Allergen-specific immunotherapy (SIT) is a long-term treatment of respiratory allergy.
ObjectiveTo look for early predictors of the effectiveness of Dermatophagoides pteronyssinus SIT.
MethodsA prospective multi-centre study was carried out in Spain. Children with D. pteronyssinus rhinitis or asthma were invited to participate. The study was divided into times: T0 (recruitment); T1 (inclusion); T2 a-f (immunotherapy times) and T3 (the end of study). Efficacy of SIT was assessed by clinical scores, visual analogue scales (VAS) and lung function tests. We performed D. pteronyssinus skin tests at T1 and T3, and determined specific serum IgE, IgG4 and IL-10 at T1, T2f and T3.
Data were analysed using Mann–Whitney and Kruskal–Wallis tests, compared using Wilcoxon and Chi-square tests, and correlated to Spearman test. All tests had a significance level of 0.05.
ResultsThirty-eight children completed the study. At T1 all had rhinitis and 34 also had asthma. At T3, 30 patients had improved, six experienced no changes and two worsened. Improvement was associated to FEV1/FVC and VAS improvement; to a reduction in D. pteronyssinus skin prick test; to a progressive increase in serum levels of D. pteronyssinus IgE, and D. pteronyssinus, Der p1 and Der p2 IgG4. IL-10 levels showed an early increase at T2f (the end of initial build-up immunotherapy phase), and then a reduction at T3 (the end of a year of immunotherapy).Improvement associated to an early increase in IL-10 and was correlated with VAS and specific IgG4 evolution.
For a century, allergen-specific immunotherapy (SIT) has been used as a desensitising therapy for allergic diseases, and is currently the only curative and specific approach to treatment.1 Allergen immunotherapy for treatment of respiratory allergy due to house dust mite allergy has also been demonstrated to be effective.2 Research on the mechanisms of immune regulation in allergy and asthma on the area of SIT has provided substantial understanding of the therapeutic and preventive mechanisms. Sherman et al.3 demonstrated in 1940 that the amount of skin-sensitising antibodies increased during the first few months of immunotherapy and subsequently decreased. Afterwards, Norman et al.4 with the ability to measure specific IgE, confirmed those findings. In addition, an allergen-specific IgG response has been demonstrated, which is restricted to IgG1 and IgG4 subclasses.5 Initially, it was thought that the role of the IgG antibodies was to block mast cell and basophile activation by competing with IgE for allergens. Recently, Wachholz et al.6 showed the possible role of IgG4 antibodies in inhibiting CD23-mediated IgE-facilitated allergen presentation to T cells. However, specific IgG4 levels do not always correlate with clinical efficacy of treatment.7,8 SIT induces an allergen-tolerant state by altering allergen-specific memory, and T and B cell responses to allergens, with the participation of Treg cells and IL-10.9
We investigated possible prognostic variables of clinical efficacy in children with respiratory allergy to house mites who were treated with SIT injections for one year. We recorded skin prick tests, rhinitis and asthma symptoms and serological responses to Dermatophagoides pteronyssinus and the relationship of IL-10 to these processes.
Material and methodsStudy designA multi-centre study was conducted in hospital allergy departments in Spain. The study was approved by ethics and research committees. Children with a diagnosis of respiratory allergy to D. pteronyssinus, rhinitis (ARIA)10 and/or asthma (Consensus Statement on the Management of Pediatric Asthma,11 were invited to participate. Informed consent was obtained from the children and their parents.
