Vestibular schwannoma (VS) is a benign, slow-growing tumour originating in the 8th cranial nerve. The treatment includes microsurgery, stereotactic radiotherapy and conservative management of tumours with periodic radiological tests.
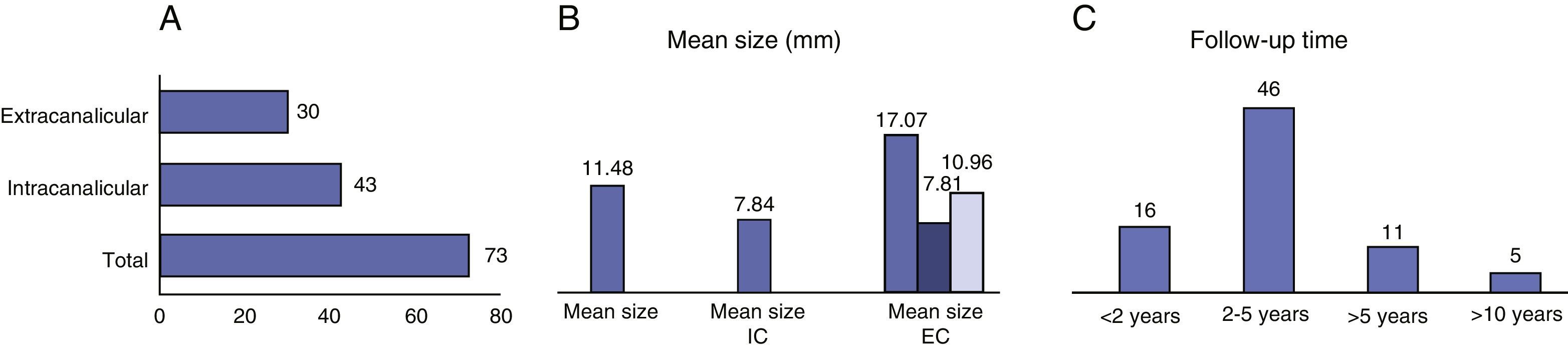
MethodsThis was a retrospective study of patients with VS following conservative management in a tertiary hospital between 1993 and 2013. A total of 73 patients were enrolled in our protocol. The mean age at diagnosis was 59.7 years. The average size was 11.9mm (4–27mm); 58.9% of the tumours were intracanalicular and 41.1%, extracanalicular. The mean follow-up period was 35.75 months.
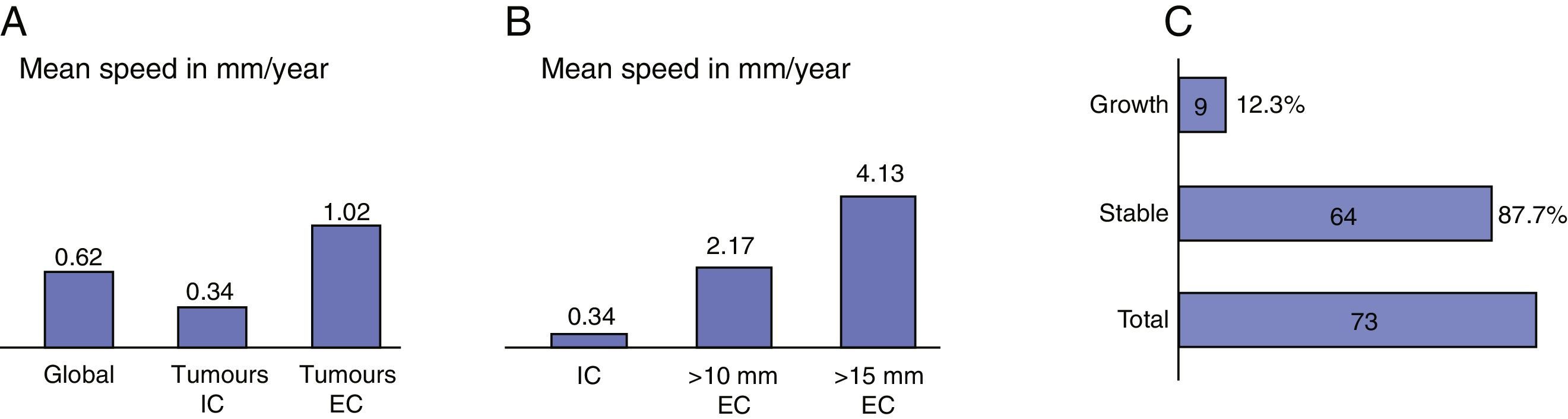
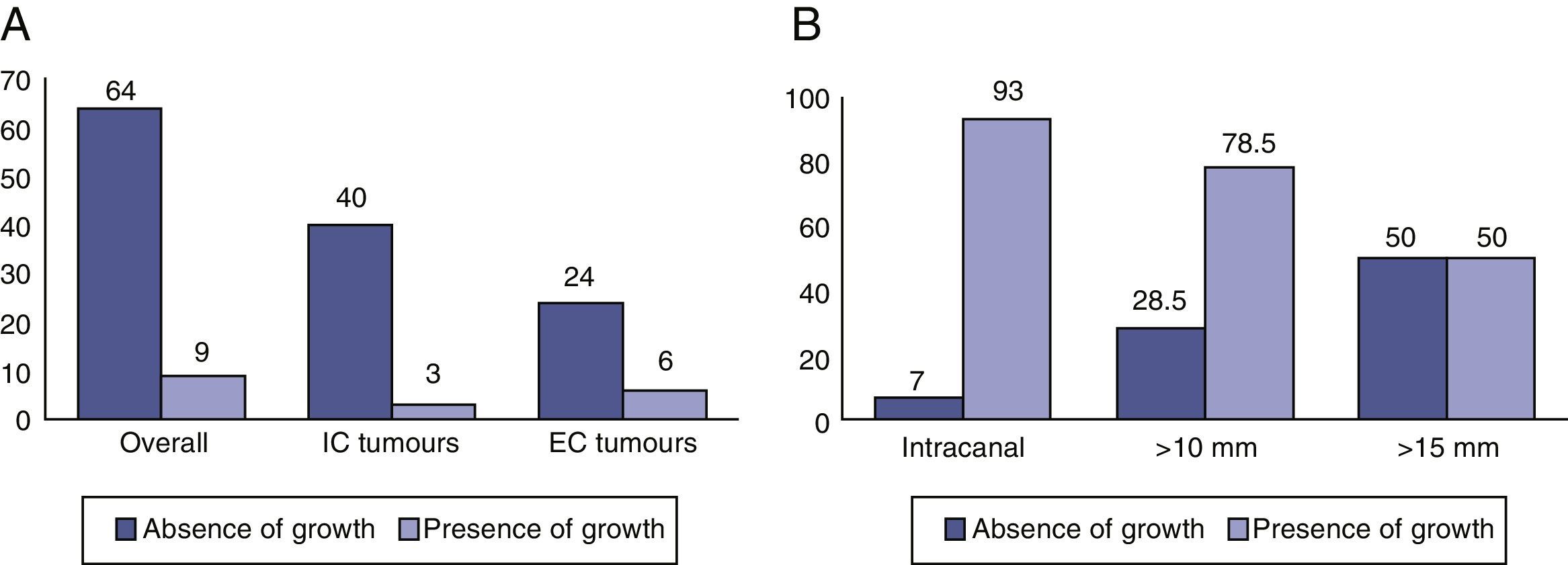
