Asthma, persistent rhinosinusitis, and/or nasal polyposis accompanying non-steroidal anti-inflammatory drug (NSAID) intolerance is defined as aspirin-exacerbated respiratory disease (AERD). Although the literature includes considerable data on comorbidities in asthma, data on comorbidities in AERD have not been previously published.
ObjectiveThis study aimed to determine the prevalence of comorbidities in AERD and compare the findings to those in asthmatic patients.
Materials and methodsThe records for 330 AERD patients that presented to our allergy clinic were reviewed. Patients with urticaria/angio-oedema type reactions to NSAIDs were included in the pseudo Samter's group (n=83) and 338 randomly selected NSAID-tolerant asthma patients constituted the control group.
ResultsGender, age at presentation, age at onset of asthma, and follow-up periods were similar in all groups. Hypertension (P=0.035), diabetes mellitus (P=0.323), gastro-oesophageal reflux (P<0.001), psychological disorders (P=0.099), obesity (P=0.003), and hyperlipidaemia (P=0.002) were significantly more prevalent in the asthma group. Interestingly, coronary artery disease (CAD) and congestive heart failure (CHF) were more common in the AERD group (P=0.178); CAD/CHF was associated with AERD (OR: 4.5; 95% CI: 1.206–16.93).
ConclusionAERD and asthma are associated with several comorbidities. Even though systemic steroid dependency and severe asthma were significantly more common in the AERD group, comorbidities occurred more frequently in the asthma group. Additional longitudinal studies are needed to more clearly discern if the risk of CAD/CHF is increased in AERD.
Aspirin-exacerbated respiratory disease (AERD) is characterised by asthma, persistent rhinitis/sinusitis, and/or nasal polyposis accompanying non-steroidal anti-inflammatory drug (NSAID) intolerance. AERD affects 0.3–0.9% of the general population, but its prevalence rises to 10–20% in asthma patients and up to 30–40% in those asthmatics with nasal polyposis.1 In AERD patients, localised inflammation in the upper respiratory tract is more prevalent and aggressive; therefore, relapse and treatment failure is common in patients with nasal polyposis and rhinosinusitis.2 Asthma is often associated with various comorbidities, but the prevalence of comorbidity varies between studies.
Comorbidity is an important factor in asthma patients, as it can negatively affect disease management and control. Rhinitis, sinusitis, gastro-oesophageal reflux disease (GERD), obstructive sleep apnoea (OSA), hormonal disorders, and psychopathologies are frequently observed in asthmatic patients.3 Analysis of large databases has shown a high prevalence of various conditions in asthma patients that do and do not affect asthma treatment outcomes.4,22 The majority of asthma patients also have allergic rhinitis, which is often undiagnosed and untreated. Patients with allergic rhinitis have a high risk of developing asthma, but allergic rhinitis also impairs asthma control, and increases symptoms and disease severity. In Soriano et al.’s data-based study the most prevalent condition in adult asthmatic patients was time-limited minor infections, whereas other conditions that were highly prevalent included depression, hypertension, diabetes mellitus (DM), ischaemic heart disease, degenerative joint disease, cardiac arrhythmia, cancer, congestive heart failure, cerebrovascular disease, and chronic obstructive pulmonary disease (COPD).5 Asthma with comorbidity results in elevated healthcare system usage and costs, and decreased quality of life and poor asthma control.
An earlier study conducted at our centre that investigated the relationship between NSAID hypersensitivity, and chronic urticaria, rhinitis/rhinosinusitis, and asthma, and identified the NSAID reaction patterns in asthmatics6 reported that AERD patients are a heterogeneous group, and proposed a new classification system (Kalyoncu classification). According to Kalyoncu classification, NSAID-induced urticaria/angio-oedema in asthma patients is defined as pseudo Samter's syndrome. The clinical characteristics of this group are unique and additional research is required to delineate the behaviour and natural course of pseudo Samter's syndrome.
Although the literature contains a great deal of data on comorbidities in asthma, comorbidities in AERD have yet to be evaluated. As such, the present study aimed to determine the prevalence of comorbidities in AERD patients and compare them with those in asthma patients, and to investigate their associations in pseudo Samter's patients.
