Eosinophils in the cerebrospinal fluid (CSF) have been observed in a variety of diseases involving the central nervous system.1,2 We describe a patient who underwent various surgical procedures for ventriculoperitoneal shunt (VPS) revision and developed latex allergy. Total and specific IgE levels to latex and its recombinant fractions were quantified in serum and CSF to determine the sensitisation profile to latex.
RPBP, a two-year-old boy, was born by normal delivery at a gestational age of 31 weeks, weighing 1800g. At five days old he underwent a colostomy due to a necrotising enterocolitis, and at 10 days old he developed intraventricular bleeding and hydrocephalus, which were treated with VPS. Owing to necrotising enterocolitis and malfunction of the VPS device, he underwent five operations in his first month of life. By two years of age he had undergone a total of 11 operations. During hospitalisation due to VPS malfunction at 12 months of age, eosinophilia in CSF (19%) was observed [CSF: 21cells/mm3 (eosinophils=4cells/mm3, monocytes=4cells/mm3, lymphocytes 10cells/mm3 and neutrophils=3cells/mm3), protein=49mg/ml, glucose=58mg/ml and no bacterial growth]. Computerised tomography (CT) showed ventricular dilatation, and the VPS was revised. A new evaluation after 15 days showed a higher number of eosinophils (47%) [CSF: 49cells/mm3 (eosinophils=23cells/mm3, monocytes=1cell/mm3, lymphocytes=21cells/mm3, neutrophils=4cells/mm3), protein=252mg/ml, glucose=39mg/ml], and no microbial growth in the CSF and normal CT. At that time there was no eosinophilia in the peripheral blood (2%) and the patient did not develop any symptoms when in contact with latex products. Serum and CSF samples were obtained and total and specific IgE to latex and its recombinant fractions were determined (Immunocap®, Thermo Fisher) (Table 1). Within three months the patient developed daily nasal itching and hives when in contact with latex products, with increased eosinophils in peripheral blood (median of 7% – range 2–13%) as well as persistent eosinophilia in the CSF (median of 9% – range 1–25%). The VPS used in this patient was latex-free and without antibiotic impregnated but latex-containing products were used in the procedures until the appearance of clinical evidence of allergy to latex. Throughout the follow-up, 39 CSF cultures were obtained and only four showed growth of a pathogen (Staphylococcus aureus, Acinetobacter baumannii, Enterococcus sp. and Escherichia coli ESBL). The patient was promptly treated with antibiotics. After the diagnosis of latex allergy, the patient was advised to avoid latex and all procedures were performed without latex. After 12 months, no malfunction of the VPS device has been documented.
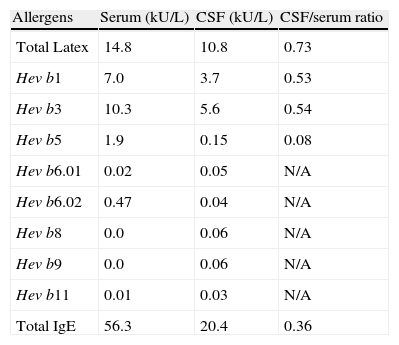
Level of total and specific IgE to latex and recombinants fractions.
| Allergens | Serum (kU/L) | CSF (kU/L) | CSF/serum ratio |
| Total Latex | 14.8 | 10.8 | 0.73 |
| Hev b1 | 7.0 | 3.7 | 0.53 |
| Hev b3 | 10.3 | 5.6 | 0.54 |
| Hev b5 | 1.9 | 0.15 | 0.08 |
| Hev b6.01 | 0.02 | 0.05 | N/A |
| Hev b6.02 | 0.47 | 0.04 | N/A |
| Hev b8 | 0.0 | 0.06 | N/A |
| Hev b9 | 0.0 | 0.06 | N/A |
| Hev b11 | 0.01 | 0.03 | N/A |
| Total IgE | 56.3 | 20.4 | 0.36 |
Hev b, Hevea brasiliensis; N/A: not applicable. Ratios cannot be calculated using values <0.1kU/L.
Eosinophilic meningitis is defined as the presence of 10 or more eosinophils/μL or levels of at least 10% in the CSF.1 This condition has several potential aetiologies, including helminthic invasion (mainly Angiostrongylus cantonensis) of the central nervous system; other parasitic infections (cerebral or spinal cysticercosis, toxocariasis, schistosomiasis); fungal, viral, and bacterial infections (Staphylococcus epidermidis [50%]); tumours (Hodgkin's lymphoma, non-Hodgkin's lymphoma, eosinophilic leukaemia); oral medication (ibuprofen, ciprofloxacin); intrathecal drugs (vancomycin, gentamicin); contrast myelography; ethylene oxide (used for sterilising VPS material); sarcoidosis; and latex allergy.2
Eosinophils are known to play an important role in tissue inflammation as a result of the release of mediators present in their cytoplasmic granules and the production of chemotactic cytokines.3 CSF eosinophils may play an important role in the obstruction of the VPS. This is partly supported by the detection of increased numbers of eosinophils in the CSF of uninfected patients with poor VPS function, compared with infected patients with a functional VPS device, and also by the fact that levels of eosinophil cationic protein (ECP) are up to 14 times higher than in patients without eosinophilia in the CSF.4
In our patient, the total IgE serum to CSF ratio was lower than 1, which reflects probable diffusion of IgE to the CSF. The same was observed when evaluating the ratio of the levels of specific IgE for total latex and recombinants Hev b 1 and 3. The presence of plasmocytes (median of 4%) in 8 of 39 samples of CSF possibly suggests local production of IgE.5
According to Niggemann et al.,5 the presence of specific IgE in the CSF might be due to local production and not to simple transudation through the blood–brain barrier. The presence of similar latex-specific IgE levels in the CSF and serum and very low levels of total IgE in the CSF in that study raised the suspicion that IgE might be produced locally.3 This was reinforced by the fact that latex-specific IgE accounted for almost all the IgE present in the CSF.5
In our study, total and specific IgE to latex and its recombinant allergens showed basically the same distribution in serum and the CSF. The lack of similar reports make it difficult to discuss in more detail the profile of the recombinant latex fractions found in the samples. However, it is well known that IgE to Hev b 1 and Hev b 3 is frequently found in the serum of patients who have undergone multiple operations.6 Although the VPS devices used in our patient were latex-free, the manipulation of the peritoneum and the meninges with latex gloves, the high number of surgical interventions, and the very presence of the VPS may explain the latex allergy.7 We think it unlikely that the dysfunctions of VPS have been secondary to the material used in valve or ethylene oxide used in the sterilisation of the valve because after the patient avoided the latex, the VPS dysfunction did not occur. Unfortunately we could not evaluate the VPS as the presence of fibroblasts as Niggemann et al. did5 and the mechanism by which the latex allergy influences the dysfunction of VPS remains unclear.
We believe that our patient became sensitised to latex during the various surgical procedures performed, leading to the production of serum IgE and subsequent transudation into the CSF. Moreover, it seems that the impaired functioning of the VPS device was not initially caused by latex allergy, but that latex allergy may actually have contributed to a greater or lesser degree of malfunction.
Further studies are needed to determine the profile of sensitisation to latex and its allergens in patients with eosinophilic meningitis as well as to determine whether IgE against latex is produced locally (CSF) or in the serum.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Sources of fundingNone of the authors have relationships to declare.