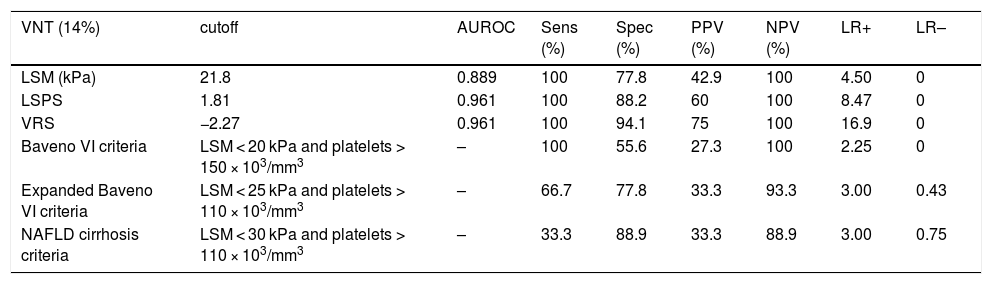
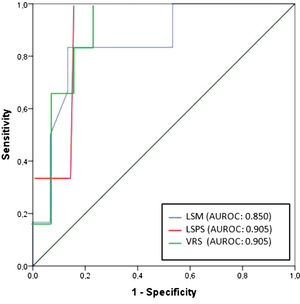
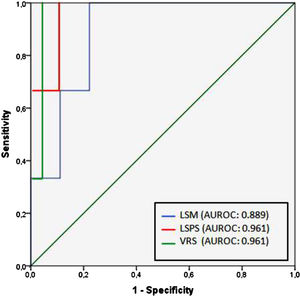
Regarding any varices, area under the ROC curve (AUROC) was 0.850 for LSM (cutoff point of 21.8 kPa) and 0.905 for both LSPS and VRS (cutoff points of 1.18 and −4.35, respectively) (Fig. 2). PSR did not reach statistical significance (p = 0.108), so the ROC curve was not performed. Regarding VNT, area under the ROC curve (AUROC) was 0.889 for LSM (again with cutoff point of 21.8 kPa) and 0.961 for both LSPS and VRS (cutoff points of 1.81 and −2.27, respectively) (Fig. 3). Although PSR reached statistical significance (p = 0.033), its AUROC was below 0.5 so it was not possible to identify cutoff for predicting VNT. The performances of all non-invasive models in predicting any varices and VNT are depicted in Tables 2 and 3, respectively.
Performance of non-invasive models in predicting any varices.
| Any varices (29%) | cutoff | AUROC | Sens (%) | Spec (%) | PPV (%) | NPV (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|---|
| LSM (kPa) | 21.8 | 0.850 | 83.3 | 86.7 | 71.4 | 92.9 | 6.26 | 0.19 |
| LSPS | 1.18 | 0.905 | 100 | 85.7 | 75 | 100 | 6.99 | 0 |
| VRS | −4.35 | 0.905 | 100 | 78.6 | 66.7 | 100 | 4.67 | 0.19 |
| Baveno VI criteria | LSM < 20 kPa and platelets > 150 × 103/mm3 | – | 83.3 | 60 | 45.5 | 90 | 2.08 | 0.27 |
| Expanded Baveno VI criteria | LSM < 25 kPa and platelets > 110 × 103/mm3 | – | 66.7 | 86.7 | 66.7 | 86.7 | 5.07 | 0.38 |
| NAFLD cirrhosis criteria | LSM < 30 kPa and platelets > 110 × 103/mm3 | – | 33.3 | 93.3 | 66.7 | 77.8 | 4.97 | 0.71 |
AUROC, area under receiver operating characteristic; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio; LSM, liver stiffness measurement; LSPS, LSM-spleen diameter to platelet ratio score; VRS, varices risk score; NAFLD, nonalcoholic fatty liver disease.
