The treatment of active moderate-severe Graves’ ophthalmopathy (GO) is based on the administration of highdose intravenous glucocorticoids. The present study compares the efficacy and safety of 2 different intravenous methylprednisolone (MTPiv) dosing regimens.
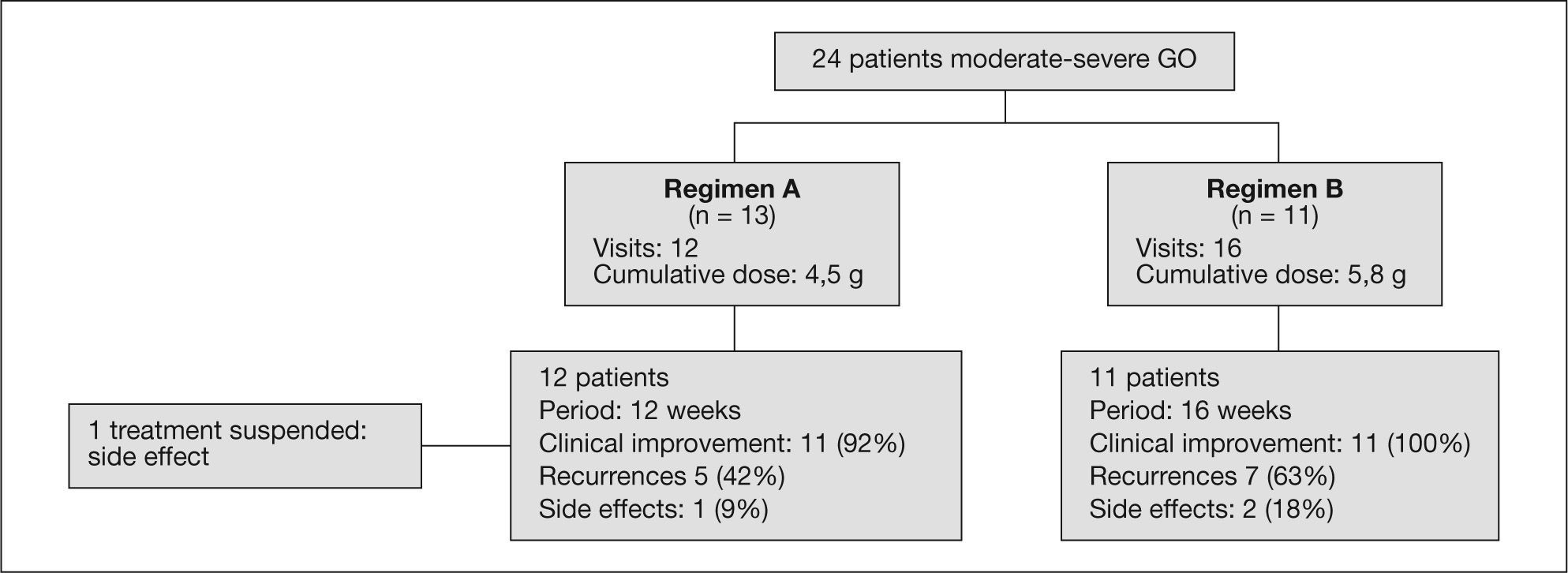
Material and methodsWe carry a retrospective descriptive study with sequential sampling of 24 patients (83% females) presenting moderatesevere GO (EUGOGO criteria) and receiving treatment in our center between January 2006 and June 2008. We use 2 dosing regimens: regimen A (12 weeks): 6 doses of 0.5g/week followed by 6 doses of 0.25g/week, for a cumulative dose of 4.5g of MTPiv (n=13); and regimen B (16 weeks): 4 cycles of 15mg/kg, followed by 4 cycles of 7.5mg/kg, for a cumulative dose of 90mg/kg (range, 4.9-7.4g) (n=11). Comparisons were made for safety (fasting glucose, cytolysis-cholestasis enzymes, lipid profile) and efficacy data (clinical improvement and recurrence).
ResultsMild-moderate liver cytolysis was recorded in four patients, one with associated moderate cholestasis and another with hyperglycemia, leading to treatment suspension – with no differences between the 2 treatment regimens. Percentage clinical improvement with regimen A was 92% (CI, 65-94%) versus 100% with regimen B (CI, 74-100%). The recurrence rate was 43% with regimen A and 63% with regimen B (p>0.05). None of the variables examined in the univariate logistic regression study were associated to a lesser treatment response or increased risk of recurrence of GO.