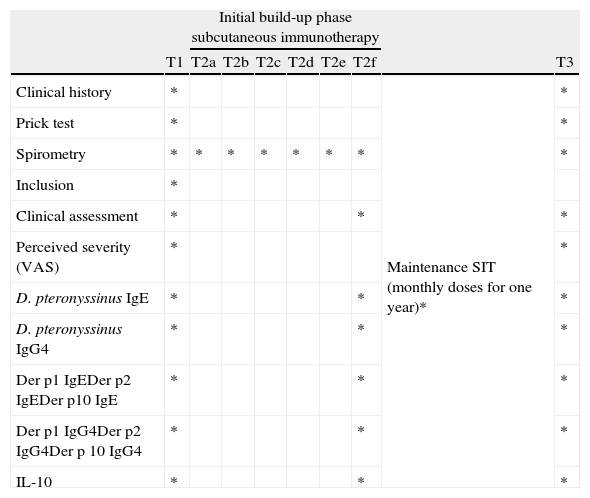
The study was divided into four different times: T0 (diagnosis and patients recruitment); T1 (inclusion time); T2 a–f (cluster initiation immunotherapy phase); T2f–T3 (maintenance immunotherapy phase, lasting one year); T3 (end of the study). We recorded the evolution of several variables at different times of the study (Table 1).
Study schedule. Times and variables determined at each time. T1 or inclusion period, T2 a–f, initial and maintenance immunotherapy phases, and T3 or end of study.
| Initial build-up phase subcutaneous immunotherapy | |||||||||
| T1 | T2a | T2b | T2c | T2d | T2e | T2f | T3 | ||
| Clinical history | * | Maintenance SIT (monthly doses for one year)* | * | ||||||
| Prick test | * | * | |||||||
| Spirometry | * | * | * | * | * | * | * | * | |
| Inclusion | * | ||||||||
| Clinical assessment | * | * | * | ||||||
| Perceived severity (VAS) | * | * | |||||||
| D. pteronyssinus IgE | * | * | * | ||||||
| D. pteronyssinus IgG4 | * | * | * | ||||||
| Der p1 IgEDer p2 IgEDer p10 IgE | * | * | * | ||||||
| Der p1 IgG4Der p2 IgG4Der p 10 IgG4 | * | * | * | ||||||
| IL-10 | * | * | * | ||||||
Inclusion criteria for patients were: age 5–14 years, with perennial symptoms of respiratory allergy for more than two years, positive skin prick test (wheal greater than 3mm×3mm) and specific serum IgE greater than 3.5kUA/mL to D. pteronyssinus. Unstable asthma, sensitisation to any other perennial environmental allergens, inclusion in any other clinical study in the last two months, previous SIT or any medical or therapeutic condition that contraindicated SIT were exclusion criteria. After diagnosis, inclusion and exclusion criteria were reviewed and signed consent was obtained during T1.
Allergen extractA biologically standardised D. pteronyssinus extract (DIATER Laboratories, Madrid, Spain) with a biological activity of 5HEP/mL (4μg Der p1+Der p2/0.8mL) was used for diagnosis and treatment.
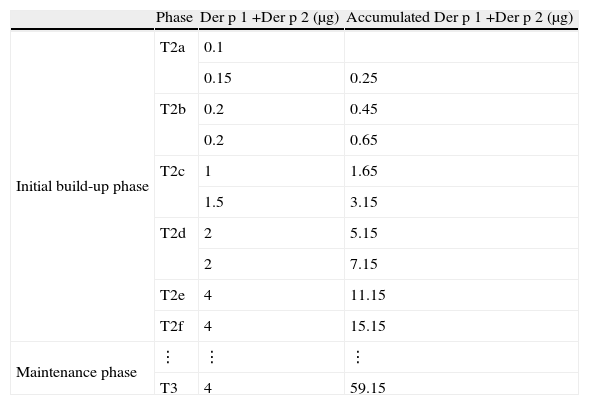
SIT protocolD. pteronyssinus subcutaneous immunotherapy began in T2. During the initial phase, for four consecutive weeks (T2a, T2b, T2c, T2d), patients were monitored weekly for 60min, and two initial schedule doses of extract were administered at each time, with a 30min interval, until reaching the maintenance dose, which was repeated after two weeks in T2e and then after one month in T2f. After that, the same dose was administered monthly for one year until T3 (Table 2). Doses administered and adverse events were recorded to assess the compliance and safety.