Performance of non-invasive models in predicting VNT.
| VNT (14%) | cutoff | AUROC | Sens (%) | Spec (%) | PPV (%) | NPV (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|---|
| LSM (kPa) | 21.8 | 0.889 | 100 | 77.8 | 42.9 | 100 | 4.50 | 0 |
| LSPS | 1.81 | 0.961 | 100 | 88.2 | 60 | 100 | 8.47 | 0 |
| VRS | −2.27 | 0.961 | 100 | 94.1 | 75 | 100 | 16.9 | 0 |
| Baveno VI criteria | LSM < 20 kPa and platelets > 150 × 103/mm3 | – | 100 | 55.6 | 27.3 | 100 | 2.25 | 0 |
| Expanded Baveno VI criteria | LSM < 25 kPa and platelets > 110 × 103/mm3 | – | 66.7 | 77.8 | 33.3 | 93.3 | 3.00 | 0.43 |
| NAFLD cirrhosis criteria | LSM < 30 kPa and platelets > 110 × 103/mm3 | – | 33.3 | 88.9 | 33.3 | 88.9 | 3.00 | 0.75 |
VNT, varices needing treatment; AUROC, area under receiver operating characteristic; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio; LSM, liver stiffness measurement; LSPS, LSM-spleen diameter to platelet ratio score; VRS, varices risk score; NAFLD, nonalcoholic fatty liver disease.
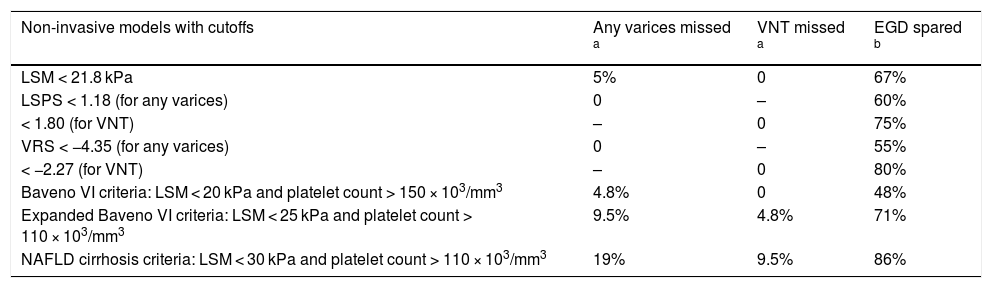
Non-invasive models were also evaluated for their ability to avoid EGD without missing any varices or VNT. All calculations were based on cutoff values obtained (LSM, LSPS and VRS) or on previously established criteria (Baveno VI, Expanded Baveno VI and NAFLD cirrhosis). With LSM, 67% of patients would not meet EGD criteria. There would be a 5% risk of missing any varices, but no risk of missing VNT. With LSPS, 60% of patients would not meet EGD criteria and there would be no risk of missing any varices. With VNT, 75% of patients would not meet EGD criteria and there would be no risk of missing varices. The VRS had similar behavior. Fifty-five percent of patients would not meet EGD criteria, and there would be no risk of missing any varices. As far as VNT was concerned, 80% of patients would not meet EGD criteria and there would be no risk of missing varices. If Baveno VI criteria were followed, 48% would not meet EGD criteria and 4.8% of patients with any varices would not be identified. No patient with VNT would be unidentified. If Expanded Baveno VI criteria were followed, 71% of patients would not meet EGD criteria, the risk of missing any varices would be 9.5% and the risk of missing VNT would be 4.8%. If NAFLD cirrhosis criteria were followed, 86% percent of patients would not meet EGD criteria, the risk of missing any varices would be 19% and the risk of missing VNT would be 9.5% (Table 4).
Performance of non-invasive models for screening EGD.
| Non-invasive models with cutoffs | Any varices missed a | VNT missed a | EGD spared b |
|---|---|---|---|
| LSM < 21.8 kPa | 5% | 0 | 67% |
| LSPS < 1.18 (for any varices) | 0 | – | 60% |
| < 1.80 (for VNT) | – | 0 | 75% |
| VRS < −4.35 (for any varices) | 0 | – | 55% |
| < −2.27 (for VNT) | – | 0 | 80% |
| Baveno VI criteria: LSM < 20 kPa and platelet count > 150 × 103/mm3 | 4.8% | 0 | 48% |
| Expanded Baveno VI criteria: LSM < 25 kPa and platelet count > 110 × 103/mm3 | 9.5% | 4.8% | 71% |
| NAFLD cirrhosis criteria: LSM < 30 kPa and platelet count > 110 × 103/mm3 | 19% | 9.5% | 86% |
VNT, varices needing treatment; EGD, esophagogastroduodenoscopy; LSM, liver stiffness measurement; LSPS, LSM-spleen diameter to platelet ratio score; VRS, varices risk score; NAFLD, nonalcoholic fatty liver disease.
