Edited by: Andrea Gallioli
Fundació Puigvert
Marco Moschini
San Raffaele Hospital
Last update: June 2025
More infoUntil recently there was no recommended adjuvant therapy for patients with lymph nodes metastasis (ypN+) following neoadjuvant chemotherapy (NAC) and radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC). The aim of the study was to describe the oncological outcomes of ypN+ patients following NAC and RC for MIBC.
MethodsThis collaborative retrospective study included 195 patients with ypN+ disease after NAC followed by RC and bilateral pelvic lymph node dissection for MIBC between 2000 and 2019 in seven centers. Patients’ demographics, clinical and pathological features were collected. Survival analyses were carried out with Kaplan-Meier estimates and a Cox model was generated.
ResultsA total of 120 patients (62%) were pN1, 51 pN2 (26%) and 24 pN3 (12%). Adjuvant radiation therapy was performed in 18 (9%), adjuvant chemotherapy in 40 (21%) and the remaining 137 (70%) patients were observed. The median follow-up time was 51 months (95%CI 44–62). Median times for recurrence-free survival, cancer-specific survival and overall survival (OS) were 18 months (95%CI 16−21), 47 months (95%CI 31−70) and 28 months (95%CI 22−34) respectively. On multivariable analysis, female gender (HR = 1.5, 95%CI 1.002–2.21, p = 0.049) and positive surgical margins (HR = 1.6, 95%CI 1.06–2.38, p = 0.026) were the only independent predictor of OS. The type of adjuvant therapy did not impact OS (adjuvant chemotherapy, p = 0.44; adjuvant radiotherapy p = 0.40).
ConclusionMIBC patients with residual node positive disease following NAC and RC have poor survival outcomes. Females and patients with positive margin status at RC carry a poorer prognosis. These results may be beneficial for clinical trial design.
Hasta hace poco tiempo no existía una terapia adyuvante recomendada para pacientes con metástasis en los ganglios linfáticos (ypN+) después de la quimioterapia neoadyuvante (QNA) y la cistectomía radical (CR) para el cáncer de vejiga músculo invasor (CVMI). El objetivo del estudio fue describir los resultados oncológicos de los pacientes ypN+ tras QNA y CR para CVMI.
MétodosEste estudio retrospectivo colaborativo incluyó a 195 pacientes con enfermedad ypN+ después de QNA seguida de CR y disección bilateral de ganglios linfáticos pélvicos para el CVMI en 7 centros entre 2000 y 2019. Se recopilaron los datos demográficos y las características clínicas y patológicas de los pacientes. Se realizaron análisis de supervivencia con estimaciones de Kaplan-Meier y se generó un modelo de Cox.
ResultadosEn total, 120 (62%) pacientes fueron pN1, 51 (26%) pN2 y 24 (12%) pN3. Se realizó radioterapia adyuvante en 18 (9%), quimioterapia adyuvante en 40 (21%), y los 137 pacientes restantes (70%) fueron sometidos a observación. La mediana del tiempo de seguimiento fue de 51 meses (IC 95%: 44–62). La mediana de la supervivencia libre de recurrencia, la supervivencia específica al cáncer y la supervivencia global (SG) fue de 18 meses (IC del 95%: 16−21), 47 meses (IC del 95%: 31−70) y 28 meses (IC del 95%: 22−34), respectivamente. En el análisis multivariable, el sexo femenino (HR = 1,5; IC 95%: 1,002−2,21; p = 0,049) y los márgenes quirúrgicos positivos (HR = 1,6; IC 95%: 1,06−2,38; p = 0,026) fueron los únicos factores predictivos independientes de la SG. El tipo de tratamiento adyuvante no influyó en la SG (quimioterapia adyuvante, p = 0,44; radioterapia adyuvante, p = 0,40).
ConclusiónLos resultados de supervivencia de los pacientes con CVMI y afectación ganglionar residual tras la QNA y la CR son desfavorecedores. El sexo femenino y los márgenes positivos en la CR se asocian a un pronóstico peor. Estos resultados pueden ser útiles para el diseño de ensayos clínicos a futuro.
Neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) including a bilateral pelvic lymph node dissection is the recommended treatment for non-metastatic muscle-invasive bladder cancer (MIBC).1–3 Complete pathological response (ypT0pN0) can be observed in up to 40% of patients depending on the chemotherapy regimen used and on the quality of the resection.4 However, approximately 10–20% of patients will harbor residual lymph node disease (ypN+) following NAC and RC.4,5 The oncological outcomes for these patients are not well known but the reported outcomes are poor with a median overall survival (OS) usually inferior to two years.6–10
Moreover, there is no consensus regarding additional chemotherapy for these ypN+ patients.11 A systematic review and meta-analysis of individual participant data from randomized controlled trials demonstrated a benefit of cisplatin-based adjuvant chemotherapy (AC) on OS with an absolute improvement in survival of 6% at 5 yr.12 However, these data cannot be applied to ypN+ patients (after NAC).
