During recent years some exotic animals have been introduced as laboratory animals1 or pets in domestic environments, increasing the risk of exposure to many unknown potential allergens which could cause respiratory allergy symptoms in the owners.2
In the case of hamster, there are various species with the same generic name but belonging to different rodent genus coming from different regions of the world without evidence of a clear cross reactivity among their allergens.2–4
Now in Spain it is possible to find different types of hamsters as pets, the most common is the golden or Syrian hamster (Mesocricetus auratus), there are dwarfs hamsters: Chinese hamster (Cricetus griseus), Siberian or Russian hamster (Phodopus sungourus), Roborowski (Phodopus roborowskii), and apparently the cross reactivity found among their epithelium allergens is very low.
We present three cases with different sensitisations:
First case: A 41-year-old woman with well-controlled pollinic asthma who began to suffer from daily asthmatic episodes and bad response to treatment with inhaled corticosteroids and B2, after buying a Russian hamster (Phodopus sungorus) (RH) for her child.
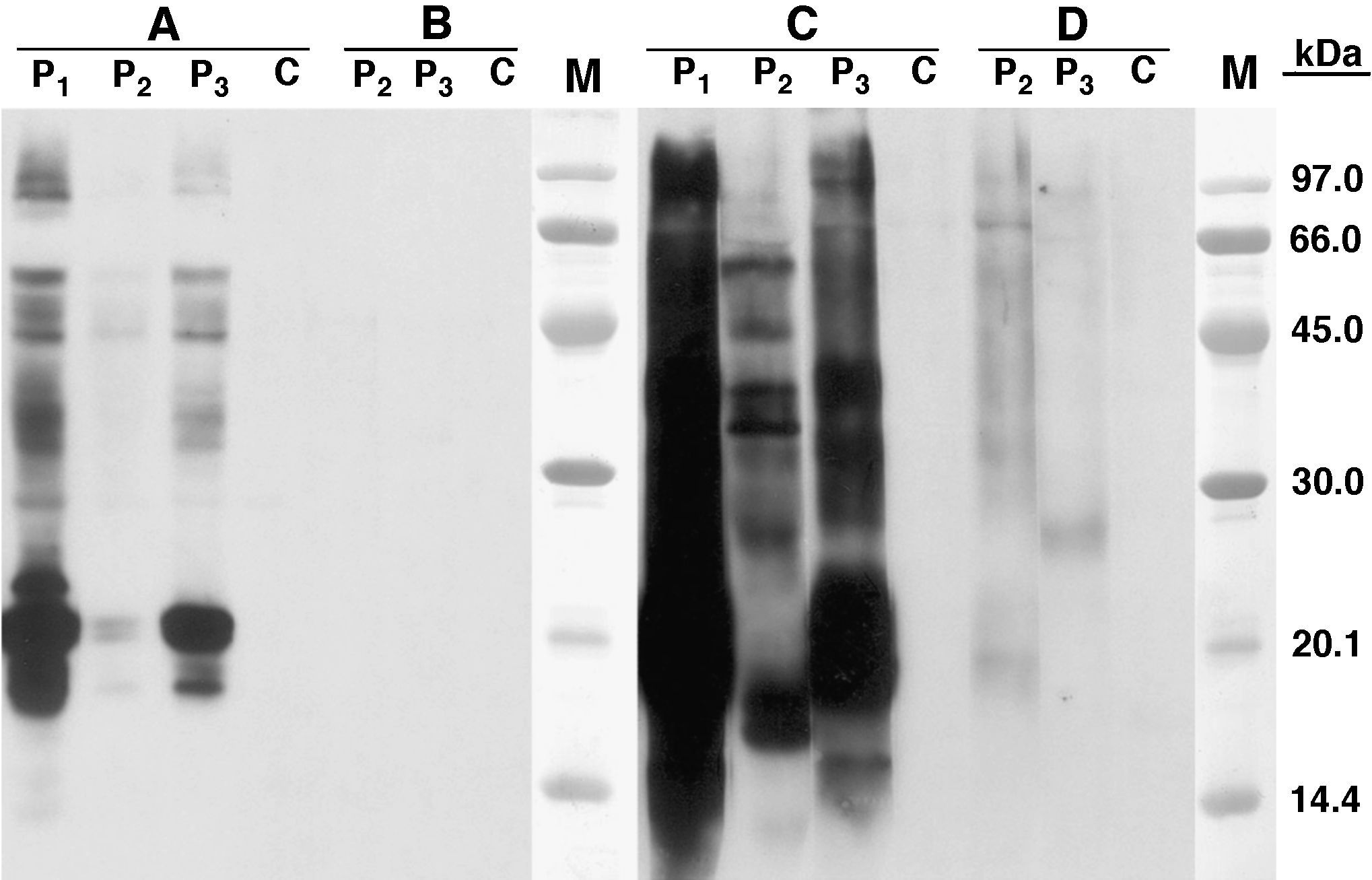
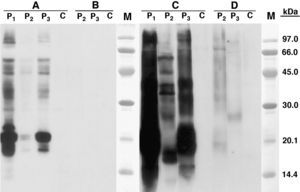
Skin prick test (SPT) with extract from RH epithelium was positive (8x7mm), however it was negative against Syrian hamster (SH) epithelium. Histamine control: 4 x 5mm. Serum specific IgE level was very high against RH epithelium 90.1kU/L and urine 86.3 kU/L, and very low against SH allergenic sources (epithelium: 0.7 kU/L; urine: 0.5 kU/L). SDS-PAGE-Immunoblotting showed an intense IgE binding band of ca. 21 kDa in RH epithelium extract and a high IgE binding area of ca. 18 - 21 kDa in RH urine extract. Some other high molecular mass IgE binding bands were observed in both extracts. No bands were revealed in extract from SH epithelium and very faint ones in SH urine (Fig. 1)
SDS -PAGE Immunoblotting results. A) Epithelium from Russian hamster B) Epithelium from Syrian hamster C) Urine from Russian Hamster D) Urine from Syrian Hamster. Lane P1: Patient 1 serum; lane P2: Patient 2 serum; lane P3: Patient 3 serum; lane C: Control serum (pool of serum from non-atopic subjects); Lane M: Molecular mass marker.
Second case: An 18–year-old woman who suffered from asthma with sensitisation to grass pollen, and horse and cat epithelium, she started with perennial asthma after buying a RH as a pet. Skin prick test with extract from RH epithelium gave a positive result (5x5mm), with negative against SH epithelium. Histamine control: 4 x 5mm.