In patients with cACLD of any etiology, it is important to identify which of them are prone to decompensate. In this context, it is necessary to recognize patients with CSPH and esophageal varices, mainly VNT. The gold standard procedures for such evaluation are HVPG measurement and EGD; however, it is known that they are invasive and, moreover, the first one has limited availability in the Brazilian public healthcare system. Besides, no more than 30–40% of patientes with cACLD have esophageal varices and a smaller proportion of them (10–20%) have VNT [14]. Accordingly, it is advisable to study non-invasive methods to predict esophageal varices, selecting patients that could be spared unnecessary EGD.
In the last decade, several non-invasive models have been proposed, mostly using LSM alone or in combination with platelet count, with or without spleen diameter. However, samples studied consisted of patients whose liver disease was principally due to viral or alcoholic etiology. The applicability of non-invasive models was therefore restricted to those etiologies [9]. This left an unmet need regarding the most prevalent chronic liver disease worldwide, NAFLD. In 2018, Petta et al. published a multicentric study that included 790 patients with NAFLD from six different countries in Europe, Asia and North America, and proposed new criteria for excluding varices, the NAFLD cirrhosis criteria [17].
Considering the high prevalence of obesity and NAFLD in Brazil and the phenotypic differences among ethnic groups, this study aimed to evaluate the performance of non-invasive models for predicting esophageal varices in Brazilian patients with NAFLD-related cACLD [18,19]. Only patients with biopsy-proven disease were included, as only biopsy can both ascertain NAFLD etiology and confirm cACLD by finding F3–F4 histologic fibrosis [9]. The studies that examined the Baveno VI [11], Expanded Baveno VI [12] and NAFLD cirrhosis criteria [17] did not establish liver biopsy as a requisite; instead, they considered LSM ≥ 10 kPa (Baveno VI and Expanded Baveno VI criteria studies) or LSM ≥ 11.0–11.5 kPa (NAFLD cirrhosis criteria study) to suspect cACLD. It is known that LSM can be overestimated by different factors, mainly inflammatory activity and, possibly, steatosis grade [22,23]. On the other hand, this strict selection criterion reduced the size of the study sample.
In this sample of patients with NAFLD with biopsy-proven cACLD, the mean body mass index was 31.4 kg/m2, and the majority of patients (71%) had metabolic syndrome or at least one of its criteria. Hyperglycemia was present in 81%, 86% had dyslipidemia and 57% had blood pressure criteria. These findings corroborate the correlation between metabolic syndrome or its components and advanced stages of NAFLD. Interestingly, even in such a small sample, the prevalence of any varices (29%) and of VNT (14%) was in line with the previous reports [14]. The mean platelet count was not reduced. All patients had Child-Pugh A as well as MELD score below 12. The mean spleen diameter was normal, too. All these parameters denote that we were dealing with a sample of patients with no major decompensation criteria, although with some risk of bleeding.
The variables most closely associated with the presence of any varices and VNT were LSM and the models combining LSM, platelet count and spleen diameter (i.e., LSPS and VRS). Platelet count and spleen diameter, which are the variables most related to the presence of CSPH and esophageal varices, did not have the same statistical behavior as LSM, LSPS and VRS. However, spleen diameter was associated with the presence of any varices and tended to be associated with the presence of VNT, as did platelet count. Interestingly, the model combining platelet count and spleen diameter without LSM (PSR) did not present the same association as the models that include LSM. The small sample size may have contributed to these results. However, even in this small sample, LSM and the models that include it showed a consistent association with any varices and VNT, which suggests the importance of LSM for predicting varices.
When the accuracy of LSM and each non-invasive model was evaluated for the prediction of any varices, LSPS and VRS presented excellent diagnostic performance, and as the objective became the ability of these tests to exclude varices, the yield was even better. For the mentioned cutoff values, the negative likelihood ratio (LR–) was 0 (zero) for LSPS and 0.19 for both VRS and LSM. Notably, the cutoff point for LSM (21.8 kPa) is in line with existing data regarding liver disease of mainly viral etiology, as acknowledged by Baveno VI consensus [9].
In the prediction of VNT, which has more practical importance, LSPS and VRS again presented excellent performance. Once more, all models studied had better performance for excluding than for diagnosing varices, with LR– values of zero for LSPS, VRS, LSM and Baveno VI criteria. Expanded Baveno VI and NAFLD cirrhosis criteria performed slightly worse.