In addition, although the use of adjuvant radiation therapy (RT) may improve OS in locally advanced MIBC (NAC was used in less than 10% of the cohort) the largest retrospective study on adjuvant RT after NAC and RC did not found an improved OS.13 Therefore none of the international guidelines support the use of adjuvant chemo- or radiation therapy after the use of preoperative systemic therapy.
Recently, the Checkmate 274 trial reported a disease-free benefit with a use of the Programmed cell Death protein 1 (PD-1) inhibitor nivolumab for high-risk MIBC patients (at least pT3 or ypT2 or N+) following RC.14 In this trial, only 43% of patients received NAC. Interestingly the subgroup analysis showed that patients with cN+ disease and those who received NAC favored nivolumab compared to patients with cN0 or without NAC respectively. Moreover, the European Medicines Agency (EMA) has approved the use of nivolumab only to PD-L1 positive patients, therefore a significant subset of patients will not benefit from such therapy.
Describing the outcomes of ypN+ patients is an unmet need since only small series are available to date. Herein, we aimed to describe the oncological outcomes of node-positive patients following NAC and RC for MIBC.
MethodsStudy designIn a collaborative group, we retrospectively reviewed all MIBC patients who underwent RC between 2000 and 2019 at seven academic centers worldwide. Each center was responsible for the approval of the study and data-sharing agreements were exchanged. Computerized databases were merged and classic data management was conducted.
Patient selection and collected informationFor this analysis, we included MIBC patients who received NAC followed by RC and in whom histopathology revealed lymph node invasion from urothelial carcinoma (ypN+). All patients received NAC with either cisplatin- or carboplatin-based regimen. We excluded patients with prior metastatic disease (cN3 and cM1) and patients with no follow-up information.
Basic demographic characteristics were obtained such as age at diagnosis, gender, Body mass index, Smoking history, ASA score, Charlson comorbidity score, clinical and node stages. Types of urinary diversion, pathological and node stages, margin status, number of lymph nodes yielded, number of positive lymph nodes, presence of concomitant carcinoma in situ (CIS) after RC were also collected. The type of adjuvant therapy was recorded (radiation therapy or chemotherapy), the remaining patients were observed. Patients were followed lifelong according to each center modality with serial cross-sectional imaging. Treatment given at recurrence was also retrieved.
OutcomesRecurrence-free survival (RFS) duration was defined as the time from cystectomy to cancer recurrence at local (cystectomy bed, pelvic lymph-nodes up to aortic bifurcation or soft tissue sites) or distant sites; patients who were recurrence-free at end of the study were censored at the last follow-up. Therefore, recurrence was defined as any local or metastatic recurrence following RC. Total follow-up time was defined by the period between RC and the last follow-up visit or date of recurrence or death. De novo urothelial carcinoma in the upper tract or urethra was not considered as a recurrence.
Cancer-specific survival (CSS) and OS were defined as the time from cystectomy to death from bladder cancer or all causes respectively; patients who were alive at end of the study were censored at the last follow-up.
Statistical analysisCategorical variables were expressed using frequency and percentage; continuous variables were expressed using the median and interquartile range (IQR). For survival analysis, patients were censored until death or recurrence. Kaplan-Meier curves were used to estimate RFS, CSS and OS. Stratified comparisons were performed with the log-rank test. The effect of different variables on OS was estimated through a stepwise Cox multivariable regression model for the variable with a p-value <0.2 in univariate analysis and with less than 30% missing values. To further assess the role of adjuvant therapy we forced the corresponding variable into the final model. Log linearity hypotheses were verified for quantitative variables and proportional hazard assumptions were verified for all variables. All statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, North Carolina, United States of America). All p-values <0.05 were considered statistically significant.