Materials and methodsThe study included 414 consecutive AERD patients that presented to the allergy clinic between January 1991 and December 2011. Among the 414 patients, we were able to obtain detailed records concerning comorbidities for 330 (79.7%). The patients were divided into two groups: AERD and pseudo Samter's. The pseudo Samter's group included asthma patients with NSAID-induced urticaria/angio-oedema (n=83, 25.1%) and the AERD group included asthma patients with NSAID-induced anaphylaxis, rhinitis/asthma, or a combination of reactions.6 The control group included 338 randomly selected NSAID-tolerant asthma patients that presented during the same time period. Demographic and clinical data were obtained from patient files. History of rhinitis, smoking status, age at onset of asthma, NSAID intolerance, nasal polyps, polypectomies, oral corticosteroid dependency, aspirin desensitisation, asthma severity, additional atopic diseases, atopy, and skin prick test results at presentation were recorded. Physician-diagnosed comorbidities during the follow-up period were also recorded. Depression and panic attacks were recorded as psychological disorder. Although obesity was defined as a body mass index >30kgm–2, data were not recorded in the patient files, but obesity as a diagnosis was recorded by the examining physicians. Atopy was defined as being positive (≥3mm diameter) for one or more allergens in skin prick test. Skin prick testing included aeroallergens of Dermatophagoides pteronyssinus, Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, pollens (Phleum pratense, Artemisia vulgaris, Parietaria officinalis, Corylus avellana, Olea europeae, and Betula verrucosa), moulds (Aspergillus fumigatus, Cladosporium herbarum, and Alternaria alternata), animal dander (dog and cat), latex, and Blatella germanica.
Diagnoses of rhinitis and asthma were made by allergists in the allergy clinic based on international and national asthma/rhinitis guidelines (GINA, ARIA, and national guidelines), and diagnosis and surgical treatment of nasal polyps were performed by otorhinolaryngologists at our centre. Patients who were oral steroid dependent or had taken high dose inhaler steroids together with two or more controller medications for the previous year were defined as having severe asthma. A reliable clinical history of or a positive oral challenge with the tested NSAIDs was required for the diagnosis of NSAID intolerance. Asthma/rhinitis, urticaria/angio-oedema, or urticaria, and angio-oedema and/or anaphylaxis induced by a single NSAID or a group of closely chemically related compounds within 24h were defined as positive reaction history.7 The challenge response was considered positive if it fulfilled ≥1 of the following criteria: (1) a FEV1 decrease ≥20%; (2) sneezing, rhinorrhoea, nasal blockage, and oropharyngeal itching; (3) pruritic and erythematous areas over normal skin; (4) macular and/or popular areas in any localisation; (5) swelling of the skin and/or external mucosa; (6) systemic anaphylaxis.8 The study protocol was approved by the Hacettepe University School of Medicine Ethics Committee. Informed consent was provided by all the participants.
Statistical analysisStatistical analysis was performed using SPSS v.15.0 for Windows. Categorical variables are expressed as frequencies, versus mean±standard deviation for continuous variables. Chi-square analysis was used to test differences for nominal variables. The association between comorbidities, and AERD and pseudo Samter's syndrome groups was adjusted for age, gender, smoking status, and follow-up period in the logistic regression analysis models. The association between comorbid cardiovascular diseases, and metabolic conditions and diseases (obesity, hyperlipidaemia, and DM), and AERD and pseudo Samter's syndrome were adjusted for age, gender, smoking status, and relevant risk factors in multiple logistic regression analysis models. Odds ratios and 95% CIs were calculated (Table 3). The level of statistical significance was set at P<0.05.