Immunotherapy schedule. Initial build-up phase: from T2a to T2d, two weekly doses were administered with a 30min interval between doses, then only one dose was administered at T2e in one week. After that, from T2f to T3 twelve monthly maintenance doses were administered.
| Phase | Der p 1 +Der p 2 (μg) | Accumulated Der p 1 +Der p 2 (μg) | |
| Initial build-up phase | T2a | 0.1 | |
| 0.15 | 0.25 | ||
| T2b | 0.2 | 0.45 | |
| 0.2 | 0.65 | ||
| T2c | 1 | 1.65 | |
| 1.5 | 3.15 | ||
| T2d | 2 | 5.15 | |
| 2 | 7.15 | ||
| T2e | 4 | 11.15 | |
| T2f | 4 | 15.15 | |
| Maintenance phase | ⋮ | ⋮ | ⋮ |
| T3 | 4 | 59.15 |
The diagnosis was evaluated by a detailed clinical history to assess, over the last two years, respiratory allergy, rhinitis and asthma, adding a lung function test (forced spirometry). Asthma and rhinitis severity were assessed according to the ARIA10 and asthma criteria of Consensus Statement on the Management of Pediatric Asthma.11
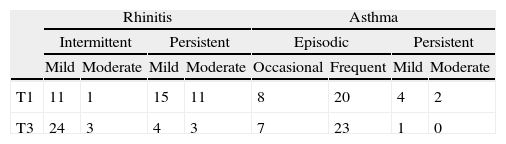
Rhinitis and asthma severity was assessed at T1 and T3 by using objective scores for rhinitis, 0–4 (intermittent or persistent, mild or moderate); and asthma, 0–4 (occasional or frequent episodic and mild and moderate or severe persistent). Rhinitis–asthma score was the sum of both scores.
Patient perceived severity (parents in cases of children under eight) was measured by a visual analogue scale (VAS), values ranged from 0 (very bad) to 100 (very good), upon examination the week prior to T1 and T3 visits.
In vivo testsLung function test (forced spirometry) was evaluated in asthmatic children and changes, according to the predicted values of FVC, FEV1 and FEV1/FVC at T1 and T3, were assessed.
Skin prick tests were performed for mites, fungi, pollens and animal dander extract allergens (DIATER Laboratories, Madrid, Spain). Results were read after 15min and contour of the wheal of D. pteronyssinus was outlined and transferred to paper for scanning and calculation of the area (Sigma Scan Pro 5.0, SPSS Inc, Chicago, Illinois, USA).
In vitro testsSerum levels of D. pteronyssinus, Der p1, Der p2 and Der p10 IgE, and IgG4, were determined by enzyme immunoassay CAP FEIA (Phadia, Barcelona, Spain) and IL-10 by Human High sensitivity IL-10 ELISA kit (GEN PROBE DIACLONE SAS, France) according to the manufacturer's instructions.
Statistical analysisResults of clinical assessment, patient perceived severity (VAS), and lung function (FEV1/FVC), in vivo and in vitro tests are expressed as mean±SD. The influence of variables on the efficacy of treatment was analysed using Mann–Whitney and Kruskal–Wallis tests. Data were compared using the Wilcoxon and Chi-square tests. p-Values<0.05 were considered statistically significant. The relationship between variables was calculated by the Spearman test and sensitivity/specificity by a receiver operating characteristic (ROC) curve (SPSS 10.0, 1999; SPSS Inc, Chicago, IL, USA).
ResultsForty-six children were included from four different allergy departments from hospitals in Spain (38% Pontevedra, 30% Sabadell, 20% Madrid, 12% Manresa). Four withdrew before beginning immunotherapy; three were withdrawn from the study because of poor compliance; and one because of change of address. Thirty-eight children, 5–15 years old (10±3) finished the study, 25 boys and 13 girls. All of them had D. pteronyssinus respiratory allergy. Twenty-four children had food allergy and/or eczema, and four had a family history of allergic disorders.
Safety and efficacyNo systemic immediate reactions were reported with the immunotherapy. Only six children had delayed local reactions,2,3 during initial build-up phase with erythema and oedema less than five cm in diameter, which resolved in 24–36h without treatment.