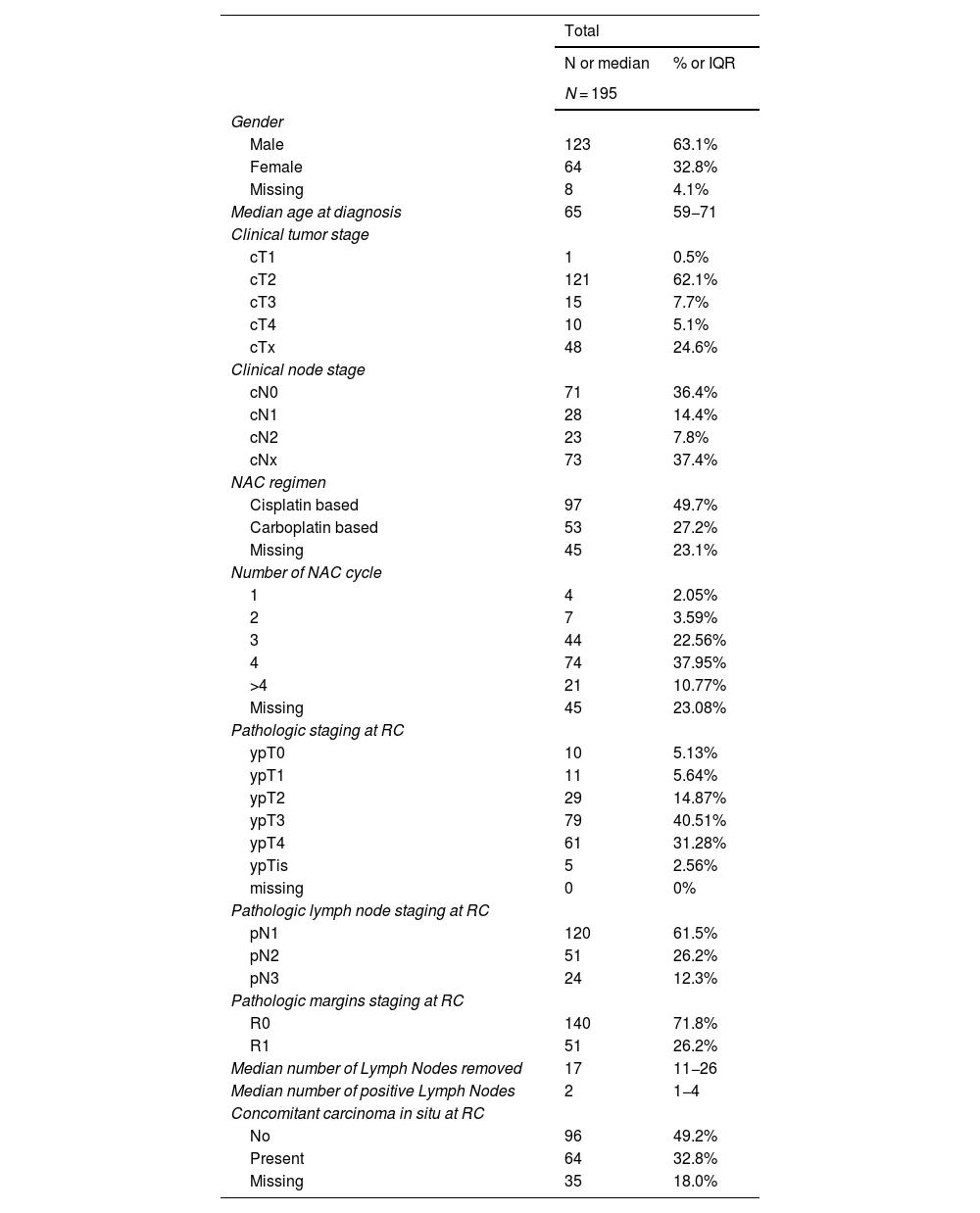
ResultsPatient characteristicsWe included 195 ypN+ patients for analysis, 123 males (63%) and 64 females (33%). Patient demographics, clinical and pathological features are summarized in Table 1. The median age at time of surgery was 65 years (IQR 59−71 years).
Patients demographics, clinical and pathological features.
| Total | ||
|---|---|---|
| N or median | % or IQR | |
| N = 195 | ||
| Gender | ||
| Male | 123 | 63.1% |
| Female | 64 | 32.8% |
| Missing | 8 | 4.1% |
| Median age at diagnosis | 65 | 59−71 |
| Clinical tumor stage | ||
| cT1 | 1 | 0.5% |
| cT2 | 121 | 62.1% |
| cT3 | 15 | 7.7% |
| cT4 | 10 | 5.1% |
| cTx | 48 | 24.6% |
| Clinical node stage | ||
| cN0 | 71 | 36.4% |
| cN1 | 28 | 14.4% |
| cN2 | 23 | 7.8% |
| cNx | 73 | 37.4% |
| NAC regimen | ||
| Cisplatin based | 97 | 49.7% |
| Carboplatin based | 53 | 27.2% |
| Missing | 45 | 23.1% |
| Number of NAC cycle | ||
| 1 | 4 | 2.05% |
| 2 | 7 | 3.59% |
| 3 | 44 | 22.56% |
| 4 | 74 | 37.95% |
| >4 | 21 | 10.77% |
| Missing | 45 | 23.08% |
| Pathologic staging at RC | ||
| ypT0 | 10 | 5.13% |
| ypT1 | 11 | 5.64% |
| ypT2 | 29 | 14.87% |
| ypT3 | 79 | 40.51% |
| ypT4 | 61 | 31.28% |
| ypTis | 5 | 2.56% |
| missing | 0 | 0% |
| Pathologic lymph node staging at RC | ||
| pN1 | 120 | 61.5% |
| pN2 | 51 | 26.2% |
| pN3 | 24 | 12.3% |
| Pathologic margins staging at RC | ||
| R0 | 140 | 71.8% |
| R1 | 51 | 26.2% |
| Median number of Lymph Nodes removed | 17 | 11−26 |
| Median number of positive Lymph Nodes | 2 | 1−4 |
| Concomitant carcinoma in situ at RC | ||
| No | 96 | 49.2% |
| Present | 64 | 32.8% |
| Missing | 35 | 18.0% |
Node staging prior RC was distributed as follow: 71 cN0 (36%), 28 cN1 (14%), 14 cN2 (7%), 9 cN3 (0.6%), and 73 missing (38%). The NAC regimens were cisplatin-based in 97 patients (50%), carboplatin-based in 53 (27.2%) and not reported in 45 (23.1%). Most patients underwent an ileal conduit urinary diversion (62%).