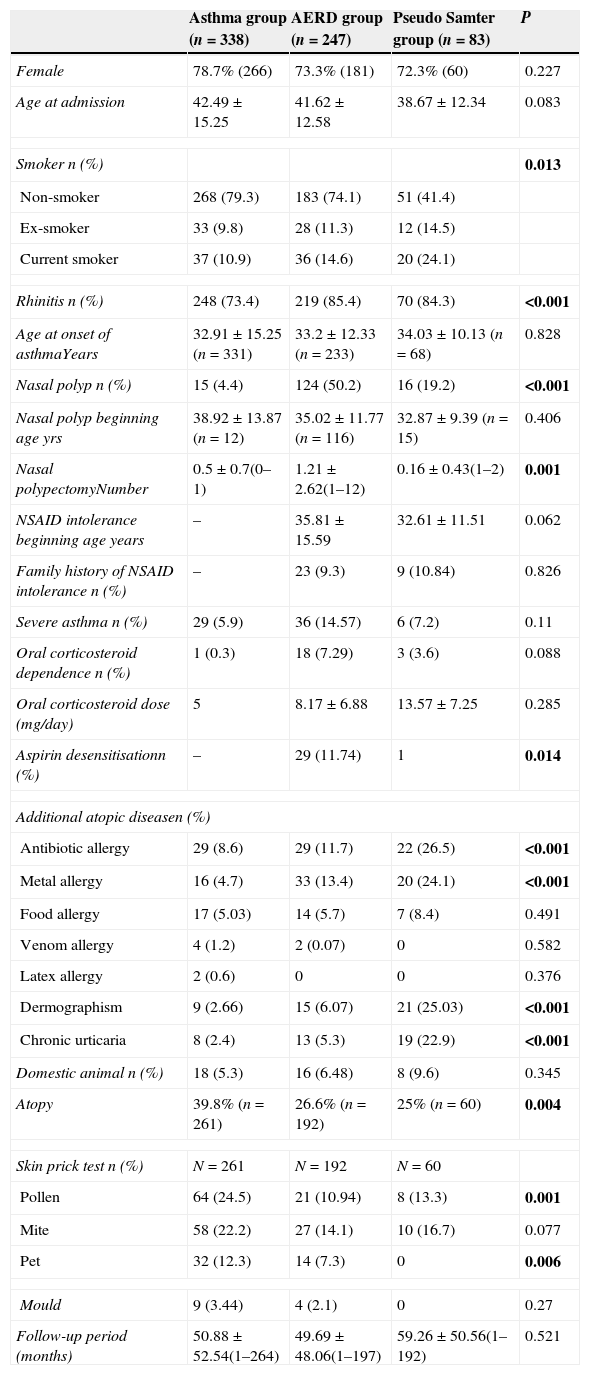
ResultsThe study included 668 patients that were followed-up at our allergy clinic (247 AERD patients, 83 pseudo Samter's patients, and 338 control asthma patients). Age, gender, age at onset of asthma, and follow-up periods did not differ significantly different between the groups (Table 1). Of note, most of the patients were female (78.7% in the control group, 73.3% in the AERD group, and 72.8% in the pseudo Samter's group). Most of the patients presented to the clinic more than once (63.9% in the control group, 64% in the AERD group, and 55.4% in the pseudo Samter's group). The occurrence of persistent rhinitis differed significantly between the groups (73.4% in the control group, 88.7% in the AERD group, and 84.3% in the pseudo Samter's group, P<0.001), as did the occurrence of nasal polyps (4.4% in the control group, 50.2% in the AERD group, and 19.2% in the pseudo Samter's group, P<0.001). Atopy was more prevalent in the control group (39.8%) than in the AERD group (26.6%) and pseudo Samter's group (25%) (P=0.004). Despite the low rates of pet ownership (5.3% in the control group, 6.48% in the AERD group, and 9.6% in the pseudo Samter's group), prick test positivity rates to animal dander were high (12.3% in the control group and 7.3% in the AERD group). Although the difference was not significant, severe asthma and oral corticosteroid dependency were more prevalent in the AERD group.