Clinical assessmentAt T1, 38 patients had allergic rhinitis and 34 of them also had asthma. At T3, 30 patients had improved their allergy respiratory; six experienced no changes in their symptoms and two worsened (Table 3). We observed statistically significant decreases in rhinitis T1 (2.70±1.13) vs. T3 (1.45±1.05) (p<0.000) and asthma scores T1 (1.74±0.682) vs. T3 (1.11±0.699) (p=0.002). Moreover, patients reported an improvement in VAS scores T1 (75.54±14.79) vs. T3 (85.54±12.63) (p<0.001).
Clinical assessment of rhinitis (mild intermittent=1; moderate–severe intermittent=2; mild persistent=3; moderate–severe persistent=4). Clinical assessment of asthma (occasional episodic=1; frequent episodic=2; mild persistent=3; moderate persistent=4) at T1and T3.
| Rhinitis | Asthma | |||||||
| Intermittent | Persistent | Episodic | Persistent | |||||
| Mild | Moderate | Mild | Moderate | Occasional | Frequent | Mild | Moderate | |
| T1 | 11 | 1 | 15 | 11 | 8 | 20 | 4 | 2 |
| T3 | 24 | 3 | 4 | 3 | 7 | 23 | 1 | 0 |
Lung function (FEV1/FVC) had improved after immunotherapy: T1 (87±25) vs. T3 (99±12) (p<0.02).
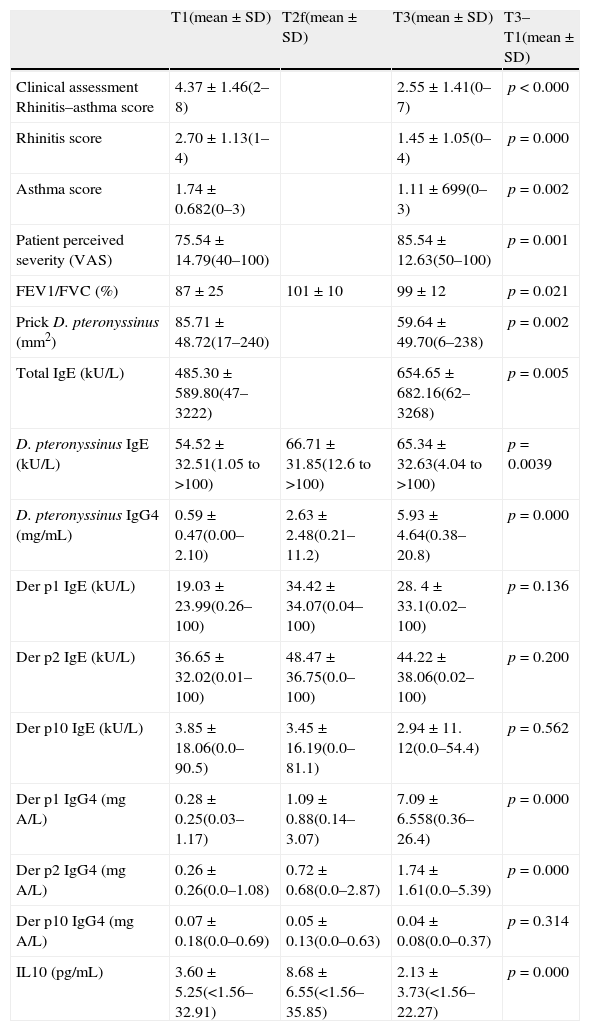
Results of the different variables (clinical assessment, VASs, lung function (FVC, FEV1, FEV1/FVC), area skin prick test (mm2) D. pteronyssinus, Der p1 and Der p2 IgE (ku/L) and IgG4 (mg/mL) and IL10 (pg/mL)) and statistical analysis at T1, T2f and T3.
