To analyze the volume of patients with chronic rhinosinusitis with nasal polyps (CRSwNP) to whom biologics would be prescribed based on the European and Spanish clinical practice guidelines, and to evaluate the impact that an increase of 1 required prior surgery (from 1 to 2) may have on the number of indications.
MethodsCross-sectional analysis evaluating the application of the European Position Paper on CRSwNP Guidelines (EPOS 2020) and the Spanish Consensus on the Management of CRSwNP Guidelines (POLINA 2.0) on an on-going prospective cohort study of consecutive patients with severe CRSwNP in a tertiary hospital.
ResultsFor a total of 103 patients with severe CRSwNP, 57.3% met EPOS 2020 criteria for biological treatment, whereas only 32% met POLINA 2.0 criteria. However, if the number of surgeries required to prescribe a biological therapy is increased to 2, only 31.1% of the patients would have indication of biological treatment, in any of the two guidelines.
ConclusionsThe differences among the POLINA 2.0 and the EPOS 2020 guidelines appear to have an impact in the proportion of patients eligible for biological therapies, with the former being stricter. Increasing the number of prior surgeries required, reduces the proportion of patients eligible for monoclonal antibodies prescription.
Comparar el porcentaje de pacientes con rinosinusitis crónica con pólipos nasales (RSCcPN) a quienes se les prescribirían tratamientos biológicos según las guías de práctica clínica europeas y españolas, y analizar el impacto que tendría un aumento de 1 cirugía previa requerida (de 1 a 2) en el número de indicaciones.
MétodosAnálisis transversal que evalúa la aplicación del Documento de Posicionamiento Europeo sobre las guías de RSCcPN (EPOS 2020) y el Consenso Español sobre el Manejo de RSCcPN (POLINA 2.0) en un estudio de cohorte prospectivo en curso de pacientes consecutivos con RSCcPN severa en un hospital terciario.
ResultadosDe un total de 103 pacientes con RSCcPN severa, el 57.3% cumplía con los criterios de EPOS 2020 para el tratamiento biológico, mientras que solo el 32% cumplía con los criterios de POLINA 2.0. Sin embargo, si se aumenta el número de cirugías requeridas para prescribir una terapia biológica a 2, solo el 31.1% de los pacientes tendría indicación de tratamiento biológico, según cualquiera de las dos guías.
ConclusionesLas diferencias entre las guías POLINA 2.0 y EPOS 2020 parecen tener un impacto en el porcentaje de pacientes elegibles para terapias biológicas, siendo la primera más estricta. Aumentar el número de cirugías previas requeridas reduce la proporción de pacientes elegibles para anticuerpos monoclonales.
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) is an inflammatory disease that affects 0.5–4.5% of the European population1 and up to 40% of them have an uncontrolled and refractory disease.2 Many of the patients with severe CRSwNP also have other comorbidities which worsen the burden of the disease, such as asthma, aspirin-exacerbated respiratory disease (AERD) and allergic rhinitis (AR). The standard-of-care for CRSwNP consists of saline nasal irrigations and intranasal corticosteroids (INCS). If these treatments are ineffective, oral corticosteroids courses or endoscopic sinus surgery (ESS) are indicated.