Demographic characteristics of study population.
| Asthma group (n=338) | AERD group (n=247) | Pseudo Samter group (n=83) | P | |
|---|---|---|---|---|
| Female | 78.7% (266) | 73.3% (181) | 72.3% (60) | 0.227 |
| Age at admission | 42.49±15.25 | 41.62±12.58 | 38.67±12.34 | 0.083 |
| Smoker n (%) | 0.013 | |||
| Non-smoker | 268 (79.3) | 183 (74.1) | 51 (41.4) | |
| Ex-smoker | 33 (9.8) | 28 (11.3) | 12 (14.5) | |
| Current smoker | 37 (10.9) | 36 (14.6) | 20 (24.1) | |
| Rhinitis n (%) | 248 (73.4) | 219 (85.4) | 70 (84.3) | <0.001 |
| Age at onset of asthmaYears | 32.91±15.25 (n=331) | 33.2±12.33 (n=233) | 34.03±10.13 (n=68) | 0.828 |
| Nasal polyp n (%) | 15 (4.4) | 124 (50.2) | 16 (19.2) | <0.001 |
| Nasal polyp beginning age yrs | 38.92±13.87 (n=12) | 35.02±11.77 (n=116) | 32.87±9.39 (n=15) | 0.406 |
| Nasal polypectomyNumber | 0.5±0.7(0–1) | 1.21±2.62(1–12) | 0.16±0.43(1–2) | 0.001 |
| NSAID intolerance beginning age years | – | 35.81±15.59 | 32.61±11.51 | 0.062 |
| Family history of NSAID intolerance n (%) | – | 23 (9.3) | 9 (10.84) | 0.826 |
| Severe asthma n (%) | 29 (5.9) | 36 (14.57) | 6 (7.2) | 0.11 |
| Oral corticosteroid dependence n (%) | 1 (0.3) | 18 (7.29) | 3 (3.6) | 0.088 |
| Oral corticosteroid dose (mg/day) | 5 | 8.17±6.88 | 13.57±7.25 | 0.285 |
| Aspirin desensitisationn (%) | – | 29 (11.74) | 1 | 0.014 |
| Additional atopic diseasen (%) | ||||
| Antibiotic allergy | 29 (8.6) | 29 (11.7) | 22 (26.5) | <0.001 |
| Metal allergy | 16 (4.7) | 33 (13.4) | 20 (24.1) | <0.001 |
| Food allergy | 17 (5.03) | 14 (5.7) | 7 (8.4) | 0.491 |
| Venom allergy | 4 (1.2) | 2 (0.07) | 0 | 0.582 |
| Latex allergy | 2 (0.6) | 0 | 0 | 0.376 |
| Dermographism | 9 (2.66) | 15 (6.07) | 21 (25.03) | <0.001 |
| Chronic urticaria | 8 (2.4) | 13 (5.3) | 19 (22.9) | <0.001 |
| Domestic animal n (%) | 18 (5.3) | 16 (6.48) | 8 (9.6) | 0.345 |
| Atopy | 39.8% (n=261) | 26.6% (n=192) | 25% (n=60) | 0.004 |
| Skin prick test n (%) | N=261 | N=192 | N=60 | |
| Pollen | 64 (24.5) | 21 (10.94) | 8 (13.3) | 0.001 |
| Mite | 58 (22.2) | 27 (14.1) | 10 (16.7) | 0.077 |
| Pet | 32 (12.3) | 14 (7.3) | 0 | 0.006 |
| Mould | 9 (3.44) | 4 (2.1) | 0 | 0.27 |
| Follow-up period (months) | 50.88±52.54(1–264) | 49.69±48.06(1–197) | 59.26±50.56(1–192) | 0.521 |
The statistical significance of bold values was set at P<0.05.