| T1(mean±SD) | T2f(mean±SD) | T3(mean±SD) | T3–T1(mean±SD) | |
| Clinical assessment Rhinitis–asthma score | 4.37±1.46(2–8) | 2.55±1.41(0–7) | p<0.000 | |
| Rhinitis score | 2.70±1.13(1–4) | 1.45±1.05(0–4) | p=0.000 | |
| Asthma score | 1.74±0.682(0–3) | 1.11±699(0–3) | p=0.002 | |
| Patient perceived severity (VAS) | 75.54±14.79(40–100) | 85.54±12.63(50–100) | p=0.001 | |
| FEV1/FVC (%) | 87±25 | 101±10 | 99±12 | p=0.021 |
| Prick D. pteronyssinus (mm2) | 85.71±48.72(17–240) | 59.64±49.70(6–238) | p=0.002 | |
| Total IgE (kU/L) | 485.30±589.80(47–3222) | 654.65±682.16(62–3268) | p=0.005 | |
| D. pteronyssinus IgE (kU/L) | 54.52±32.51(1.05 to >100) | 66.71±31.85(12.6 to >100) | 65.34±32.63(4.04 to >100) | p=0.0039 |
| D. pteronyssinus IgG4 (mg/mL) | 0.59±0.47(0.00–2.10) | 2.63±2.48(0.21–11.2) | 5.93±4.64(0.38–20.8) | p=0.000 |
| Der p1 IgE (kU/L) | 19.03±23.99(0.26–100) | 34.42±34.07(0.04–100) | 28. 4± 33.1(0.02–100) | p=0.136 |
| Der p2 IgE (kU/L) | 36.65±32.02(0.01–100) | 48.47±36.75(0.0–100) | 44.22±38.06(0.02–100) | p=0.200 |
| Der p10 IgE (kU/L) | 3.85±18.06(0.0–90.5) | 3.45±16.19(0.0–81.1) | 2.94±11. 12(0.0–54.4) | p=0.562 |
| Der p1 IgG4 (mgA/L) | 0.28±0.25(0.03–1.17) | 1.09±0.88(0.14–3.07) | 7.09±6.558(0.36–26.4) | p=0.000 |
| Der p2 IgG4 (mgA/L) | 0.26±0.26(0.0–1.08) | 0.72±0.68(0.0–2.87) | 1.74±1.61(0.0–5.39) | p=0.000 |
| Der p10 IgG4 (mgA/L) | 0.07±0.18(0.0–0.69) | 0.05±0.13(0.0–0.63) | 0.04±0.08(0.0–0.37) | p=0.314 |
| IL10 (pg/mL) | 3.60±5.25(<1.56–32.91) | 8.68±6.55(<1.56–35.85) | 2.13±3.73(<1.56–22.27) | p=0.000 |
Cutaneous reactivity (skin prick tests) to D. pteronyssinus showed a clear decrease from T1 to T3 (p=0.002).
In vitro testsSerum D. pteronyssinus, Der p1, Der p2, Der p10 IgE and IgG4, and IL-10 levels were determined in all patients at T1 and T3. Only 25 patients had these tests at T2f too. D. pteronyssinus antibodies, IgE and IgG4, increased in T2f and T3 with p=0.0039 and p=0.000, respectively. Der p1 and Der p2 IgE had no significant increase at T2f, and then both decreased at T3. However, Der p1 and Der p2 IgG4 serum were increased at T2f and T3 (p=0.000). No changes were observed in Der p10 IgE and IgG4.