ConclusionsThe treatment of GO with MTPiv is safe and effective, with a lower recurrence rate when using dosing regimen A.
El tratamiento de la oftalmopatía de Graves (OG) moderadagrave se basa en la administración de corticoides por vía intravenosa. El presente estudio compara la eficacia y la seguridad de dos regímenes de tratamiento intravenoso con metilprednisolona (MTPiv).
Material y métodoSe realizó un estudio descriptivo, retrospectivo, con muestreo secuencial de 24 pacientes (el 83% mujeres) que presentaban OG moderadagrave (criterios EUGOGO) y recibieron tratamiento en nuestro centro entre enero de 2006 y junio de 2008. Se utilizaron los dos regímenes siguientes: A (12 semanas), 6 dosis de 0,5g/semana seguidas de 6 dosis de 0,25g/semana, con una dosis acumulada de 4,5g de MTPiv (n=13); B (16 semanas), 4 ciclos de 15mg/kg, seguidos de 4 ciclos de 7,5mg/kg, para una dosis acumulada de 90mg/kg (intervalo, 4,9-7,9g) (n = 11). Se compararon las variables de seguridad (glucemia basal, enzimas de colestasiscitólisis, perfil lipídico) y de eficacia (mejoría clínica y recurrencia).
ResultadosSe observó citólisis hepática de leve moderada en 4 pacientes, una de ellas asociada a colestasis moderada y otra a hiperglucemia, que determinaron la suspensión del tratamiento, sin diferencias entre regímenes. Hubo mejoría con el régimen A en el 92% (intervalo de confianza [IC] del 95%, 65-94) frente al 100% con el régimen B (IC del 95%, 74-100). La tasa de recurrencia fue del 43% con el régimen A y el 63% con el B (p>0,05). Ninguna de las variables analizadas en el estudio univariable de regresión logística se asoció a menor respuesta al tratamiento o mayor recurrencia de OG.
ConclusionesEl tratamiento de la OG mediante MTPiv es seguro y efectivo, con menor tasa de recurrencia con la dosificación del régimen A.
The treatment of active moderate-severe Graves’ ophthalmopathy (GO) is based on the administration of high-dose intravenous glucocorticoids (GCiv). Different studies have shown the greater efficacy and safety of GCiv compared with oral prednisone1-4 and placebo5. As a result, the latest consensus report published by the European Group on Graves Orbitopathy (EUGOGO)6 recommends as treatment of choice the administration of intravenous methylprednisolone (MTPiv) at doses of under 8 g, with a level I B of evidence and recommendation grade A. However, such therapy is not without complications, and fulminant liver failure may occur, apparently related to the GCiv dose accumulated in each cycle6-11.
The main objective of the present study is to compare the safety profiles of two different MTPiv dosing regimens. A secondary objective is assessment of the efficacy of the two treatment regimens.
MATERIAL AND METHODSWe performed a retrospective descriptive study of patients with GO treated with MTPiv in our center between January 2006 and June 2008.
PatientsSequential sampling was made of patients with moderatesevere GO treated in our center. The study included patients between 16-72 years old not previously subjected to immunosuppressor therapy, radiotherapy or eye surgery. We excluded patients with contraindications for MTPiv pulse therapy (heart arrhythmias, unexplained gastric pain, a history of pulmonary tuberculosis, active liver disease, HIV infection, uncontrolled hypertension). The diagnosis was based on the typical clinical characteristics of the disease, evaluating activity and severity according to the criteria established by the EUGOGO in 20086 (moderate to severe ophthalmopathy being defined by the presence of one or more of the following: palpebral retraction > 2 mm, moderate or severe soft tissue involvement, exophthalmos > 3 mm above normal for the race and sex, and inconstant or constant diplopia; sight-threatening GO like dysthyroid optic neuropathy or corneal rupture).