Classically, relapse rates for CRSwNP are estimated to range from 40–90%, contingent on comorbidities, such as asthma and AERD.3 Moreover, the reintervention index stands at approximately 20–40% over a 7.5-year and 12-year follow-up, respectively.4–6 Nevertheless, neither the criteria for indicating surgery nor the role that different types of surgery play in the disease relapse are well defined.5,7–9
In recent years, biological treatments against type-2 (T2) inflammation have been approved for patients with severe CRSwNP. These therapies, which were first used to treat patients with severe asthma, were seen to be effective on CRSwNP.10 In the last 20 years, several guidelines have been suggested for the management of the disease.11 The first international clinical guideline which provided some criteria for prescription and monitoring of the biological treatment in patients with CRSwNP was the consensus of the European Forum for Research and Education in Allergy and Airways Disease (EUFOREA) in 2019.12 Later, in February 2020, European Position Paper on Rhinosinusitis with Nasal Polyps Guidelines (EPOS 2020)1 defined more precisely their use and, from then on, different countries have established their own guidelines. These regional guidelines aim to adapt the international ones in the different settings they are addressed to13 and thus they might show differences with the general consensus. The Spanish Consensus on the Management of Chronic Rhinosinusitis With Nasal Polyps (POLINA 2.0 guideline) was published in 2023.14 It was developed in collaboration with Otorhinolaryngology, Pneumology, Allergology, Pharmacology, Pharmacy and General Practitioners societies, as well as the members of the Spanish association of patients with CRSwNP.
Both EPOS 2020 and POLINA 2.0 guidelines require at least one prior ESS for CRSwNP before prescribing biological therapy. In addition, the POLINA 2.0 guideline established one criteria as crucial to select patients who are eligible for biological treatment: severe impairment of quality of life (QoL), measured either with a Sino-Nasal Outcome Test (SNOT-22) higher than 50 points out of 110 or with a Visual Analog Scale (VAS) of the overall nasal symptoms higher than 7 out of 10, whereas the EPOS 2020 guideline considers this criteria non-obligatory (Table 1).
EPOS 2020 and POLINA 2.0 criteria for biological treatment in patients with severe bilateral nasal polyps who have undergone previous ESS*.
| Guideline | EPOS 2020 | POLINA 2.0 |
|---|---|---|
| Criteria required for the use of biologics | At least 3 additional criteria | VAS > 7 cm and/or SNOT-22 > 50 + At least 1 additional criteria |
| Additional criteria | Cut-off points | |
| Type 2 inflammation | Blood eosinophilia ≥ 250 cells/µl and/or IgE ≥ 100 UI/ml and/or tissular eosinophilia ≥ 10 eosinophils/high-power-field (eos/hpf). | Blood eosinophilia ≥ 300 cells/µl and/or IgE > 100 UI/ml and/or tissular eosinophilia ≥ 10 eos/hpf. |
| Significant loss of smell | Anosmia (smell test) | VAS of smell loss > 7 cm or severe hyposmia/anosmia (smell test) |
| Need for oral oral corticosteroids or contraindication to systemic corticosteroids | >2 courses per year or long term >3 mo. | ≥2 courses per year (5 days at a dose of 0.5–1 mg/kg/day) |
| Comorbidities | Diagnosis of comorbid asthma needing regular inhaled corticosteroids. | Diagnosis of comorbid asthma and/or AERD needing regular inhaled corticosteroids. |
| Impairment of quality of life | SNOT-22 ≥ 40 | |
The European Medicines Agency (EMA) approved Dupilumab in 2019 as add-on therapy with INCS for the treatment of patients with CRSwNP for whom systemic corticotherapy and/or surgery does not provide adequate control, followed by Omalizumab in 2020 and Mepolizumab in 2021.15 In Spain Mepolizumab and Dupilumab are currently approved by the Spanish Agency for Medicines and Medical Devices (AEMPS) for patients with CRSwNP who had undergone at least 2 prior EES.16
In 2022, in coordination with the Multidisciplinary Team of the Severe Asthma Unit of our hospital, the Department of Otorhinolaryngology developed and implemented a monographic clinic (BIOL clinic) for patients with severe CRSwNP candidates for biological treatment.
The aim of this study was to compare the proportion of patients with CRSwNP to whom biologics would be prescribed based on the European and Spanish guidelines, as well as to analyze the impact that an increase of 1 required prior surgery (from 1 to 2) may have on the number of indications.
Materials and methodsStudy design and populationWe performed a cross-sectional analysis of an on-going prospective cohort study of consecutive patients with primary chronic rhinosinusitis with nasal polyps visited at BIOL clinic at a tertiary hospital in Spain, from February 2022 until February 2024. Patients were referred from the general Otorhinolaryngology outpatient clinics, as well as from the Allergology and Pneumology outpatient clinics.