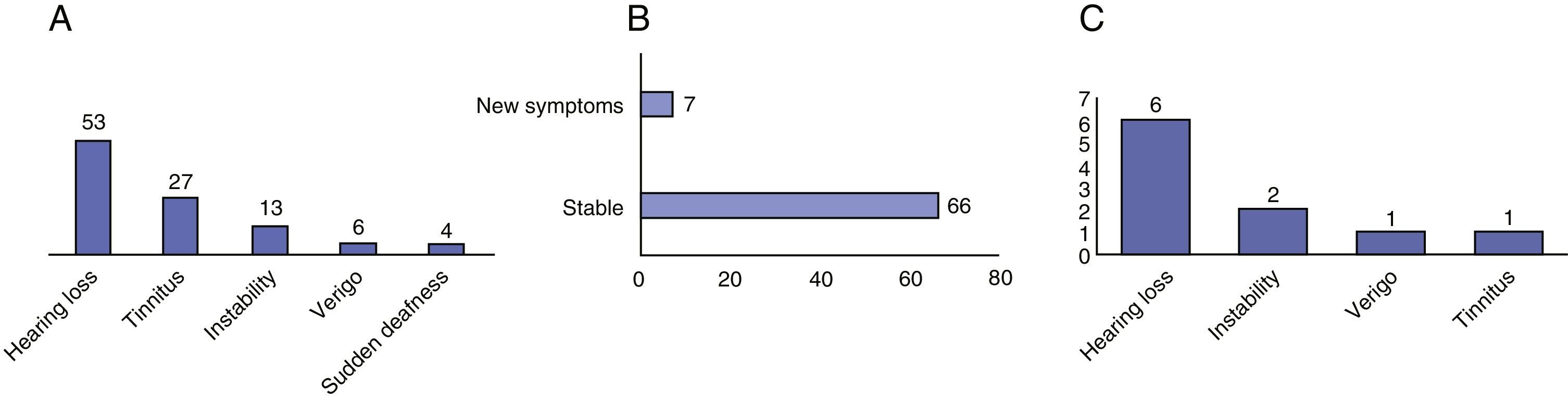
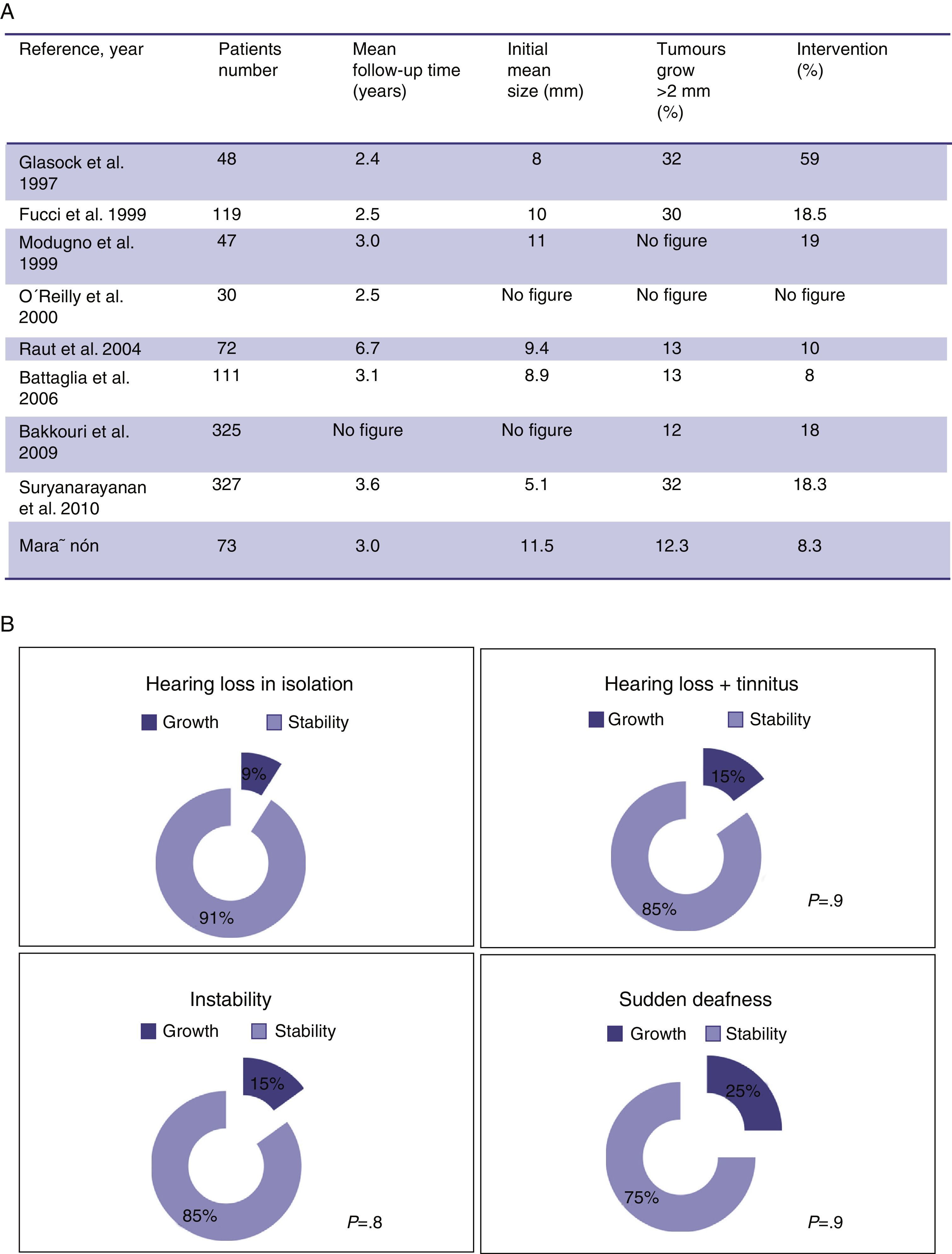
ResultsIn 87.7% of patients there was no evidence of tumour growth. A total of 9 tumours (12.3%) increased in size. The average growth rate was 0.62mm/year. The percentage of extracanalicular tumours that grew (20%) was higher than that of intracanalicular tumours (7%). Seven patients (9.5%) experienced significant changes in their symptoms and 6 of these (8.2%) experienced a loss of useful hearing. Six patients (8.2%) left follow-up and underwent surgery.
ConclusionsPeriodic monitoring of vestibular schwannomas with magnetic resonance imaging represents an option for management, because most small tumours experience little or no growth over time.