Treatment regimensTwo MTPiv regimens were compared. Regimen A, which is currently used in our center, involves the administration of 6 doses of 0.5 g/week followed by 6 doses of 0.25 g/week, for a cumulative dose of 4.5 g of MTPiv over 12 consecutive weeks3. Regimen B was used in our center until late 2006, and was based on the administration of four cycles of 15 mg/kg of MTPiv, followed by another four cycles of 7.5 mg/kg (each cycle consisted of two MTPiv infusions on alternate days —Monday and Wednesday— at two-week interval), with a cumulative dose of 90 mg/kg (total administered dose per patient ranged between 4.9-7.4 g) of MTPiv over a period of 16 weeks1 (fig. 1).
Safety evaluationThe blood samples were collected after a 10-hour fasting period, with quantification of the following before and after treatment: blood glucose, liver cytolysis enzymes (aspartate aminotransferase [ASAT], alanine aminotransferase [ALAT]) and lipid profile (total cholesterol, LDL-cholesterol, triglycerides), based on the VisUV Coban 711® enzyme-photometric assay; and cholestasis markers (gamma-glutamyl transpeptidase [GGT], alkaline phosphatase [AP]), based on the VisUV Coban 711® kinetic-photometry technique. The determination of anti-TSH receptor antibodies (TRACK) was carried out by Medizym® TRA enzyme immunoanalysis (reference value [vr] < 14 U/l), while antiperoxidase antibodies (antiTPO) were assessed with Axsym Abbott® electro-chemiluminescence (vr < 34 U/ml).
Efficacy evaluationAll patients were evaluated by the same endocrinologist before and after treatment. We establish a cutoff point for repeat evaluation of 11 weeks (range, 6-24), to evaluate the primary study objective (safety). Because of such early evaluation, many patients had not yet been re-evaluated by the Service of Ophthalmology. For this reason, clinical improvement was assessed on the basis of subjective perception of both, the patient and the Endocrinologist, registered in the case history. We recorded too the cases of recurrence and the need for additional MTPiv cycles or posterior radiotherapy or eye surgery.
Statistical analysisThe study results were analyzed on an intent-to-treat (ITT) basis, using the SPSS version 15.0 statistical package. The quantitative variables were analyzed with the Mann–Whitney U-test. The quantitative variables corresponding to paired samples in turn were analyzed with the Wilcoxon test. The χ2 test was used to evaluate differences between the categorical variables in 2 × 2 contingency tables. Univariate logistic regression analysis was performed to identify the risk factors associated with the absence of treatment response and a greater probability of GO relapse. Statistical significance was considered for p < 0.05.
RESULTSTwenty-four patients (17% males and 83% females) with a mean age at the start of treatment of 45.3 ± 14.7 years were included in the study. Of these subjects, one presented euthyroid Graves’ disease (4%; 95% CI, 1-20), two suffered Hashimoto's disease (8%; 95% CI, 2-26), and the rest presented Graves’ disease with hyperthyroidism (88%; 95% CI, 69-96). All patients presenting Graves’ disease with hyperthyroidism received antithyroid therapy, associating radioiodine in 6 cases, surgery in 4, and all 3 therapeutic modalities in one patient. Radioiodine administration preceded the development of GO in 2 patients (in which GO developed 18 and 88 months after treatment), while another 4 patients presented moderate GO at the time of radioiodine therapy. During follow-up after treatment with MTPiv, one patient required metabolic therapy with radioactive iodine to control the thyroid disease.
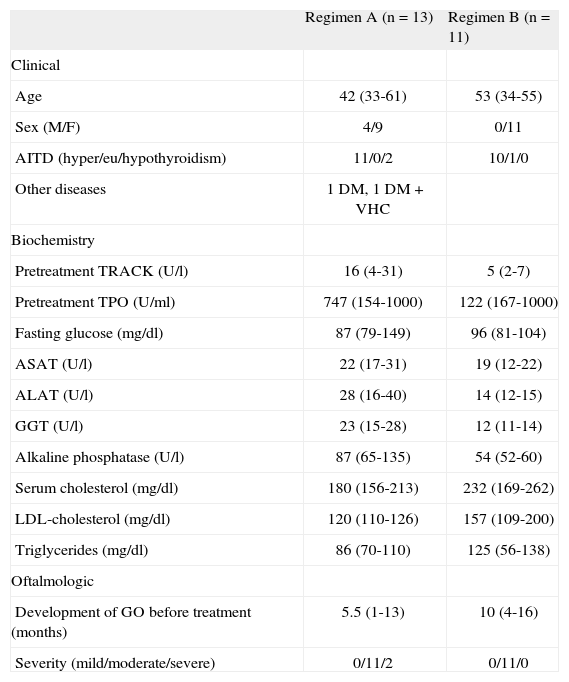
There were no significant differences in baseline characteristics between the 2 groups. All patients receiving dosing regimen B were women, while regimen A included 4 men (table 1).
