The pathogenesis of asthma involves both airway inflammation and an oxidant/antioxidant imbalance. It is demonstrated in asthmatic adults that exercise programmes improve lung function, a mechanism yet to be elucidated. The purpose of this study was to investigate the possible beneficial effects of physical exercise on antioxidant status in asthmatic children which may lead to ameliorated lung function.
MethodsThe study enrolled thirteen control and thirty asthmatic children. The asthmatic group was subdivided into two: the first group receiving only pharmacological treatment (n=15) and the second receiving pharmacological treatment with exercise programme (n=15) for 8 weeks. Blood samples were drawn from the subjects before and after treatment periods. As oxidant stress markers blood levels of malondialdehyde (MDA) and total nitric oxide (NO), and as antioxidant status, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) enzyme activities were assessed.
ResultsBefore any treatment was initiated, MDA and NO levels in the asthmatic group were significantly higher than the controls (3.40±0.96 nmol/ml vs 2.46±0.58 nmol/ml, and 12.53±2.10 vs 9.40±1.39 micromol/L, respectively). Both SOD (p=0.0001) and GSH-Px (p=0.023) activities were significantly lower in the asthmatic group. Pharmacological treatment and exercise programme together significantly improved lung performance and decreased the levels of oxidant stress markers, in concordance with a significantly increase in antioxidant enzyme activity measures when compared to the pharmacological treatment.
ConclusionStructured exercise programme in asthmatic children resulted in better lung function, which may be attributed to its effect on antioxidant status.
The pathogenesis of chronic obstructive lung diseases such as asthma is complex. It involves both airway inflammation and an oxidant/antioxidant imbalance.1 The link between chronic inflammation and oxidative stress is activated inflammatory cells, including neutrophils and eosinophils, which generate reactive oxygen species (ROS).2 Pro-inflammatory cytokines released from activated cells stimulate the production of reactive oxygen species, which act as signalling mediators for a variety of signal transduction pathways and gene expression.3,4 Elevated levels of ROS such as hydroxyl radicals, superoxides, and peroxides in inflammatory conditions have been reported in various inflammatory diseases.5 These molecules exert a number of toxic effects that have been demonstrated in many different biological systems.6 The ROS are likely to play a vital role in the pathogenesis of asthma because they have been shown to be associated with many pathophysiological changes that are relevant in asthma, such as increased lipid peroxidation, increased airway reactivity and secretions, increased production of chemoattractants, and increased vascular permeability.7
Multiple defence systems, collectively called antioxidants, are present in the human body to prevent the damage caused by the ROS.8 These defence systems include enzymatic antioxidants such as superoxide dismutase (SOD), which degrades superoxide anion (O2_), and glutathione peroxidase (GSH-Px), which detoxifies hydrogen peroxide (H2O2)9 and non-enzymatic antioxidants such as the GSH (glutathione) redox system1,10 However, when the production of harmful ROS exceeds the capacity of the body's antioxidant defences to detoxify them, a condition known as oxidative stress occurs. Oxidative stress leads to changes such as modification of receptor activity and signalling as well as the release of endogenous mediators of inflammation.
Children and adults with asthma may avoid exercise because of their respiratory symptoms and they may have reduced exercise tolerance due to the increased sensation of dyspnoea.11,12 The results of previous studies on the influence of exercise on spirometry are contradictory, some reporting improvement.13 The basic pathogenetic mechanisms underlying these changes in asthmatic patients taking exercise are unclear.
The current study was designed to investigate the possible beneficial effect of physical exercise on antioxidant status in asthmatic children which may lead to ameliorated lung function.
Materials and methodsSubjectsThirty children aged 8-13 years diagnosed with asthma (Group 1) in the Pediatric Allergy and Pulmonology Department of the Medical Faculty of Celal Bayar University were enrolled in the study. During the selection process, potential candidates for inclusion in the asthmatic group of the study were asked about their general sporting habits. Those children who stated that they regularly participated in exercise and/or were part of a sports team were excluded from the study. The diagnosis of asthma was based on history of recurrent cough and wheezing with prolonged expiration time which demonstrated clinical reversibility with a short acting beta-2 agonist bronchodilator therapy. Immunocompromised patients, patients with a history of chronic inflammation/rheumatological disorder, and patients with autoimmune diseases were excluded. Asthma patients had not been receiving any medication and had not had any symptoms of lower or upper respiratory tract infection or asthma exacerbation during the period four weeks prior to the study.