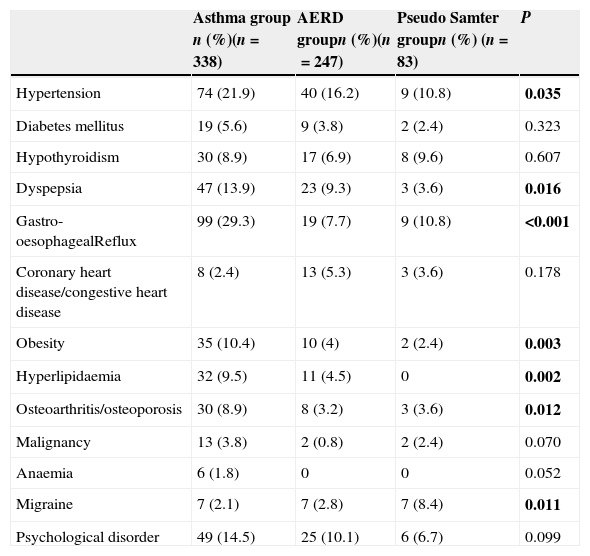
The prevalence of the following comorbidities differed significantly between the three groups: hypertension (21.9% in the control group, 16.2% in the AERD group, and 10.8% in the pseudo Samter's group, P=0.035); dyspepsia (13.9% in the control group, 9.3% in the AERD group, and 3.6% in the pseudo Samter's group, P=0.016); gastro-oesophageal reflux (29.3% in the control group, 7.7% in the AERD group, and 10.8% in the pseudo Samter's group, P<0.001); obesity (10.4% in the control group, 4% in the AERD group, and 2.4% in the pseudo Samter's group, P=0.003); hyperlipidaemia (9.5% in the control group, 4.5% in the AERD group, and 0% in the pseudo Samter's group, P=0.002); osteoarthritis/osteoporosis (8.9% in the control group, 3.2% in the AERD group, and 3.6% in the pseudo Samter's group, P=0.012). The frequency of DM, hypothyroidism, malignancy, anaemia, and psychological disorders did not differ significantly between the groups (Table 2).
Prevalence of comorbidities in study groups.
| Asthma group n (%)(n=338) | AERD groupn (%)(n=247) | Pseudo Samter groupn (%) (n=83) | P | |
|---|---|---|---|---|
| Hypertension | 74 (21.9) | 40 (16.2) | 9 (10.8) | 0.035 |
| Diabetes mellitus | 19 (5.6) | 9 (3.8) | 2 (2.4) | 0.323 |
| Hypothyroidism | 30 (8.9) | 17 (6.9) | 8 (9.6) | 0.607 |
| Dyspepsia | 47 (13.9) | 23 (9.3) | 3 (3.6) | 0.016 |
| Gastro-oesophagealReflux | 99 (29.3) | 19 (7.7) | 9 (10.8) | <0.001 |
| Coronary heart disease/congestive heart disease | 8 (2.4) | 13 (5.3) | 3 (3.6) | 0.178 |
| Obesity | 35 (10.4) | 10 (4) | 2 (2.4) | 0.003 |
| Hyperlipidaemia | 32 (9.5) | 11 (4.5) | 0 | 0.002 |
| Osteoarthritis/osteoporosis | 30 (8.9) | 8 (3.2) | 3 (3.6) | 0.012 |
| Malignancy | 13 (3.8) | 2 (0.8) | 2 (2.4) | 0.070 |
| Anaemia | 6 (1.8) | 0 | 0 | 0.052 |
| Migraine | 7 (2.1) | 7 (2.8) | 7 (8.4) | 0.011 |
| Psychological disorder | 49 (14.5) | 25 (10.1) | 6 (6.7) | 0.099 |
The statistical significance of bold values was set at P<0.05.
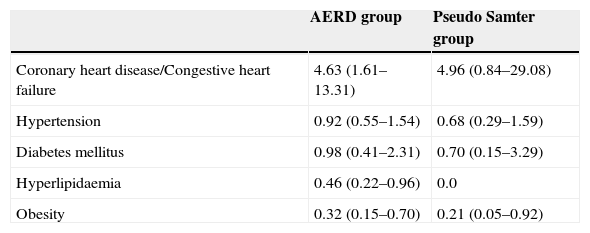
Multiple logistic regression analysis models were built to adjust for the associations between comorbidities, and AERD and pseudo Samter's syndrome using the asthma group selected as the reference group. After adjusting for age, sex, smoking, obesity, hyperlipidaemia, and hypertension, coronary heart disease/congestive heart failure was associated with AERD (OR=4.63; 95% CI: 1.61–13.31). Hypertension and DM were not associated with AERD or pseudo Samter's syndrome. Obesity was associated with AERD (OR=0.32; 95% CI: 0.15–0.70) and pseudo Samter's syndrome (OR=0.21; 95% CI: 0.05–0.92) after adjusting for age, sex, and oral corticosteroid dependence (Table 3).