Baseline characteristics of the study population
| Regimen A (n = 13) | Regimen B (n = 11) | |
| Clinical | ||
| Age | 42 (33-61) | 53 (34-55) |
| Sex (M/F) | 4/9 | 0/11 |
| AITD (hyper/eu/hypothyroidism) | 11/0/2 | 10/1/0 |
| Other diseases | 1 DM, 1 DM + VHC | |
| Biochemistry | ||
| Pretreatment TRACK (U/l) | 16 (4-31) | 5 (2-7) |
| Pretreatment TPO (U/ml) | 747 (154-1000) | 122 (167-1000) |
| Fasting glucose (mg/dl) | 87 (79-149) | 96 (81-104) |
| ASAT (U/l) | 22 (17-31) | 19 (12-22) |
| ALAT (U/l) | 28 (16-40) | 14 (12-15) |
| GGT (U/l) | 23 (15-28) | 12 (11-14) |
| Alkaline phosphatase (U/l) | 87 (65-135) | 54 (52-60) |
| Serum cholesterol (mg/dl) | 180 (156-213) | 232 (169-262) |
| LDL-cholesterol (mg/dl) | 120 (110-126) | 157 (109-200) |
| Triglycerides (mg/dl) | 86 (70-110) | 125 (56-138) |
| Oftalmologic | ||
| Development of GO before treatment (months) | 5.5 (1-13) | 10 (4-16) |
| Severity (mild/moderate/severe) | 0/11/2 | 0/11/0 |
ALAT: alanine aminotransferase; ASAT: aspartate aminotransferase; AITD: autoimmune thyroid disease; DM: diabetes mellitus; F: female; GGT: gamma-glutamyl transpeptidase; GO: Graves’ oftalmopathy; HCV: hepatitis C virus; LDL: low density lipoproteins; M: male; TPO: antiperoxidase antibodies; TRACK: anti-TSH receptor antibodies. Median (percentile 25-75) and absolute number.
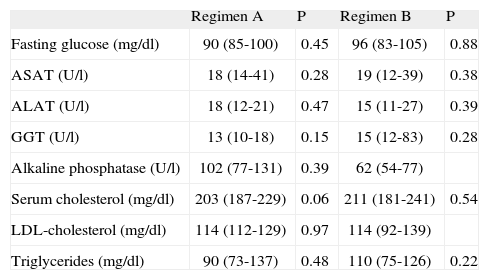
MTPiv was well tolerated, with no detection of immediate side effects. Blood pressure remained stable during treatment. No significant differences were seen in the biochemical parameters at baseline or after the administration of both regimens – with the exception of a decrease in GGT concentration with regimen A (table 2). There was no correlation between the cumulative dose of MTPiv and the ASAT/ALAT ratio.
Biochemical characteristics following intravenous methylprednisolone treatment
| Regimen A | P | Regimen B | P | |
| Fasting glucose (mg/dl) | 90 (85-100) | 0.45 | 96 (83-105) | 0.88 |
| ASAT (U/l) | 18 (14-41) | 0.28 | 19 (12-39) | 0.38 |
| ALAT (U/l) | 18 (12-21) | 0.47 | 15 (11-27) | 0.39 |
| GGT (U/l) | 13 (10-18) | 0.15 | 15 (12-83) | 0.28 |
| Alkaline phosphatase (U/l) | 102 (77-131) | 0.39 | 62 (54-77) | |
| Serum cholesterol (mg/dl) | 203 (187-229) | 0.06 | 211 (181-241) | 0.54 |
| LDL-cholesterol (mg/dl) | 114 (112-129) | 0.97 | 114 (92-139) | |
| Triglycerides (mg/dl) | 90 (73-137) | 0.48 | 110 (75-126) | 0.22 |
ASAT: aspartate aminotransferase; ALAT: alanine aminotransferase; GGT: gamma-glutamyl transpeptidase; LDL: low density lipoproteins. Median (percentile 25-75).