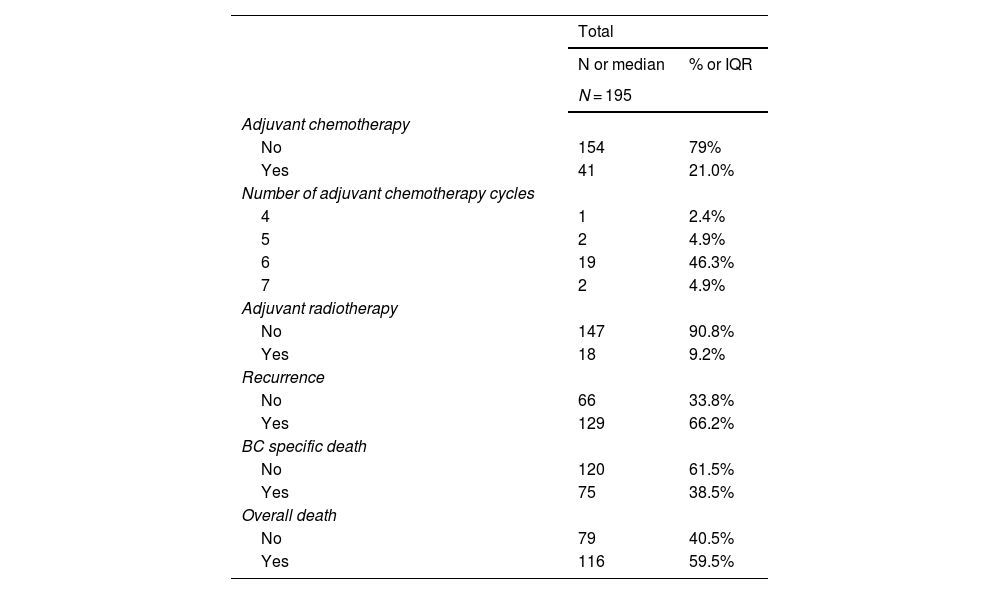
A total of 10 patients (5%) were ypT0 on final pathology, 16 (8%) had a residual non-MIBC and 169 (87%) had residual MIBC. Most of the patients were pN1 (n = 120; 62%) with a median number of lymph nodes removed of 17.11–26 Positive margins were identified in 53 (27%). Eighteen patients (9%) underwent adjuvant radiation, 40 patients (21%) received adjuvant chemotherapy, and the remaining 137 (70%) patients were observed (Table 2).
Patients postoperative management and oncological outcomes.
| Total | ||
|---|---|---|
| N or median | % or IQR | |
| N = 195 | ||
| Adjuvant chemotherapy | ||
| No | 154 | 79% |
| Yes | 41 | 21.0% |
| Number of adjuvant chemotherapy cycles | ||
| 4 | 1 | 2.4% |
| 5 | 2 | 4.9% |
| 6 | 19 | 46.3% |
| 7 | 2 | 4.9% |
| Adjuvant radiotherapy | ||
| No | 147 | 90.8% |
| Yes | 18 | 9.2% |
| Recurrence | ||
| No | 66 | 33.8% |
| Yes | 129 | 66.2% |
| BC specific death | ||
| No | 120 | 61.5% |
| Yes | 75 | 38.5% |
| Overall death | ||
| No | 79 | 40.5% |
| Yes | 116 | 59.5% |
The median follow-up time in censored patients was 51 months (95%CI 44–62). Recurrence occurred in 129 patients (66%), cancer-specific deaths in 75 (39%) and deaths in 116 (60%). Most patients (68%) underwent chemotherapy at the time of recurrence. Patient’s oncological outcomes are summarized in Table 2. Systemic progression happened in 70% of patients who recurred.