The control group (Group 2) consisted of 13 age-matched healthy children. They were chosen from those referred to the paediatric outpatient clinic in the University Hospital, where all children periodically undergo check-ups for their growth and development. Control patients were evaluated with regard to chronic and/or severe infections, rheumatological and autoimmune disorders, familial and personal history of atopy and also by laboratory tests. Children were included in the control group if they had no personal and familial history of atopy and no signs of atopic disorder.
All patients were examined physically and their forced vital capacity (FVC) and forced expiratory volume 1 (FEV1) was measured. The study was approved by the Institutional Ethical Review Board and informed consent was taken from the parents or guardians of all children taking part in the study.
Study designAll thirty asthmatic patients started to receive the standard pharmacological treatment (fluticazone 250μg/day). The patient group was randomly divided into two equal groups. One group (Group 1a) received only the pharmacological treatment for eight weeks. The other group (Group 1b) received the pharmacological treatment and was assigned to an exercise programme carried out by the Physiotherapy and Rehabilitation Department. Group 1b children exercised twice a week for an hour on a bicycle for eight weeks. The rate of exercise was determined according to the resting heart rate of the children. Their submaximal heart rate was determined as 50% higher than their resting heart rate. Their target heart rate during exercise was determined as 80% of the submaximal heart rate. The pedalling rate required to reach the target heart rate was determined using a pulse oximeter during exercise. During the eight week exercise programme, 15minutes of warm-up exercise was followed by 45minutes of cycling at the target heart rate.
Blood samplingFor baseline values, venous blood samples were drawn from Group 1 and 2 at the beginning of the eight week period. At the end of eight weeks of pharmacological treatment or pharmacological treatment in addition with an exercise programme, post treatment samples were obtained from the subjects in Groups 1a and 1b.
After an overnight fast, blood samples were collected into evacuated plain and K3EDTA containing tubes. Samples were centrifuged at 4,000rpm for 10minutes within 2hours after sampling. The plasma/sera were removed and frozen at −80°C until analysis. To minimise the influence of analytical variation, all the samples were analysed on the same day for each measurement.
Measurement of malondialdehyde (MDA)Analyses of plasma malondialdehyde (MDA) levels were performed by a spectrophotometrical (Shimadzu UV-1201V, Japan) method described by Ohkawa et al.14 An external standard curve was prepared using 1,1,3,3-tetraethoxypropane. Plasma MDA levels were expressed as nmol/mL.
Measurement of GSH-PxPlasma GSH-PX activities were determined by the method of Paglia.15 Cumene hydroperoxide was added as a substrate. Oxidised glutathione produced by the action of GSH-PX present in the sample was then reduced by glutathione reductase in the presence of NADPH. The decrease in NADPH was recorded at 340nm spectrophotometrically. The results were expressed as IU/L.
Measurement of superoxide dismutase (SOD)SOD enzyme activities were determined in plasma by the spectrophotometeric modified methods of Sun et al. and Durak et al.16,17 Superoxide formation via the xanthine/xanthine oxidase system depends on the reduction of nitroblue tetrazolium (NBT). Superoxide radicals form coloured formazan compounds by reducing NBT, with a maximum absorbance at a wavelength of 560 nm. This reduction is maximal in the absence of this enzyme, with a significant blue-purple colour formation. The results were expressed as U/mL.