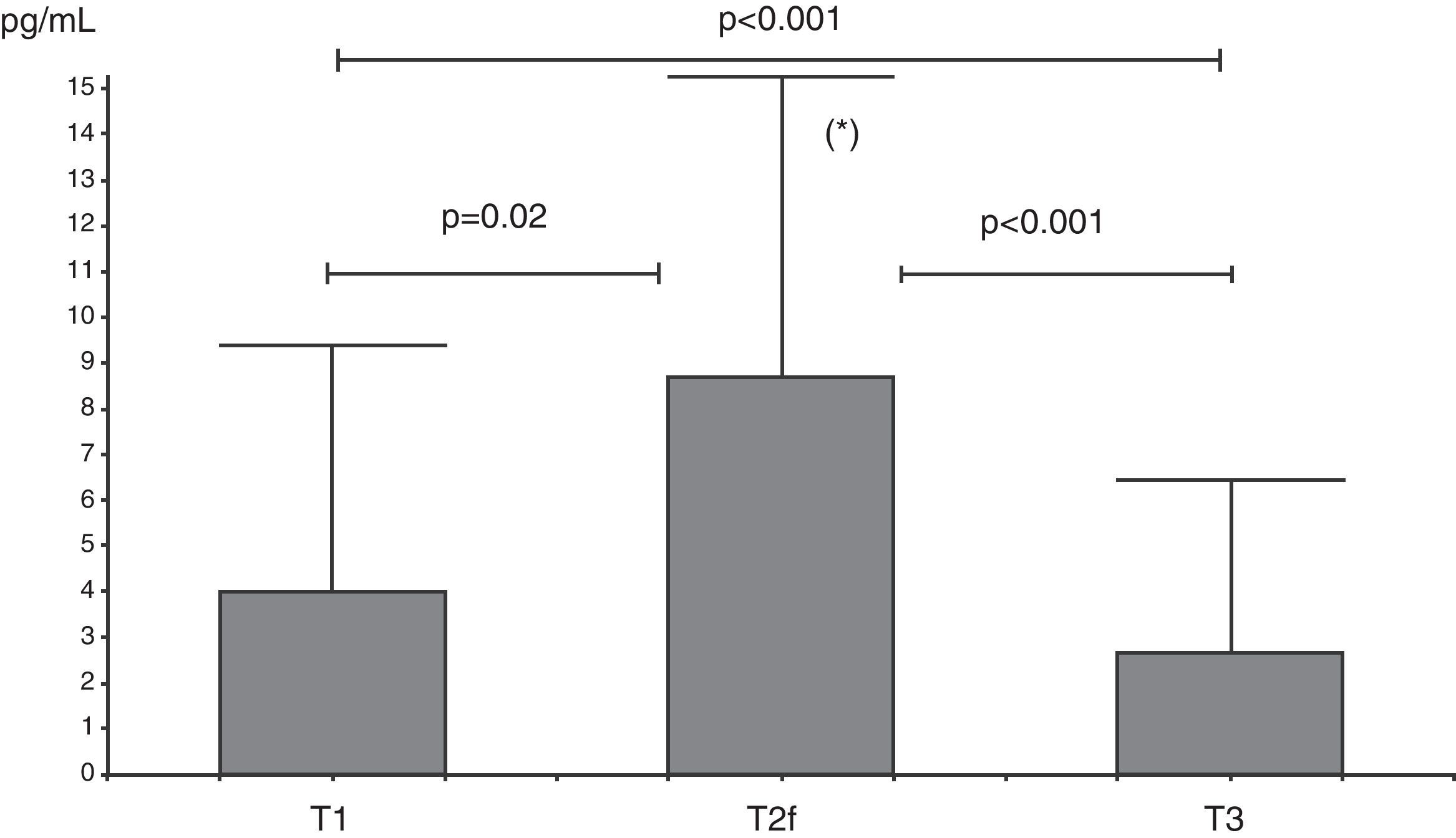
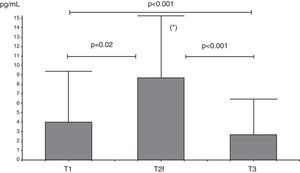
IL-10 levels increased from T1 to T2f (p=0.02), and then decreased at T3 (p=0.001) with levels at this time below T1 levels (Fig. 1).
Analysis of serum IL-10 (pg/mL) levels at T1 (before beginning immunotherapy), at T2f (beginning of maintenance D. pteronyssinus immunotherapy phase) and at T3 (after one year of D. pteronyssinus immunotherapy). There was an early increase of IL-10 levels at T2f followed by a decrease at T3, below levels at T1.