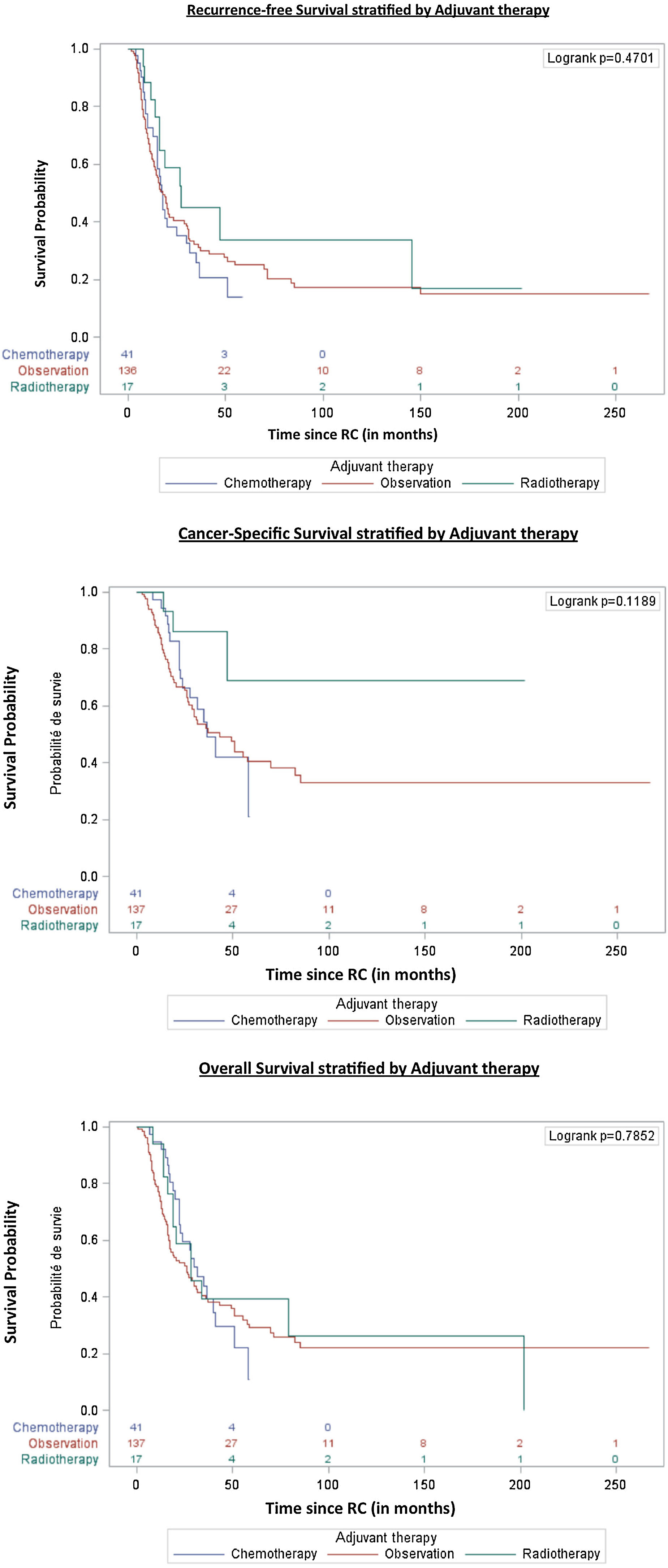
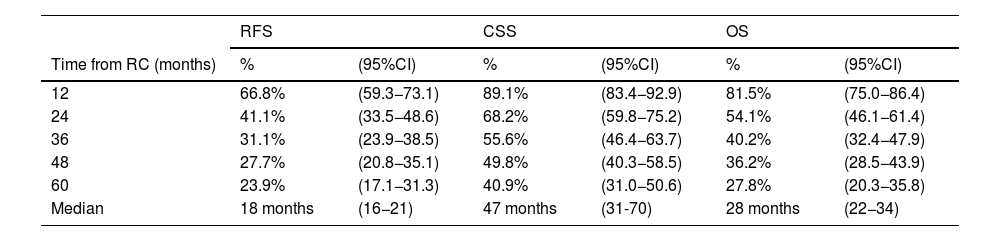
In overall population, RFS at 1, 3 and 5 years was 67% (95%CI 59–73), 31% (95%CI 24–39) and 24% (95%CI 17–31) respectively. CSS at 1, 3 and 5 years was 89% (95%CI 83–93), 56% (95%CI 46–64) and 41% (95%CI 31–50) respectively. OS at 1, 3 and 5 years was 82% (95%CI 75–86), 40% (95%CI 32–48) and 28% (95%CI 20–36) respectively (Table 3).
