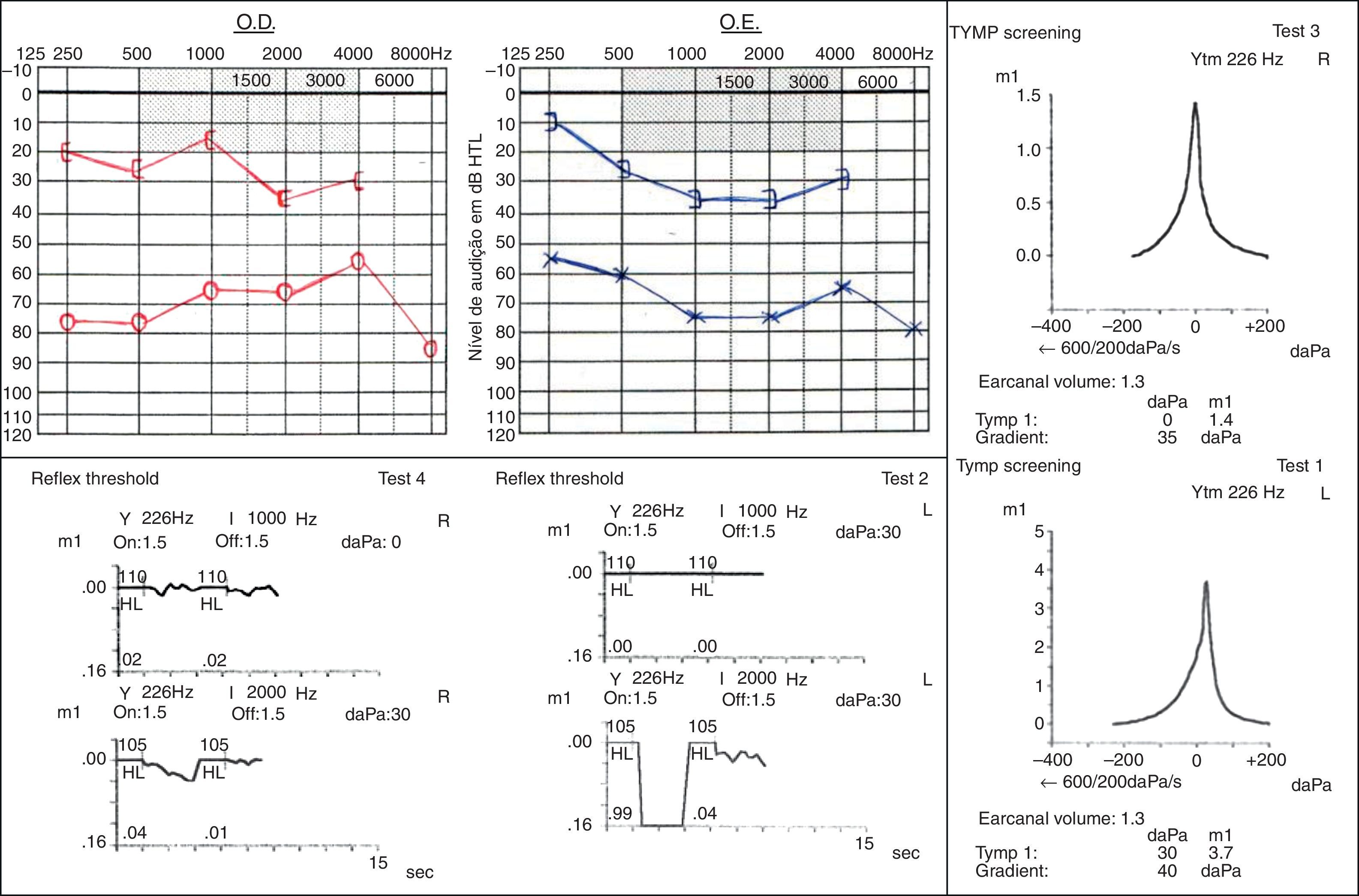
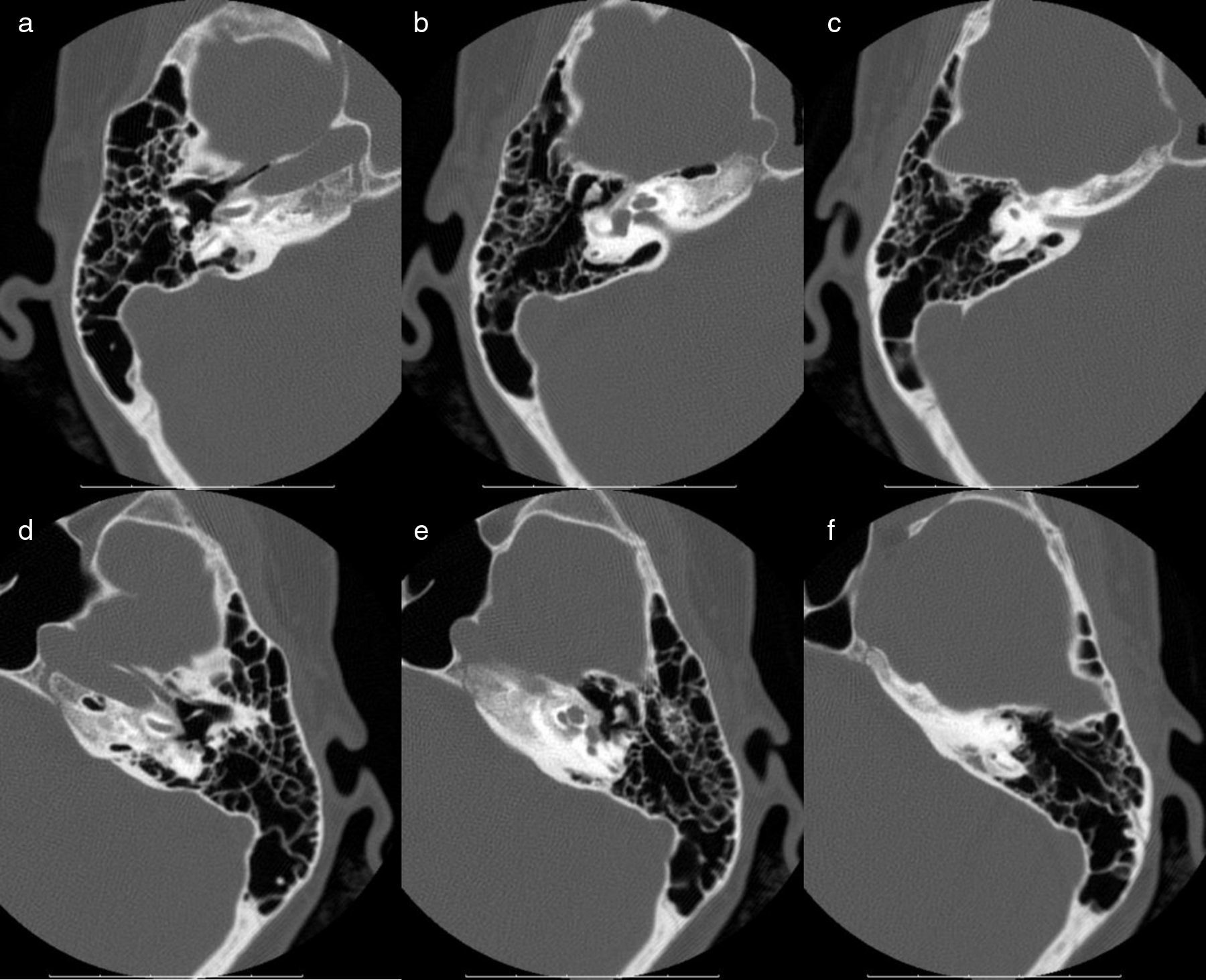
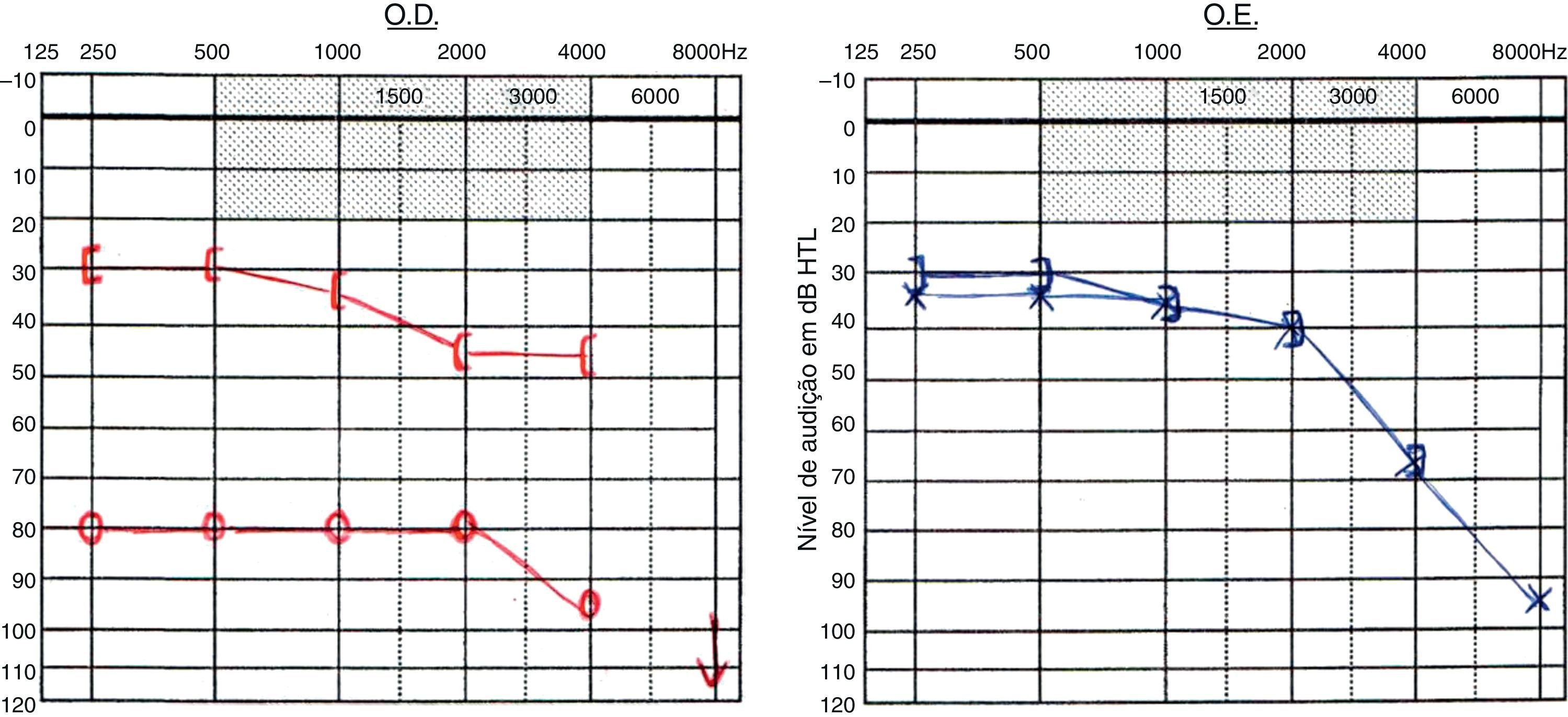
Osteogenesis imperfecta is the commonest connective tissue hereditary disease. Its clinical presentation has a wide spectrum of characteristics, which includes skeletal deformities and hearing loss. We describe three case reports of individuals carriers of this disease presenting with different patterns of hearing loss.
Hearing loss prevalence and patterns are variable and have no clear relation with genotype. Its assessment at initial evaluation and posterior monitoring is essential to provide the best therapeutic alternatives.
La osteogénesis imperfecta es la enfermedad hereditaria del tejido conectivo más frecuente. Su presentación clínica tiene un amplio espectro de características, que incluyen deformidades esqueléticas e hipoacusia. Se describen 3 casos clínicos de pacientes portadores de esta enfermedad, que se presentan con diferentes patrones de hipoacusia.
La prevalencia y los patrones de la hipoacusia son variables y no tienen una relación clara con el genotipo. Su evaluación en la exploración inicial y posterior seguimiento es esencial para ofrecer las mejores alternativas terapéuticas.