Measurement of total nitric oxide (NO)Serum nitrite (NO2−) and nitrate (NO3−) levels may be used to estimate NO production. Total NO levels were determined by using a previously described method based on Griess reagent.18 After deproteinisation with Somogy reagent19 to eliminate any interference, the samples were subjected to nitrite analysis by Griess reagent. In order to reduce the nitrate to nitrite, the samples were incubated with copper-coated cadmium granules for 90minutes. By using these incubated samples for nitrite analysis, the total NO levels (nitrate+nitrite) were calculated. The results were expressed as μmol/L.
Statistical analysisData were analysed using the SPSS for Windows computing program (Version 11.0). All values were expressed as mean±standard deviation. Group to group comparisons were performed by Kruskal Wallis and Mann–Whitney U-tests and, within group comparisons were performed by Wilcoxon signed ranks test for continuous variables. Chi square test was used for frequency comparisons. Statistical significance was defined for p values of less than 0.05.
ResultsSociodemographic characteristics of the study populationIn the asthmatic group (Group 1) the mean age was 9.8±1.8 years, consisting of 22 male and 8 female children. In the control group (Group 2) the mean age was 10.3±2.0 years consisting of 6 male and 7 female children. There was no difference between Groups 1 and 2 regarding age or gender (p=0.33 and p=0.086, respectively).
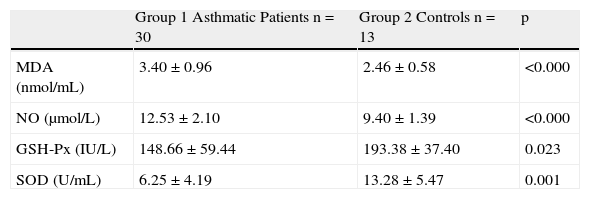
Comparison of MDA, NO levels and GSH-Px, SOD enzyme activities between asthmatic and control groups before interventionMDA and NO levels in the asthmatic group were significantly higher than the control group (3.40±0.96 nmol/mL vs. 2.46±0.58 nmol/mL, and 12.53±2.10μmol/L vs. 9.40±1.39μmol/L respectively, p=0.0001). GSH-Px and SOD activities were significantly lower in the asthmatic group compared to the control group (p=0.023 and p=0.001, respectively) (Table 1).
Comparison of MDA, NO levels and GSH-Px, SOD enzyme activities between asthmatic and control groups before intervention.
| Group 1 Asthmatic Patients n=30 | Group 2 Controls n=13 | p | |
| MDA (nmol/mL) | 3.40±0.96 | 2.46±0.58 | <0.000 |
| NO (μmol/L) | 12.53±2.10 | 9.40±1.39 | <0.000 |
| GSH-Px (IU/L) | 148.66±59.44 | 193.38±37.40 | 0.023 |
| SOD (U/mL) | 6.25±4.19 | 13.28±5.47 | 0.001 |
MDA: malondialdehyde, NO: total nitric oxide, GSH-Px: glutathione peroxidase, SOD: superoxide dismutase.
Before any treatment was initiated, the asthmatic children were divided into two equal groups randomly. Group 1a received only pharmacological treatment whereas Group 1b received both pharmacological treatment and an exercise programme for 8 weeks. When these two groups were compared before any treatment was initiated, none of the parameters differed significantly (data not shown).
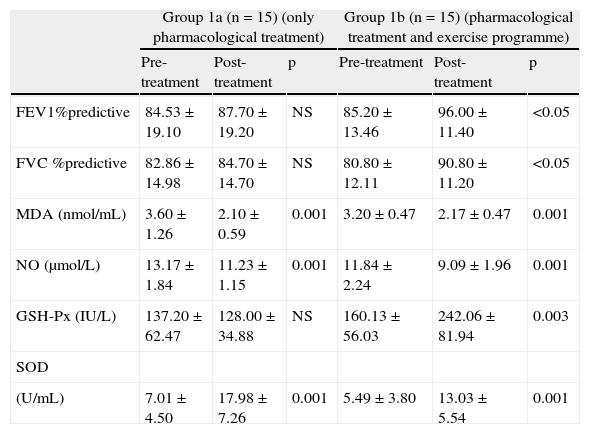
Comparison of pre and post treatment MDA, NO levels and GSH-Px, SOD enzyme activities within Group 1a.Group 1a received only pharmacological treatment for 8 weeks. When pre and post treatment MDA, NO levels and GSH-Px, SOD enzyme activities were compared, MDA and NO levels significantly decreased whereas SOD activity increased by the end of 8 weeks. No difference was observed in GSH-Px activities and lung function tests (Table 2).