Kaplan Meier Estimation of Recurrence-free (RFS), cancer-specific (CSS) and overall survivals (OS) after a median follow-up of 51 months 95%CI (44–62).
| RFS | CSS | OS | ||||
|---|---|---|---|---|---|---|
| Time from RC (months) | % | (95%CI) | % | (95%CI) | % | (95%CI) |
| 12 | 66.8% | (59.3−73.1) | 89.1% | (83.4−92.9) | 81.5% | (75.0−86.4) |
| 24 | 41.1% | (33.5−48.6) | 68.2% | (59.8−75.2) | 54.1% | (46.1−61.4) |
| 36 | 31.1% | (23.9−38.5) | 55.6% | (46.4−63.7) | 40.2% | (32.4−47.9) |
| 48 | 27.7% | (20.8−35.1) | 49.8% | (40.3−58.5) | 36.2% | (28.5−43.9) |
| 60 | 23.9% | (17.1−31.3) | 40.9% | (31.0−50.6) | 27.8% | (20.3−35.8) |
| Median | 18 months | (16−21) | 47 months | (31-70) | 28 months | (22−34) |
Median times to RFS, CSS and OS were 18 months (95%CI 16−21), 47 months (95%CI 31−70) and 28 months (95%CI 22−34) respectively. We did not observe any survival differences among types of adjuvant therapy after NAC and RC (Fig. 1).
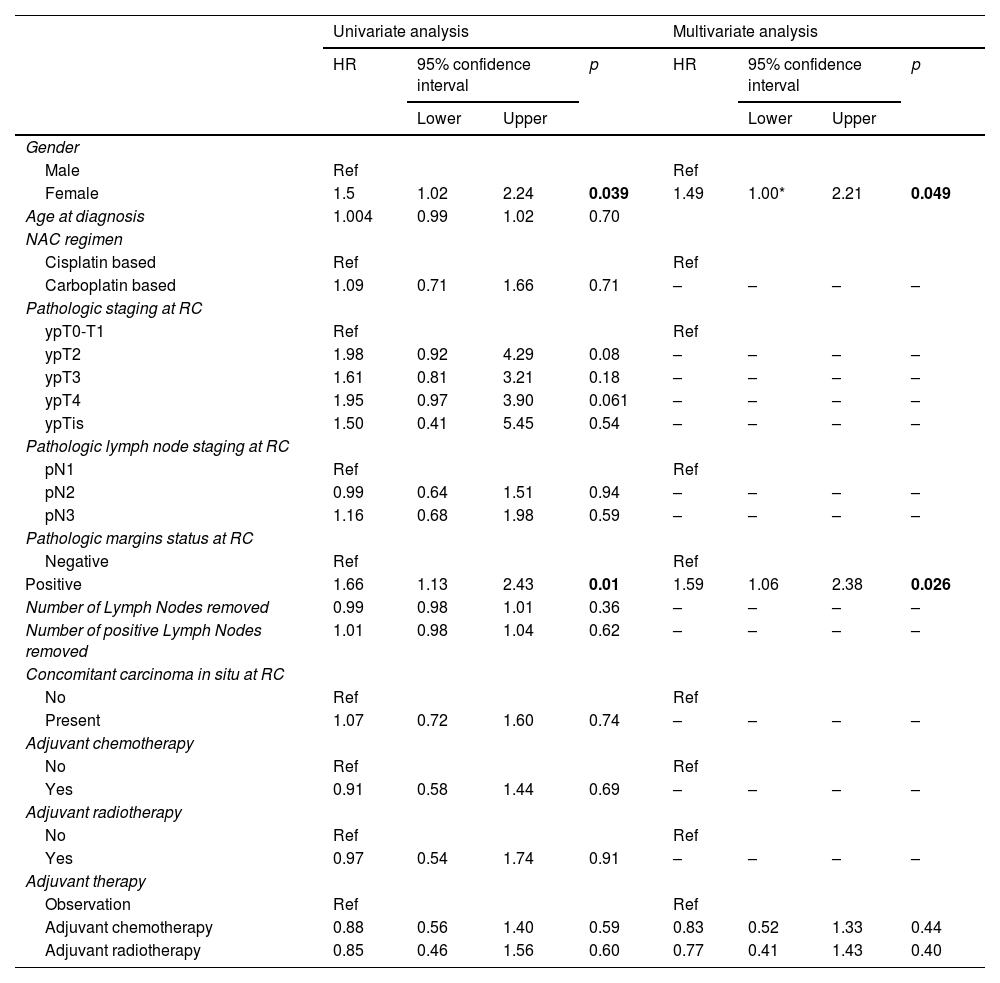
Multivariate analysesA multivariate cox model was designed. A female gender (HR = 1.5, 95%CI 1.002–2.21, p = 0.049) and positive margins at the time of RC (HR = 1.6, 95%CI 1.06–2.38, p = 0.026) were the only independent predictor of overall survival (Table 4). The type of adjuvant therapy did not impact OS: HR = 0.8, 95%CI 0.52–1.33, p = 0.44 and HR = 0.8, 95%CI 0.41–1.34, p = 0.4 for adjuvant chemotherapy and radiotherapy, respectively.