Adjusted odds ratios and 95% confidence interval of comorbidities in AERD and Pseudo Samter groups.
| AERD group | Pseudo Samter group | |
|---|---|---|
| Coronary heart disease/Congestive heart failure | 4.63 (1.61–13.31) | 4.96 (0.84–29.08) |
| Hypertension | 0.92 (0.55–1.54) | 0.68 (0.29–1.59) |
| Diabetes mellitus | 0.98 (0.41–2.31) | 0.70 (0.15–3.29) |
| Hyperlipidaemia | 0.46 (0.22–0.96) | 0.0 |
| Obesity | 0.32 (0.15–0.70) | 0.21 (0.05–0.92) |
Asthma is the reference group.
Multiple logistic regression analysis models adjusted for: coronary heart disease/Congestive heart failure: age, sex, smoking, obesity, hyperlipidaemia and hypertension.
Hypertension: age, sex, smoking, obesity and hyperlipidaemia.
Diabetes mellitus: age, sex, obesity and oral corticosteroid dependence.
Hyperlipidaemia: age, sex and obesity.
Obesity: age, sex, and oral corticosteroid dependence.
According to the literature, rhinosinusitis, gastro-oesophageal reflux, infections, psychological disorders, and obstructive sleep apnoea are the most frequent comorbidities in asthma patients.3,4,22 These comorbidities can affect the diagnosis of asthma and its treatment. AERD is a well-defined phenotype of asthma, yet the literature does not include any data about comorbidities in this phenotype of asthma. To the best of our knowledge, the present study is the first to compare the frequency of comorbidities between asthma, AERD, and pseudo Samter's patients.
AERD is a clinical syndrome characterised by a sequence of symptoms: persistent rhinitis, followed by asthma, NSAID intolerance, and nasal polyps. Although some variation occurs, there is a close relationship between age and the order in which the symptoms appear.16,17 The predominance of AERD in females is well known.16,18,28 In the present study age at presentation, age at onset of asthma, the prevalence of nasal polyps, age at onset of nasal polyps, and age at onset of analgesic intolerance were similar to those reported earlier. In the present study's AERD and Pseudo Samter's groups, 9.7% of the patients had a family history of analgesic intolerance, which is higher than the 6% reported by Szczeklik et al.17 The atopy rate was higher in the control group, whereas other allergic conditions, such as antibiotic allergy, metal allergy, dermographism, and chronic urticaria were more prevalent in the pseudo Samter's group, as previously reported.18
The majority of patients with asthma report GERD symptoms and/or have an abnormal 24-h oesophageal pH test9,10; however, the effect of GERD on asthma remains unclear because improvement in asthma following GERD treatment is variable. Field et al. reported that anti-reflux surgery helped alleviate the symptoms of asthma without changing pulmonary function. Another study reported improvement in asthma-related quality of life and reduced asthma exacerbation in moderate to severe persistent asthma patients treated with lansoprazole for 24 weeks; however, the treatment did not improve asthma control of pulmonary function, and did not lower the rescue medication use rate. As such, the effects of GERD on asthma might vary by patient. In the National Heart, Lung, and Blood Institute Cohort more severe asthma patients with aspirin intolerance had GERD than non-severe asthma patients (41% versus 12–16%).25 In the present study the prevalence of GERD was 29.3%, 10.8%, and 7.7% in the control, pseudo-Samter's, and AERD groups, respectively. Even though the frequency of severe asthma was high and some of the patients were aspirin desensitised, the prevalence of GERD was low in AERD group.
Psychiatric disorders, such as anxiety, depression, and panic disorder, occur more frequently in asthma patients. Psychiatric disorders can trigger asthma symptoms and can affect patients’ perceptions of those symptoms and medication compliance; therefore, they should be detected and treated.11 The prevalence of depression and panic disorder in the present study ranged between 6.7% and 14.5%, but did not differ significantly between the groups.