Serum specific IgE level was positive against RH epithelium: 1.8 kU/L and urine: 1.7 kU/L, and very low against SH allergenic sources (epithelium: 1.2 kU/L, urine: 0.5 kU/L)
SDS-PAGE-Immunoblotting showed IgE binding band of ca. 21 kDa in RH epithelium extract, and 17.5 - 16 kDa in RH urine extract. (Fig. 1)
Third case: A 40-year-old woman with asthma with sensitisation to grass and olive pollen who developed perennial asthma when introducing a new pet (RH) to home. Skin prick test with RH epithelium was positive (6 x 5mm) and negative for SH epithelium. Histamine control (4 x 4mm)
SDS-PAGE-Immunoblotting showed IgE binding band of ca. 21 kDa in RH epithelium extract, and 18-21 kDa in RH urine extract. (Fig. 1)
Patients’ symptoms improved after the hamsters were removed from their house and now they are well controlled using treatment only for spring symptoms, all of them improved the spirometric values (FEV1 and the FEV1%FVC), and the asthma was controlled only with the animal removal.
In the last years two main allergens have been described in rat (Rattus norvegicus): Rat n 1A (20-21 kDa) and Rat n 1B (16-17 kDa), as well as in mouse (Mus musculus) Mus m 1 (19 kDa), Mus m 2 (16 kDa), all of them are lipocalins.
There are reports on allergy to Syrian Hamster (Mesocricetus auratus) in patients who work in laboratories with animals, and in pet owners. All these publications described an allergen between 15 to 21 kDa, a range of size similar to that of the lipocalins, however the identity of these hamster allergens has not been assessed.
Torres JA et al. described the presence of several Russian hamster (Phodopus sungoris) allergens with molecular mass between 18 – 23 kDa in various allergenic sources from RH (epithelium, faeces and urine).
There is a case report of anaphylaxis after hamster bites (Lim et al. and Nitsuma et al.)5,6 where a specific IgE-binding component of 21kD was detected in the hamster saliva. ELISA inhibition tests showed partial cross-reactivity with Dermatophagoides pteronyssinus extract.
Although the protein sequences of the Russian hamster (Phodopus sungoris) allergens have not been assessed, the molecular masses of them, 18- 23 kDa,3–5 and the general knowledge about the identity of rodent allergens let us suppose they must be lipocalins.
In conclusion it is important to take into account the presence of a pet in the daily environment of a patient with asthma, and if the animal is a hamster and we want to carry out a reliable prick test assay, we should know the hamster species as the lack of allergen cross reactivity between the allergens from different hamster species could give us an erroneous result.