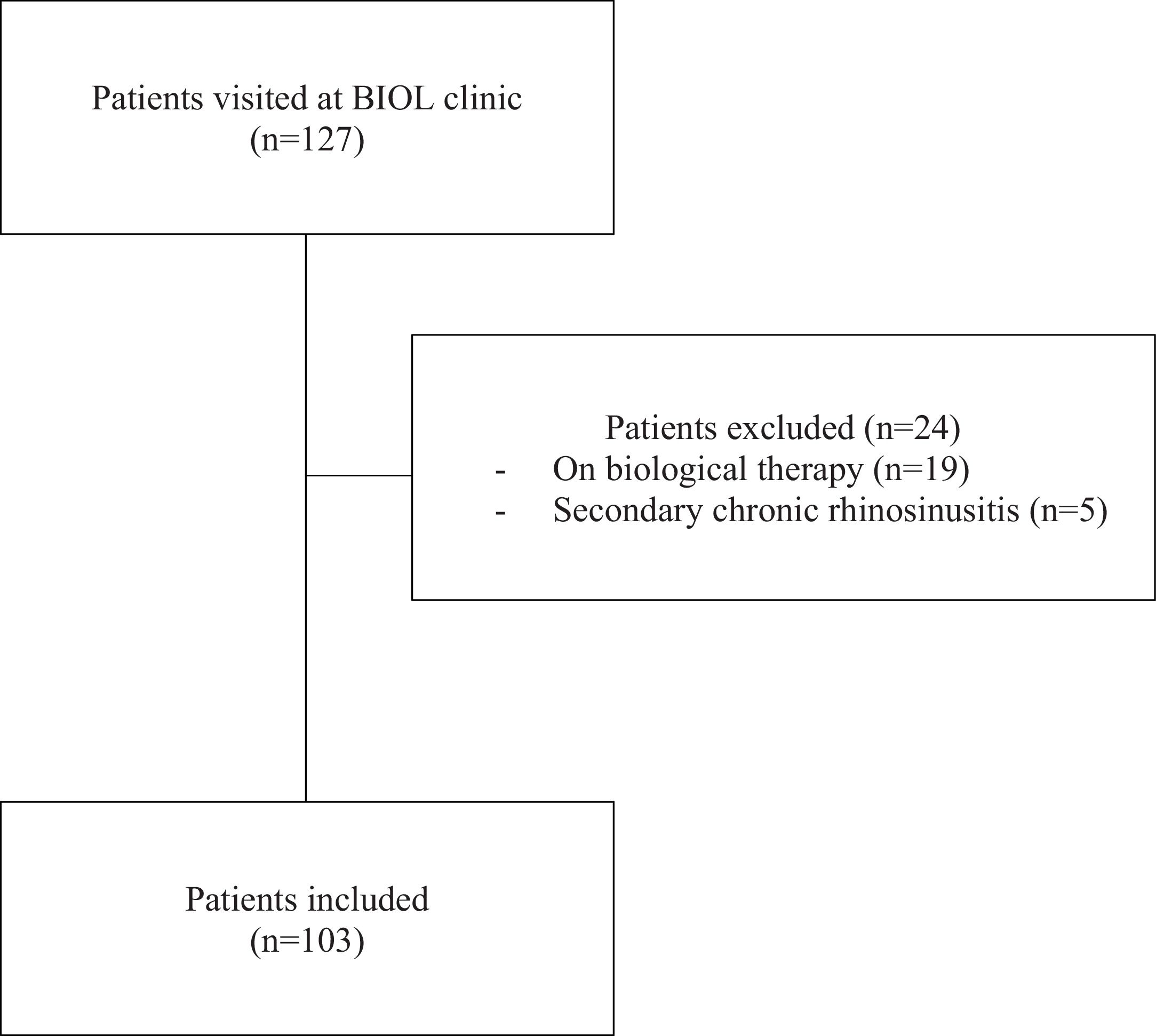
Exclusion criteria for this analysis were patients with secondary chronic rhinosinusitis17 and patients who were already being treated with biological therapy for T2 inflammatory respiratory disease at baseline (Fig. 1).