Overall survival uni- and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% confidence interval | p | HR | 95% confidence interval | p | |||
| Lower | Upper | Lower | Upper | |||||
| Gender | ||||||||
| Male | Ref | Ref | ||||||
| Female | 1.5 | 1.02 | 2.24 | 0.039 | 1.49 | 1.00* | 2.21 | 0.049 |
| Age at diagnosis | 1.004 | 0.99 | 1.02 | 0.70 | ||||
| NAC regimen | ||||||||
| Cisplatin based | Ref | Ref | ||||||
| Carboplatin based | 1.09 | 0.71 | 1.66 | 0.71 | – | – | – | – |
| Pathologic staging at RC | ||||||||
| ypT0-T1 | Ref | Ref | ||||||
| ypT2 | 1.98 | 0.92 | 4.29 | 0.08 | – | – | – | – |
| ypT3 | 1.61 | 0.81 | 3.21 | 0.18 | – | – | – | – |
| ypT4 | 1.95 | 0.97 | 3.90 | 0.061 | – | – | – | – |
| ypTis | 1.50 | 0.41 | 5.45 | 0.54 | – | – | – | – |
| Pathologic lymph node staging at RC | ||||||||
| pN1 | Ref | Ref | ||||||
| pN2 | 0.99 | 0.64 | 1.51 | 0.94 | – | – | – | – |
| pN3 | 1.16 | 0.68 | 1.98 | 0.59 | – | – | – | – |
| Pathologic margins status at RC | ||||||||
| Negative | Ref | Ref | ||||||
| Positive | 1.66 | 1.13 | 2.43 | 0.01 | 1.59 | 1.06 | 2.38 | 0.026 |
| Number of Lymph Nodes removed | 0.99 | 0.98 | 1.01 | 0.36 | – | – | – | – |
| Number of positive Lymph Nodes removed | 1.01 | 0.98 | 1.04 | 0.62 | – | – | – | – |
| Concomitant carcinoma in situ at RC | ||||||||
| No | Ref | Ref | ||||||
| Present | 1.07 | 0.72 | 1.60 | 0.74 | – | – | – | – |
| Adjuvant chemotherapy | ||||||||
| No | Ref | Ref | ||||||
| Yes | 0.91 | 0.58 | 1.44 | 0.69 | – | – | – | – |
| Adjuvant radiotherapy | ||||||||
| No | Ref | Ref | ||||||
| Yes | 0.97 | 0.54 | 1.74 | 0.91 | – | – | – | – |
| Adjuvant therapy | ||||||||
| Observation | Ref | Ref | ||||||
| Adjuvant chemotherapy | 0.88 | 0.56 | 1.40 | 0.59 | 0.83 | 0.52 | 1.33 | 0.44 |
| Adjuvant radiotherapy | 0.85 | 0.46 | 1.56 | 0.60 | 0.77 | 0.41 | 1.43 | 0.40 |
P-value in blod are considered statiscally significant.
Variable with > 30% Missing values are excluded, * 1.002.
Our study showed that patients with residual lymph node disease following NAC and RC have poor survival outcomes with a median time to recurrence of 18 months and a median time to death of 28 months. No adjuvant therapy appears to be beneficial on OS. Females and patients with positive margin status on cystectomy specimens had a worse prognostic.
To date, this cohort is the largest reported investigating ypN+ patients. Several cohorts, also limited in size, have investigated the outcomes of patients with adverse pathological findings following NAC and RC.5–7,9,10,15–18 Fewer reports focused on ypN+ patients. Kassouf et al. reported a median RFS of 6 months and a median OS of 13 months with a 50-month median of follow-up in 37 patients.7 Interestingly, they found on multivariate analysis that surgical margin status, gender and variant histology were significantly associated with OS, whereas variant histology and use of adjuvant chemotherapy were significantly associated with RFS. In our cohort margin status and gender were also significantly associated with worse OS in the multivariate analysis. However, we found no benefit for adjuvant chemotherapy on RFS, CSS or OS. Moreover, we were not able to report on variant histology as our study has included only pure urothelial carcinoma, which may explain most likely the difference with our reported outcomes. Jeong et al. also reported on this particular population with a median follow-up of 68 months, including 53 patients.6 In their analysis, the median RFS was 8.5 months and the median OS was 16.2 months. Only age was reported to significantly impact OS and adjuvant chemotherapy did not improve RFS or OS.