El schwannoma vestibular (SV) es un tumour benigno de lento crecimiento originado en el VIII par craneal, en cuyo tratamiento entran a formar parte la microcirugía, la radioterapia estereoatáxica, y el manejo conservador de los tumores con controles radiológicos periódicos.
Material y métodosEstudio retrospectivo de pacientes con SV siguiendo un manejo conservador en un hospital de tercer nivel entre los años 1993–2013.
Un total de 73 pacientes fueron incorporados a nuestro protocolo de seguimiento de SV. La edad media al diagnóstico fue de 59,7 años. El tamaño medio de 11,9mm (4–27mm), siendo el 58,9% intracanaliculares y el 41,1% extracanaliculares. El periodo de seguimiento medio fue de 35,75 meses.
ResultadosEn el 87,7% no hubo evidencia de crecimiento tumoral. Un total de 9 (12,3%) tumores incrementaron sus dimensiones. La velocidad media de crecimiento fue de 0,62mm/año. El porcentaje de tumores extracanal que crecieron (20%) fue mayor que el de los tumores intracanal (7%). Siete pacientes experimentaron cambios significativos en su sintomatología (9,5%) y 6 de estos una pérdida de la audición útil (8,2%). Seis pacientes salieron del seguimiento y fueron intervenidos quirúrgicamente (8,2%).
ConclusiónEl seguimiento del SV con controles periódicos de resonancia magnética nuclear representa una opción válida de manejo, dado que la mayoría de los tumores de pequeño tamaño experimentan poco o nulo crecimiento a lo largo del tiempo.