Data collectionData on demographics, comorbidities and laboratory tests were collected. In order to assess the severity of the disease, the following data was collected: VAS of overall nasal symptoms (range 0–10); SNOT-22 test (range 0–110); endoscopic nasal polyp score (range 0–8); inflammation type-2 markers (total IgE, blood eosinophilia and tissular eosinophilia); nasal peak inspiratory flow (NPIF), and loss of smell assessment by Sniffin’ Sticks Smell Test (SSST, range 1–48) (Burghardt®, Wedel, Germany) and by VAS (range 0–10). In patients with comorbid asthma, the Asthma Control Test (ACT, range 5–25) was also evaluated.
DefinitionsCriteria to be eligible for biological therapy were evaluated with both EPOS 20201 and POLINA 2.014 guidelines (Table 1).
On the one hand, in the EPOS 2020 guidelines, bilateral nasal polyps (NP) and at least one prior ESS are required to be eligible for biologic treatment. In addition, at least three of the following criteria have to be met: evidence of type 2 inflammation (tissular eosinophilia ≥ 10 eosinophils/high-power-field (hpf) and/or total IgE ≥ 100 Ul/ml and or blood eosinophilia ≥ 250 cells/µl); significant loss of smell (anosmia on smell test); comorbid asthma needing regular inhaled corticosteroids; significantly impaired QoL (SNOT ≥ 40), or either need for ≥ 2 courses of systemic corticosteroids per year or long term use (> 3 mo). In patients without prior surgery due to comorbidities, 4 of the above criteria are required.
On the other hand, POLINA 2.0 requires that a patient has undergone at least one prior ESS, has bilateral NP and has a detrimental QoL (SNOT > 50 or VAS of overall symptoms > 7). In addition, at least one of the following criteria has to be met to indicate a biological therapy: evidence of type 2 inflammation (tissular eosinophilia ≥ 10 eosinophils/hpf and/or total IgE > 100 Ul/ml and or blood eosinophilia ≥ 300 cells/µl); significant loss of smell (VAS > 7 or severe hyposmia/anosmia on smell test); comorbid asthma and/or AERD needing regular inhaled corticosteroids, or need for systemic corticosteroids ≥2 courses per year.
Statistical analysisCategorical variables were expressed as frequencies (n) and percentages (%). Quantitative variables were assessed for normal distribution with Kolmogorov-Smirnov test; those with normal distribution were expressed as mean and standard deviation (SD), while non-normal distributed variables were expressed as median and interquartile range (25th–75th percentile). The Student T test was used to compare quantitative variables with normal distribution. All statistical analyses were performed using SPSS version 27.0.1.0.
Ethical aspectsThe research was conducted in accordance with both Declarations of Helsinki and Istanbul. The study protocol was approved by the Ethics Committee of Bellvitge University Hospital in September 2023 (protocol code EOM024/23). All patients included in the study signed an informed consent for data collection and analysis, in accordance with the Regulation of the European Parliament EU 20216/697.
ResultsPatient populationOne hundred and twenty-seven patients were visited at the BIOL clinic during the study period. Of those, 24 were excluded from this study because they were either already receiving biological treatment for a different indication or had secondary chronic rhinosinusitis. One hundred and three patients were included in this study (Fig. 1). Seventy-five (72.8%) patients had previously been diagnosed with asthma and 27 (26.2%) had AERD. Most patients included (n = 82, 80%) had undergone at least one prior surgery, and up to 43 (42.2%) had had 2 or more. Patients’ demographics, main clinical characteristics and associated comorbidities are shown in Table 2.