Importantly, for the prediction of any varices, the cutoff point for LSPS (1.18) also corresponded to the best value for excluding varices according to AUROC (NPV 100%), and had an LR– value of zero. In previous studies, Colecchia et al. and Berzigotti et al. found LSPS cutoff points of 1.32 (LR– value of 0.03) and 2.06 (LR– value of 0.13) for excluding any varices, respectively [6,7]. For the prediction of VNT, the cutoff points for LSM (21.8 kPa), LSPS (1.81) and VRS (−2.27) also corresponded to the best values for excluding VNT (NPV 100% and LR– value of zero for all of these parameters). In the study that formulated the LSPS score, Kim et al. found cutoff point for excluding VNT of 3.5 (NPV 94.7%; LR– not reported) [5].
These differences in cutoff values maybe related to differences in etiologies, stages of liver disease and inclusion criteria of each study. In the study by Colecchia et al., both compensated and decompensated cirrhotic patients were included, and the etiology was chronic hepatitis C [6]. In the study by Berzigotti et al., only compensated cirrhotics with viral or alcoholic etiology were evaluated [7]. Kim et al. included compensated and decompensated cirrhotic patients secondary to chronic hepatitis B [5].
Regarding the possibility of sparing EGDs, it is acceptable to miss less than 10% of any varices as suggested by the “Anticipate” Investigators [10]. Assuming this recommendation, the Expanded Baveno VI criteria could spare the largest number of EGDs, followed by LSM alone using the 21.8 kPa cutoff point and, after it, LSPS using the 1.18 cutoff point. NAFLD cirrhosis criteria could spare the maximum number of EGDs; however, it presented an unacceptable 19% risk of missing any varices, making this model inappropriate. The very satisfactory performance of LSM as an isolated method should be emphasized.
For VNT, the model that could spare the largest number of EGDs while missing an acceptable number of VNT (below 5%, as per Baveno VI consensus recommendations [9]) was VRS, followed by LSPS and, after it, Expanded Baveno VI criteria. Once more, the model that could spare the maximum number of EGDs was NAFLD cirrhosis criteria, but with an unacceptable 9.5% risk of missing VNT. Again, LSM alone exhibited good performance.
Despite the great performance of both LSPS and VRS, the variability of cutoff values in different studies hampers the clinical applicability of these models. Moreover, LSPS and mainly VRS are calculated using not so simple mathematical formulas. Thus, from a practical point of view, the Expanded Baveno VI criteria proved to be a reliable and simple way to avoid screening EGDs in this NAFLD-related cACLD population. LSM alone, without platelet count or spleen diameter, also had a good performance.
This study has limitations. Despite a sample universe of 319 patients, only 21 were eligible, which made the final sample size small. There were several reasons for excluding patients. Even after selecting 31 patients with biopsy-proven cACLD secondary to NAFLD, it was decided to exclude 9 of them because the time between elastography and EGD was longer than 12 months. One patient with LSM of 46.4 kPa and no varices on EGD was also excluded; this patient had a spontaneous splenorenal shunt on Doppler ultrasound and it was postulated that decompression of the portal system by the shunt could account for the absence of esophageal varices, configuring a confounding factor in the application of non-invasive models.
Another point refers to the lack of standardization of the type of Fibroscan® probe to be used. All tests were initially attempted with the M probe; in case of failure (mainly due to obesity), the XL probe was used. Of the 21 LSM performed, 13 were performed with the M probe and 8 with the XL probe. It should be emphasized that, in relation to NAFLD cirrhosis criteria, we decided to evaluate only the proposed model for probe M (LSM < 30 kPa and platelet count > 110 × 103/mm3) due to the small sample of our study, and also because the XL probe model is exactly the same as the Baveno VI Expanded criteria (LSM < 25 kPa and platelet count > 110 × 103/mm3); in addition, two recent large studies have shown no significant difference between LSM obtained with M and XL probes [24,25].
In conclusion, this study showed that non-invasive methods/models for predicting esophageal varices in a sample of Brazilian patients with NAFLD-related cACLD performed as well as they did for cACLD of viral or alcoholic etiology in other ethnic groups. They were better at excluding than at diagnosing varices. The absence of validated cutoff points and the need to use mathematical formulas to calculate LSPS and VRS limit their applicability. The Expanded Baveno VI criteria proved to be both reliable and practical for clinical use in this situation. In this study, LSM cutoff point was in line with the values acknowledged by the Baveno VI consensus for viral etiology, and can be used for future comparisons.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interestNone.
The authors would like to thank the Brazilian Society of Hepatology and the Brazilian Association of Hepatitis Patients for providing Fibroscan® 502 equipment in order to perform LSM.