After eight weeks of treatment, comparisons of pre- and post-treatment values of FEV1, FVC results, MDA, NO levels and GSH-Px, SOD enzyme activities in Groups 1a and 1b.
| Group 1a (n=15) (only pharmacological treatment) | Group 1b (n=15) (pharmacological treatment and exercise programme) | |||||
| Pre-treatment | Post-treatment | p | Pre-treatment | Post-treatment | p | |
| FEV1%predictive | 84.53±19.10 | 87.70±19.20 | NS | 85.20±13.46 | 96.00±11.40 | <0.05 |
| FVC %predictive | 82.86±14.98 | 84.70±14.70 | NS | 80.80±12.11 | 90.80±11.20 | <0.05 |
| MDA (nmol/mL) | 3.60±1.26 | 2.10±0.59 | 0.001 | 3.20±0.47 | 2.17±0.47 | 0.001 |
| NO (μmol/L) | 13.17±1.84 | 11.23±1.15 | 0.001 | 11.84±2.24 | 9.09±1.96 | 0.001 |
| GSH-Px (IU/L) | 137.20±62.47 | 128.00±34.88 | NS | 160.13±56.03 | 242.06±81.94 | 0.003 |
| SOD | ||||||
| (U/mL) | 7.01±4.50 | 17.98±7.26 | 0.001 | 5.49±3.80 | 13.03±5.54 | 0.001 |
FEV1: forced expiratory volume 1; FVC: forced vital capacity; MDA: malondialdehyde; NO: total nitric oxide; GSH-Px: glutathione peroxidise; SOD:superoxide dismutase; NS: not significant.
Group 1b received both pharmacological treatment and an exercise programme for 8 weeks. When pre- and post-treatment MDA, NO levels and GSH-Px, SOD enzyme activities were compared, MDA and NO levels were significantly lower; whereas both SOD and GSH-Px activities were higher at the end of 8 weeks compared to the pre-treatment measurements. Both post-treatment FVC and FEV1 scores were also higher (Table 2).
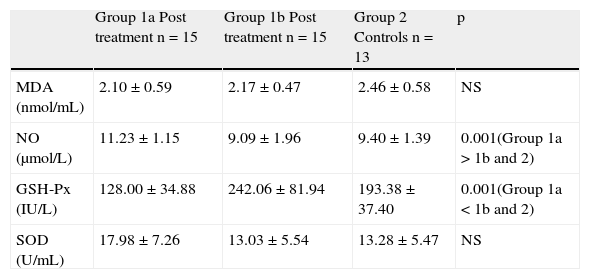
Comparison of post-treatment MDA, NO levels and GSH-Px, SOD enzyme activities between Group 1a, Group 1b and Group 2Total nitric oxide levels and GSH-Px levels were significantly different between the three groups. Comparison analysis between groups revealed that although post-treatment NO levels decreased with pharmacological therapy, they were still significantly higher than the controls and Group 1b. However, decreased post-treatment levels of NO in Group 1b were similar to the controls. Similarly, in Group 1b, increased post-treatment GSH-Px activities were even higher than those of the controls (Table 3).
After eight weeks treatment, comparison of post treatment MDA, NO levels and GSH-Px, SOD enzyme activities between Groups 1a, 1b and controls.
| Group 1a Post treatment n=15 | Group 1b Post treatment n=15 | Group 2 Controls n=13 | p | |
| MDA (nmol/mL) | 2.10±0.59 | 2.17±0.47 | 2.46±0.58 | NS |
| NO (μmol/L) | 11.23±1.15 | 9.09±1.96 | 9.40±1.39 | 0.001(Group 1a>1b and 2) |
| GSH-Px (IU/L) | 128.00±34.88 | 242.06±81.94 | 193.38±37.40 | 0.001(Group 1a<1b and 2) |
| SOD (U/mL) | 17.98±7.26 | 13.03±5.54 | 13.28±5.47 | NS |
MDA: malondialdehyde; NO: total nitric oxide; GSH-Px: glutathione peroxidise; SOD: superoxide dismutase; NS: not significant.