Patient’s characteristics.
| Variables | Total, n = 103n (%) |
|---|---|
| Females | 45 (43.7) |
| Age, years, mean (SD) | 52.2 (11.1) |
| Ethnicity, caucasian | 92 (89.3) |
| BMI, kg/m2, median (IQR) | 26.2 (23–30) |
| Current smoker | 12 (11.7) |
| Asthma | 75 (72.8) |
| AERD | 27 (26.2) |
| Prior EES ≥1 | 82 (80.4) |
| Prior EES ≥2 | 43 (42.2) |
| ACT, median (IQR) | 20 (12.7–22–0) |
| NPS, median (IQR) | 5 (3–6) |
| Inflammation type 2 markers* | 82 (79.6) |
| Blood eosinophilia, cells/µl, mean (IQR) | 400 (250–610) |
| Serum IgE, UI/ml, mean (IQR) | 138 (73.5–261.0) |
| Tissular eosinophilia ≥ 10 eos/hpf | 68 (89.6%) |
| VAS overall nasal symptoms, mean (IQR) | 7 (6–9) |
| NPIF, ml, mean (IQR) | 80 (50–105) |
| VAS loss of smell, mean (IQR) | 9 (9–10) |
| Lund- Kennedy, mean (IQR) | 5 (4.0–6–5) |
| SNOT-22, mean (SD) | 52.16 (22.58) |
| SSST, mean (IQR) |
|
Data expressed as frequencies and percentages in parenthesis unless otherwise stated.
BMI: body mass index; AERD: aspirin exacerbated respiratory disease; ACT: asthma control test; NPS: nasal polyp score; VAS: visual analogue scale; NPIF: nasal peak inspiratory flux; SNOT-22: sino-nasal outcome test 22 items; SSST: sniffin’ sticks smell test. *Defined by: Blood eosinophilia ≥ 250 cells/µl and/or IgE ≥ 100 UI/ml and/or tissular eosinophilia ≥ 10 eosinophils/high-power-field (eos/hpf).
We assessed potential combinations of criteria for indicating biological therapy based on both the EPOS 2020 and the POLINA 2.0 guidelines. This yielded 10 possible sets of criteria for the EPOS 2020 guideline and 4 for the POLINA 2.0. Results are displayed in Table 3.
Patients eligible for biological therapy implementing EPOS 2020 and POLINA 2.0 guidelines.
| Guideline | Criteria | n (%) |
|---|---|---|
| EPOS 2020 (≥1 prior surgery) | Smell-L + SNOT-22 ≥ 40 + T2 | 43 (41.7) |
| Smell-L + SNOT-22 ≥ 40 + Asthma* | 33 (32.0) | |
| Smell-L + T2 + Asthma* | 33 (32.0) | |
| SNOT-22 ≥ 40 + T2 + Asthma* | 27 (26.2) | |
| Smell-L + SNOT-22 ≥ 40 + OCS | 25 (24.3) | |
| Smell-L + T2 + OCS | 20 (19.4) | |
| SNOT-22 ≥ 40 T2 + OCS | 19 (18.4) | |
| Smell-L + Asthma* + OCS | 18 (17.5) | |
| SNOT-22 ≥ 40 + Asthma* + OCS | 18 (17.5) | |
| T2 + Asthma* + OCS | 14 (13.6) | |
| Total | 59 (57.3) | |
| POLINA 2.0 (≥1 prior surgery AND SNOT-22 > 50 or VAS > 7) | Smell-L | 33 (32.0) |
| T2 | 26 (25.2) | |
| Asthma* | 21 (20.4) | |
| OCS | 14 (13.6) | |
| Total | 33 (32.0) | |
| EPOS 2020 and/or POLINA 2.0 | Total | 63 (61.1) |
Results are expressed in absolute number (n) and in percentages (%).