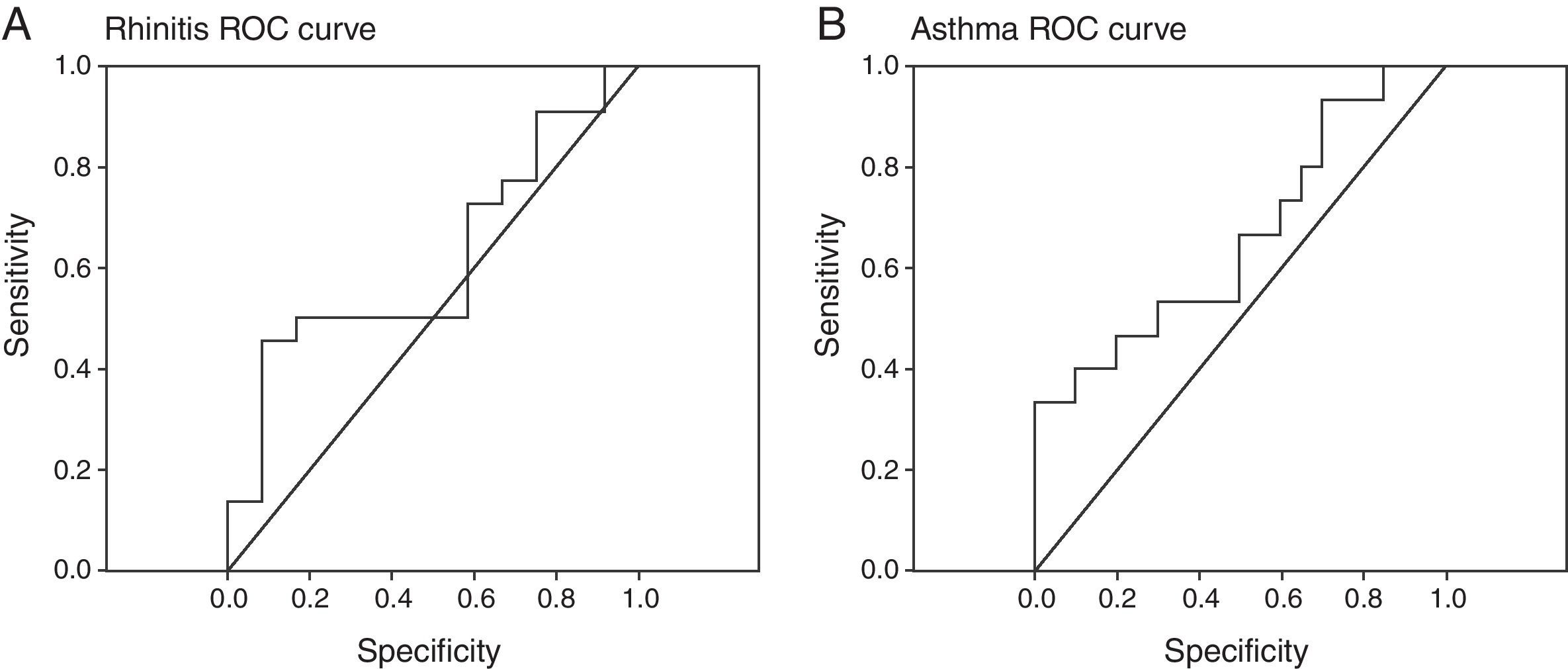
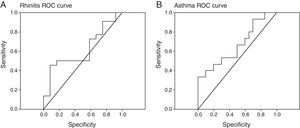
The improvement of asthma and rhinitis was correlated with the improvement of patient perceived severity (VAS) (p=0.02, r=−0.392) and D. pteronyssinus IgG4 evolution (p=0.01, r=−0.436). However, a low sensitivity and specificity of specific IgG4 for an early prediction of immunotherapy efficacy was found (Fig. 2). We cannot demonstrate a significant correlation between IL-10 variations and a favourable clinical course.
D. pteronyssinus IgG4 response is correlated to asthma evolution (p<0.01, r=−0.472) and rhinitis evolution (p<0.01, r=−0.435). A. Rhinitis and B. asthma rreceiver-operating characteristic (ROC) curves showed low sensitivity and specificity of this variable in the prediction of the efficacy of immunotherapy.
In 1998, the World Health Organization12 and the European Academy of Allergology and Clinical Immunology (EAACI)13 affirmed the clinical effectiveness of SIT by injection or local nasal or sublingual administration in the management of allergic rhinitis and asthma when standardised extracts are used in adequate amounts.