Interestingly some patients will harbor a differential response in the bladder and in the nodes. In our study and reported series,6,7 a small proportion of patients (5% in our cohort) will have a complete bladder response (ypT0) but would still be ypN+. This highlights the beneficial role of pelvic lymph node dissection at the time of RC. Even if the LEA German randomized controlled trial failed to show a significant advantage of extended over limited lymph node dissection in RFS, CSS, and OS it is important to note that none of the patients received NAC and therefore the results of this trial should not be extrapolated to this population.19 The results of the SWOG 1011 (NCT01224665) were recently reported during international conferences reporting similar results but the main publication is eagerly awaited. However, given the rarity of the disease (ypN+) it is unlikely that this trial may answer the question of a therapeutic benefit for an extended lymph node dissection in this subgroup population. Identifying lymph node metastasis is critical as these patients harbor a poor prognosis regardless of the number and location of the positive nodes. In our series, we did not find any OS difference among ypN1, ypN2 or ypN3 patients.
The role of adjuvant RT is also under debate. A systematic review evaluating the efficacy of adjuvant RT for BC found no clear OS benefit,20 even if some large multicenter retrospective propensity score analyses have reported an OS benefit in pT4 or pN+ patients.21 However, the extrapolation of this meta-analysis is challenging since most studies did not investigate ypN+ patients specifically. Lewis et al. did not found any difference in OS when investigating the role of adjuvant RT using the National Cancer Data Base for patients with pT3-4N0-3M0 urothelial carcinoma of the bladder that received NAC and RC (n = 1646 patients).13 In a subgroup analysis, they reported no difference in OS with adjuvant RT for patients with ypN2-N3 disease (16.6 months vs. 15.1 months, p = 0.205) in line with our results. Moreover, in a phase II trial evaluating the toxicity and local control rate after adjuvant RT following RC (only 47% of patients received NAC and 65% were pN+), the 2-year OS was 52%,22 which is equivalent to our results (2-year OS 54%). Several trials are ongoing to address this issue,23 notably Bladder-ART (GETUG-AFU 30, NCT03333356) the Egyptian NCI trial (NCT04740866) and the BART trial (NCT02951325).
Interestingly, a randomized phase 2 trial investigated the role of adjuvant chemotherapy and RT vs adjuvant chemotherapy alone for locally advanced bladder cancer after RC without NAC.24 Although the 2-year outcomes and overall adjusted hazard ratios (HRs) favored chemotherapy plus RT vs chemotherapy alone for locoregional recurrence-free survival (96% vs 69%; HR, 0.08; 95% CI, 0.02−0.39; P < .01), this trial failed to show an OS benefit of the combined adjuvant therapy (71% vs 60%; HR, 0.61; 95% CI, 0.33–1.11; P = .11). We can hypothesize that adding chemotherapy or RT following RC and NAC will not be beneficial for ypN+ patients.
Three trials have been conducted to investigate the role of adjuvant PD-1 or PD-L1 blockade. The Checkmate 274 trial randomized high-risk (at least pT3 or ypT2 or N+) MIBC patients following RC to one year of adjuvant nivolumab (PD-1 inhibitor) vs placebo.14 This trial showed an improvement in disease-free survival in the intent-to-treat population (HR, 0.70; 95% CI, 0.54−0.89; P < .001). These results have led to FDA approval in 2021.25 In this trial, only patients with negative margins were enrolled and only 43% of patients received NAC. Interestingly the subgroup analysis showed that patients with N+ disease (HR 0.64; 0.48−0.85) and those who received NAC (HR 0.52; 0.38−0.71) favored nivolumab. In contrast in the IMvigor010 trial randomizing patients for 1-year atezolizumab vs observation did not meet the primary endpoint of improvement in disease-free survival in the intent-to-treat population.26 In the subgroup analysis in patients with pN+ or those who received NAC did not benefit from atezolizumab. The AMBASSADOR trial (NCT03244384) randomizing patients to 1 year of adjuvant pembrolizumab vs observation is also positive but the publication is awaited. None of the two reported trials included patients with positive margins at RC, while in our study 27% of included patients had positive margins. This very high-risk population should be investigated in clinical trials as they harbor adverse oncological outcomes and will not be eligible for adjuvant nivolumab.
Our study has several limitations besides its retrospective design. This represents a heterogeneous group of patients, treated in multiple different hospitals (according to local protocols) during a relatively long period of time. Even if this cohort represents the largest reported so far, we remain underpowered to identify a benefit of any adjuvant therapy in this setting. Moreover, the prolonged inclusion period may bring heterogeneity among the type of treatment received, including treatment at recurrence which could have an impact on overall survival analyses. In addition, a quarter of included patients received carboplatin-based NAC due to renal function impairment. Current standards advice against the use of carboplatin-based NAC. Lastly, given the relatively small number of patients who received adjuvant therapy and the heterogeneous cohort, no conclusions can be drawn regarding adjuvant treatment strategies. However, results of our study do underline the need to further investigate the use of adjuvant treatment and for selection of patients who will likely benefit most.
ConclusionMIBC patients with residual lymph node disease following NAC and RC have poor survival outcomes. Females and patients with positive margin status at RC carry a poorer prognosis. Our results may be beneficial for clinical trials and for selection of potential adjuvant strategies.