Smell-L (smell loss): sniffin’ sticks smell test < 30 and/or visual analogue scale (VAS) of smell loss > 7; SNOT-22 (sino-nasal outcome test); T2 (inflammation type-2 markers): Blood eosinophilia ≥ 250 cells/µl and/or IgE ≥ 100 UI/ml and/or tissular eosinophilia ≥ 10 eosinophils/high-power-field; *Asthma in treatment with chronic inhaled corticosteroids; OCS (oral corticosteroids) >2 courses per year or long term >3 months.
Using the EPOS 2020 guideline, in a total of 59 patients (57.3%) a biological therapy would be prescribed. The combination of criteria with the highest number of candidates for biological therapy was that including severe impairment of the smell, T2 inflammation markers and SNOT-22 > 40 (43 patients [41.7%]).
When applying the POLINA 2.0 guideline, 33 patients (32%) would meet criteria for biological treatment. The combination of criteria that resulted in the highest number of potential biological treatment prescriptions was the addition of smell impairment based on VAS or SSST scores to the impairment of QoL.
Impact of increasing the number of required prior surgeriesAs previously stated, to prescribe biological therapy to patients with CRSwNP in Spain, the AEMPS requires that patients have undergone at least 2 ESS.16Table 4 shows the results applying the criterion of 2 previous interventions. Patients with a minimum of 2 prior ESS in our cohort (n = 43) exhibited a clear trend towards higher SNOT-22 scores compared to patients with only 1 prior surgery (n = 38) (mean SNOT-22: 58.49, SD ± 23.68 vs 50.00, SD ± 19.90, respectively [p = 0.087]).
Patients eligible for biological therapy if at least 2 prior surgeries are required. The results are also separated based on the application of EPOS 2020 and POLINA 2.0 guidelines.
| Guideline | Criteria | n (%) |
|---|---|---|
| EPOS 2020 | Smell-L + SNOT-22 ≥ 40 + T2 | 25 (24.3) |
| Smell-L + SNOT-22 ≥ 40 + Asthma* | 22 (21.4) | |
| Smell-L + T2 + Asthma* | 21 (20.4) | |
| SNOT-22 ≥ 40 + T2 + Asthma* | 19 (18.4) | |
| Smell-L + SNOT-22 ≥ 40 + OCS | 14 (13.6) | |
| SNOT-22 + Asthma* + OCS | 12 (11.7) | |
| Smell-L + Asthma* + OCS | 12 (11.7) | |
| Smell-L + T2 + OCS | 11 (10.7) | |
| SNOT-22 ≥ 40 + T2 + OCS | 11 (10.7) | |
| T2 + Asthma* + OCS | 10 (9.7) | |
| Total | 32 (31.1) | |
| POLINA 2.0 | Smell-L | 32 (31.1) |
| T2 | 25 (24.3) | |
| Asthma* | 21 (20.4) | |
| OCS | 14 (13.6) | |
| Total | 32 (31.1) | |
| EPOS 2020 and/or POLINA 2.0 | Total | 36 (35.0) |
Results are expressed both in absolute number (n) and as percentages (%).
Smell-L (smell loss): sniffin’ sticks smell test < 30 and/or visual analogue scale (VAS) of smell loss > 7; SNOT-22 (sino-nasal outcome test); T2 (inflammation type-2 markers): Blood eosinophilia ≥ 250 cells/µl and/or IgE ≥ 100 UI/ml and/or tissular eosinophilia ≥ 10 eosinophils/high-power-field; *Asthma in treatment with chronic inhaled corticosteroids; OCS (oral corticosteroids) >2 courses per year or long term >3 months.
When applying the EPOS 2020 guidelines with a minimum of 2 prior ESS, the indication for biologics decreases from 59 (57.3%) to 32 (31.1%) patients. Conversely, the number of patients eligible according to the POLINA 2.0 guideline remains stable (from 32.0% to 31.1%). Therefore, in the setting of a regulation requiring 2 prior surgeries, such as that of the Spanish healthcare system, the number of patients meeting criteria for biological therapy is similar regardless of the guidelines used. Currently, otorhinolaryngologists in Spain have the option of using either EPOS 2020 or POLINA 2.0 for indicating biological therapy. Consequently, we also assessed the number of patients from our cohort who would meet criteria for this treatment in any of the two guidelines. Sixty-three patients (61.1%) would qualify for biological therapy as per current guidelines, whereas only 36 (35%) would under the Spanish regulation of at least 2 prior ESS.