A meta-analysis including 54 clinical trials that assessed the efficacy of SIT in asthma, led to the conclusion that SIT significantly reduces asthma symptoms, medication required to control symptoms, and worsening of asthma.14 SIT has also been proven to reduce specific bronchial reactivity and to prevent the development of asthma in patients with allergic rhinitis.2,15–17
However, SIT is a long-term treatment, and its duration has important consequences for security of patients and compliance and economy of their families. Tabar et al.18 in a recently study evaluated the differences in clinical efficacy of SIT as a result of the extension of the treatment, and found clinical improvement in rhinitis and asthma obtained with three years of D. pteronyssinus SIT, finding an additional benefit in rhinitis only after a further two years of SIT.
More than three quarters of the patients in our study had improved symptoms of both rhinitis and asthma after one year of D. pteronyssinus immunotherapy; lung function showed an improvement over theoretical values; and the perceived severity for patients and their parents was improved too. Moreover, there were only few local reactions to the treatment. Our results confirm the effectiveness and safety of SIT as previously demonstrated in prospective, blinded and placebo-controlled studies with different allergens in allergic rhinitis and asthma treatment.19–21 In addition to clinical improvement, we found a decrease in immediate response in skin prick tests after one year of SIT. Although not all studies have observed this decline,22,23 our results support the efficacy of treatment.
Clinical improvement in our patients was accompanied by an increase in specific IgE and IgG4, which was maintained and increased at the end of one year of SIT, as occurred in other studies.24–26 This confirms the immunogenicity of the extract.27 However, we observed an early increase of specific IgE to major allergens (Der p1 and Der p2) at the end of the build-up phase of SIT, and after that a decrease at the end of study. Changes in clinical evolution, skin prick test responses and D. pteronyssinus, Der p1 and Der p2 IgE and IgG4, were preceded by an early and transient increase of IL-10 at the end of build-up phase of SIT.
At the end of the 1990s, the first evidence was found that SIT with insect venom induced IL-10-production. Later, similar data were obtained for inhalant allergies, and it was confirmed that SIT with house dust mite or grass pollen extract induces CD4+CD25+ T cells that produce IL-10 and show suppressive capacity.9,28 This suppression could be partially blocked by the addition of anti-IL-10 or anti-TGF-β antibodies.29,30 IL-10 was also shown to be important for the induction of specific unresponsiveness (anergy) in peripheral T cells. As a consequence, the up-regulation of Treg cells is now considered the cardinal event of SIT.31–33 Bohle, in a recent review of the mechanisms of SIT, suggested that the induction of IL-10-producing Treg actively suppresses allergen-specific T cell proliferation and cytokine production. In addition, the early increase in IL-10-producing Treg cells also results in increased IL-10 levels, which suggests early production of allergen-specific IgG4.34 Antigen-induced Treg cells have been shown to have a short life span and to be rapidly removed from periphery tissues.35 Bohle observed that the presence of Treg cells faded over time during SIT. This reduction in Treg cells may represent an important regulatory feature of the human immune system: in response to pathogens, we need to set up a defending immune response quickly. However, after some time this response needs to be stopped again in order to avoid chronic and potentially self-destroying immune responses.
Finally, we determined specific IgE to Der p10 (tropomyosin of D. pteronyssinus) which shared more than 65% of identical residues with other invertebrate tropomyosins. van Ree et al.36 observed induction of food allergy during mite immunotherapy. In our study, no significant changes in specific IgE to Der p10 levels were observed and none of our patients developed food allergies along the study. These results confirm the findings of Rossi et al.37 who did not find neo sensitisation to tropomyosin of shrimp in patients treated with mite sublingual immunotherapy.
We suggest that the early IL-10 response with an increase in specific IgG4 levels and an associated beginning of the decline in Der p1 and Der p2 IgE levels in the first year of treatment could be efficacy predictors of the allergen specific immunotherapy. The limitations of this study are the relatively small number of patients studied, and thus further studies are necessary to confirm our results.
Conflict of interestThe authors have no conflict of interest to declare.