Asthma is one of the most common chronic inflammatory diseases of childhood. It has been demonstrated in adult asthmatic patients that an exercise programme improves FEV1 levels, although the mechanism has yet to be elucidated. The current study was designed to investigate the possible beneficial effect of physical exercise on the antioxidant status in asthmatic children which may lead to amelioration of lung function. At the beginning of the study, asthmatic patients demonstrated significantly higher ROS levels (MDA and NO) and lower antioxidant enzyme (SOD and GSH-Px) activity compared to the controls.
The pharmacological therapy with/without an exercise programme for 8 weeks decreased MDA and NO levels. However, addition of the exercise programme to pharmacological therapy produced better NO values, as the post-treatment values in this group were indistinguishable from the controls. Both treatment protocols increased SOD activity so that levels were similar to controls; however no change was observed in post-treatment GSHPx activity in the group receiving pharmacological monotherapy. As in NO levels, exercise improved GSH-Px activities in Group 1b even compared to the controls, when applied with pharmacological therapy (Table 3). Significant improvements in lung function were obtained with the addition of an exercise programme to the standard monotherapy.
The pathogenesis of chronic obstructive lung diseases like asthma involves both airway inflammation and an oxidant/antioxidant imbalance.20 Many observations suggest that levels of oxidative stress are increased in children and in adults with asthma, not only in the lungs but also in the blood compartments.1 Several studies published have put forward the idea that when the oxidant system increases, there is a depletion of consumable antioxidants resulting in decreased antioxidant systems.21 Our pre-treatment results of higher ROS levels and lower antioxidant enzyme activity in the asthmatic groups are in concordance with these previous data.
Inhaled corticosteroids are the standard care in asthma and they successfully inhibit airway inflammation.22 They may have direct effects on oxidative stress by decreasing the number and/or activity of cells involved in ROS production.23,24 Secondly, they may act on the antioxidant enzyme activity by acting on transcription and/or through post-translational modifications, however reports of them being capable of elevating the antioxidant enzyme activity are not unanimous.22,25,26 Our results support the steroid effect on decreasing ROS production demonstrated by lower MDA and NO levels in post-treatment samples in both groups. Although a significant increase was observed regarding SOD activity in both groups, steroid monotherapy did not seem to alter GSH-Px activity. We believe this difference might be due to different capacities of antioxidant defence in individuals, which are in part genetically determined.
Regular physical activity is known to increase the efficiency of the antioxidant defence system and thus to reduce oxidative stress.27 These effects are reported to be associated with the amount of physical activity.28 There is a general agreement that low levels of exercise causes less free radical production, whereas strenuous exercise increases oxidative stress and may impair the antioxidant defence.29 Structured exercise programmes not only regulate antioxidant enzyme activity, but also increase oxidative capacity.30 Therefore, in our asthmatic group 1b, the physical activity was structured according to each individual's heart rate. Reports on the SOD and GSH-PX activities related to exercise are contradictory.31,32 When the literature is reviewed, the main causes of these conflicting results seem to be different samplings and analytical methods. Our results suggest that exercise significantly improves oxidant capacity, which is shown by increases in enzyme activity, which in turn leads to diminished ROS levels, shown by decreases in MDA and NO. The beneficial effects of exercise on lung functions were not observed in the group receiving the steroid monotherapy alone.
In conclusion, our study results reveal that the use of constructed exercise programmes in asthmatic children ameliorates lung function. The underlying mechanism may be partially the consequence of an increase in oxidant capacity, resulting in a decrease in oxidative burden, together strengthening the anti-inflammatory effects of steroids.
Conflict of interestThe authors have no conflict of interest to declare.