DiscussionAt present, we are entering a new phase in treating patients with CRSwNP with biological therapies, but their widespread use is restricted in many countries due to their high costs and the need for long-term administration. Various articles have compared international and regional guidelines for CRSwNP and its biological treatment,8,9,13,18,19 but to our knowledge, this is the first study comparing the application of the EPOS 2020 and the POLINA 2.0 guidelines to assess how many patients could benefit from biological treatment in a tertiary care hospital in Spain. In our study, 57% of patients considered would start receiving biologic drugs according to EPOS 2020, while only 32% would do so if POLINA 2.0 was used. These results highlight that POLINA 2.0 is more restrictive than EPOS 2020. The main difference among these two guidelines lies in the mandatory requirement of a higher SNOT-22 score in POLINA 2.0 (cutoff 10 points higher). It is important to highlight that the POLINA 2.0 guideline represents a broad consensus among a wide range of scientific societies, including those in otolaryngology, pulmonology, allergology, pharmacology, pharmacy, general medicine, and even representatives from the patient association for CRSwNP. This inclusive collaboration reflects the latest advances in research and clinical practice, with key contributions from studies such as the pivotal trials on biologics and systematic reviews on chronic rhinosinusitis with nasal polyps (CRSwNP),20 which have demonstrated the efficacy of targeted treatments in improving patient outcomes. This broad-based agreement lends significant credibility and strength to the guideline. The process of reconciling different perspectives from such a wide array of disciplines and stakeholders might have led to a more restrictive approach in its framework. However, this may not be the only contributing factor, as other influences or considerations might also have played a role, leaving the exact reasons unclear.
Regarding the specific combination of criteria, the one with the greatest potential to enroll candidates for biological treatment at EPOS 2020 is the one that includes severe loss of smell, type-2 inflammation markers and SNOT-22 ≥ 40. This is an interesting finding given that type-2 inflammation markers available in clinical practice are nonspecific and do not ensure the presence of type-2 inflammation disease.21,22 In addition, the severe impairment of the smell, which affects QoL the most, is a sensitive but nonspecific symptom of severe CRSwNP. Our results show that the smell loss is the secondary criteria that recruits the most patients using both guidelines. This finding is noteworthy and highlights the importance of emphasizing that achieving a substantial clinical improvement in olfactory function is a key outcome of biologic treatment. Although the SSST stands out as one of the most comprehensive tests to assess smell loss,23 it is time-consuming. On the other hand, the VAS test is quick but seems to be extremely subjective and lacks accuracy for inter-patient comparison. Although some studies indicate a correlation between the VAS and other psychophysical olfactory tests in patients with CRSwNP,24 it seems that the optimal approach would be to associate it with a threshold and identification test.19,25 Future studies should evaluate the cost-effectiveness of using one test or the other.
Regarding the requirement of prior surgeries to be eligible for biological treatment, it is yet to be determined the optimal number, if any, for which the benefits outweigh the risks.8 However, the requirement of a prior surgery, in the absence of contraindications, is common in most guidelines.19 The number of surgeries varies, ranging from a simple recommendation to a requirement of 3 in specific circumstances.8 Our results show that increasing the required number of surgeries (from at least 1 to at least 2) leads to a significant reduction in the percentage of patients that qualify for biological treatment according to the EPOS 2020 guideline, dropping from 57% to 31%. However, according to the POLINA 2.0 guideline, the reduction in percentage is negligible, with only a slight decrease, from 32% to 31%. In addition, we observed that patients who underwent 2 or more surgeries had a trend towards higher SNOT-22 scores compared to patients with 1 prior surgery. These findings suggest that an increase in the number of surgeries demanded may be equivalent to requesting higher SNOT-22 scores. Nevertheless, timing after surgery is an important factor to take into consideration. While the evidence may not yet be robust enough to define the most appropriate timing for initiation of biological treatment, it seems to be a critical factor to consider. Although patients with 2 or more surgeries may qualify for biologics under both guidelines, POLINA 2.0 mandates that patients experience significant symptom recurrence (SNOT-22 ≥ 50). In contrast, EPOS 2020 allows eligibility based on fulfilling three criteria (Table 1), enabling treatment immediately after the second surgery.