Obesity is increasing worldwide and it has become apparent that obesity is a risk factor for specific asthma phenotype (low lung volume, less eosinophilic inflammatory process, low-grade systemic inflammation, reduced response to corticosteroids).23 Mosen et al.24 reported that obese adults were more likely to have poor asthma-specific quality of life, poor asthma control, and a higher number of asthma-related hospitalisations. Comorbidities associated with obesity, such as dyslipidaemia, GERD, OSA, type 2 DM, and hypertension, can cause asthma or exacerbate it. In the present study the prevalence of obesity was 10.4% in the control group and 4% in the AERD group, but the prevalence of obesity may have actually been higher because the body mass index was not recorded, which is a limitation of the study.
Despite a body of evidence, the importance of asthma as a risk factor for atherosclerotic vascular disease remains unclear. Studies have linked potent inflammatory mediators (leukotrienes) and genes that regulate them to both asthma and atherosclerosis. Other studies have suggested that asthma itself may be a risk factor for coronary heart disease and stroke, particularly in females with adult onset asthma.12–15 A large population-based retrospective study reported that asthma was weakly associated with cardiovascular and hypertensive diseases, depression, DM, dyslipidaemia, and osteoporosis,3 and that the prevalence of hypertension, DM, dyslipidaemia, and osteoporosis were 28.3%, 8.4%, 4.2%, and 10.3%, respectively in the asthma group, which are higher prevalences than observed in the present study. Even though hypertension and DM were not associated to AERD or pseudo Samter's syndrome, coronary heart disease/congestive heart failure was associated with AERD.
Yigla et al.19 conducted a prospective cohort study that included 20 patients with severe unstable asthma and observed an unexpectedly high prevalence of OSA in those that received long-term chronic or frequent bursts of oral corticosteroid therapy. OSA is associated with both upper and systemic airway inflammation.20,21 Pharyngeal inflammation in OSA may promote upper airway collapse, while systemic inflammation may increase cardiovascular morbidity. A major limitation of the present study is that body mass index and OSA status were not recorded. AERD patients with aggressive inflammation in the upper airway and severe asthma patients with OSA must be evaluated carefully. Nasal polyps and persistent rhinitis, which cause severe nasal congestion, should immediately be treated medically or surgically, and opening of nasal passages should be provided.
Earlier studies have suggested a link between asthma and severe headache, and between migraine and wheezing.26 Analysis of the British Birth Cohort showed that the incidence of asthma was higher in those with a history of migraine at follow-ups. This association was observed only in those without a history of hay fever, allergic rhinitis, or eczema (non-atopic patients).27 In the present study migraine was more prevalent in the pseudo Samter's group, but a causal link was not determined.
The present study has some limitations. This retrospective study obtained data from the patients’ medical records. Incomplete documentation and variation in the quality of data recorded are limitations of any retrospective chart review. Our clinic is a tertiary care centre and as such there may have been a bias towards severe patients. The most important limitation, though, is that the body mass index, obesity, and OSA status were not recorded, as mentioned previously. Coronary heart disease/congestive heart failure was more common in the AERD group and after adjusting for age, gender, smoking status, obesity, hypertension, and hyperlipidaemia remained associated with AERD (OR=4.63; 95% CI: 1.61–13.31); The range of the 95% CI in the present study is such that it decreases the power of the study. Additionally, treatment modalities were not examined, which could be factors that affect the prognosis of asthma.
In conclusion, numerous comorbidities (rhinitis, obesity, GERD, OSA, migraine, and psychiatric disorders) are frequently associated with asthma, and may negatively affect the clinical expression and severity of the disease. Asthma is a heterogeneous disease with many recognised phenotypes. In AERD patients, localised inflammation in the upper respiratory tract is more prevalent and aggressive, and may have differential effects on systemic inflammation and OSA. Even though the prevalence of metabolic syndrome components (DM, hypertension, and hyperlipidaemia) was not higher in AERD patients, coronary heart disease/congestive heart failure was associated with AERD. In order to more clearly understand the common underlying mechanism of severe asthma and coronary heart disease/congestive heart failure, large prospective studies that include use of polysomnography are needed.
Conflict of interestThe authors declare no conflicts of interest relevant to this study. No financial support for the research was received. Thanks for English editing to Scott Evans.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.