Moreover, our results indicate that in the setting of the Spanish regulation, which requires a minimum of 2 prior surgeries, the use of either guideline results in a similar number of indications. Additionally, assessing the indication of biological therapy with both guidelines, that is meeting criteria in one or the other, does not significantly increase the number of candidates (from 31% to 35%).
Our study has two main limitations that should be acknowledged. Firstly, the cohort of patients with CRSwNP treated at our BIOL clinic might differ from what general otorhinolaryngologists typically encounter, as most BIOL clinic patients present with more severe disease and have poor responses to medical treatments and surgeries. Consequently, our results might not be applicable to other settings apart from tertiary care. Secondly, the cross-sectional nature of the study does not allow for assessment of the impact of using either guideline in terms of clinical outcomes or costs. However, this was not the aim of the current study and, therefore, it should be evaluated in future studies with different designs. The main strengths of our study are its prospective methodology with inclusion of consecutive patients, reducing the risk for selection bias, and the exhaustive evaluation of clinical manifestations using various tests and scales.
ConclusionsThe differences among the POLINA 2.0 and the EPOS 2020 guidelines appear to have an impact in the percentage of patients eligible for biological therapies, with the former being stricter. Increasing the number of prior surgeries required, which seems to be equivalent to requesting higher SNOT-22 scores, reduces the proportion of patients eligible for monoclonal antibodies targeting T2 inflammation. However, the impact that these measures may have on clinical and economic outcomes should be comprehensively evaluated in future studies.
CRediT authorship contribution statementMireia Golet, Núria Padullés-Zamora, Alejandro Portillo, José María Caballero, Mariana Muñoz Esquerre, Joaquín Sastre, Isam Alobid, and Xavier González-Compta have all contributed substantially to the following aspects of the manuscript:
- -
Conception or design of the work, including acquisition, analysis, or interpretation of data, AND
- -
Drafting the manuscript or revising it critically for important intellectual content, AND
- -
Providing final approval of the version to be published, AND
- -
Agreeing to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors declare no financial interest related to the work submitted for publication.
The authors did not receive support from any organization for the submitted work. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
X.G.C has received honoraria for consultancy, conferences or clinical trials from AstraZeneca, Aldo Sanofi, GlaxoSmithKline, Merck and Novartis.
MG has received honoraria for conferences from Sanofi and GlaxoSmithKline.
J.S. has received honoraria for consultancy and conferences from Sanofi, GlaxoSmithKline, Faes Farma, Mundipharma, ALK, and Novartis.
I.A. has received honoraria for consultancy and conferences from Viatris, Roche, Sanofi, GlaxoSmithKline, MSD, Menarini, Salvat, Medtronic, Storz, Olympus and Novartis.
M.M.E has received honoraria for consultancy, conferences, clinical trials or grants from AstraZeneca, ALK-Abello, Chiesi, Ferrer, GlaxoSmithKline, Novartis, Regeneron, Palobiofarma, Sanofi and TEVA, and Sanofi.
The rest of the authors have no conflicts of interest to declare.
We would like to thank Marta Pulido for her thorough review of the manuscript. Her feedback and suggestions have improved the clarity of our work.
We also thank the CERCA Program/Generalitat de Catalunya for institutional support.