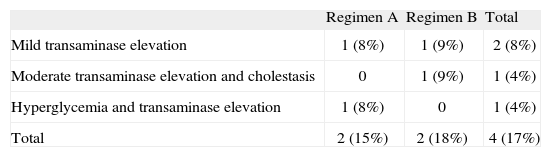
Side effects were recorded in 4 patients (table 3). Two patients of regimen A presented type 2 diabetes mellitus and one of these subjects, previously treated with insulin, was the only patient that needed to suspend the treatment after two cycles due to poor blood glucose control. Two patients (one belonging to each dosing regimen) presented transaminase elevation that did not double the normal value. Two patients showed transaminase elevation to 2-3 times the normal value: the diabetic patient in whom treatment was suspended and who proved positive for hepatitis C virus infection (regimen A), and a second patient who also showed liver cholestasis enzyme elevation with no associated comorbidity (regimen B). There were no significant differences in side effects between the two treatment groups.
Side effects observed in relation to intravenous methylprednisolone treatment
| Regimen A | Regimen B | Total | |
| Mild transaminase elevation | 1 (8%) | 1 (9%) | 2 (8%) |
| Moderate transaminase elevation and cholestasis | 0 | 1 (9%) | 1 (4%) |
| Hyperglycemia and transaminase elevation | 1 (8%) | 0 | 1 (4%) |
| Total | 2 (15%) | 2 (18%) | 4 (17%) |
Absolute number (percentage with respect to regimens A/B and total population).
Repeated assessment of ocular activity following the completion of treatment was carried out after a median of 11 weeks (percentile 25, 8; percentile 75, 16). The cumulative dose in regimen A was 4.5 g of MTPiv. Percentage subjective clinical improvement in regimen A was 92% (11 out of 12 patients; 95% CI, 65-94%). There were 5 recurrences in the group of patients that responded to treatment: 3 (25%) patients required an additional MTPiv cycle; 1 (9%) patient received orbital radiotherapy; and another patient (9%) required decompressive eye surgery in the course of follow-up.
The cumulative dose in regimen B was 5.76 g (percentile 25, 5.13; percentile 75, 6.12). In regimen B all patients experienced improvement of the symptoms and ophthalmological signs. However, 7 recurrences were recorded during follow-up: 5 (45%) patients required additional MTPiv cycles, including 2 (18%) patients who received a total of three MTPiv cycles, one of them also required orbital radiotherapy (having previously received 3 MTPiv cycles, with a total MTPiv cumulative dose of 15 g), and 2 (18%) patients were referred for orbital surgery and had received a single MTPiv treatment cycle. Regimen B showed a higher recurrence risk, although without statistical significance (odds ratio [OR] = 2.78; 95% CI, 0.48-16).
The univariate logistic regression analysis did not associate success or failure of MTPiv treatment during follow-up to any of the evaluated clinical variables (i.e., age, age over 50 years, sex, severity of GO, duration of the ocular disease, TRACK antibody titer, maximum TRACK titer > 40, TPO titer, maximum TPO titer > 1000, type of treatment prior to thyroid disease, impairment of visual acuity, diplopia prior to treatment).
DISCUSSIONThe present study confirms the safety of MTPiv pulse therapy in patients with moderate to severe GO. No serious adverse events were recorded in relation to MTPiv therapy. The percentage of treatment complications was 16%, which is lower than the previously reported in the literature (17-56%1,3,4). Maximum liver cytolysis enzyme elevation was recorded between 1.5-3 months after treatment1. This justifies the early time of re-evaluation of our patients, though stricter laboratory control could have detected a larger number of mild adverse events. In contrast to the work of Kahaly et al3, where no cytolysis enzyme elevation was recorded in patients treated with a cumulative dose of 4.5 g of MTPiv, in our series there were two cases of enzyme elevation with the same intravenous GC dose: in one patient the elevation was less than twice the upper limit of normal, while in the other patient (with positive hepatitis C serology) the increase was over three times the upper limit of normal.
Different studies have shown a relationship between the recorded side effects and an increased cumulative MTPiv dose per cycle1,3,4,7. However, in our series no such association was noted, since there were no significant differences in the number of adverse events between the 2 treatment regimens. This may be because the existing evidence suggests that most side effects occur with doses of over 6-8 g of MTPiv per cycle, and we used a maximum dose of 7.4 g. The existence of positive serological findings for hepatotropic viruses has been related to an increased presence of adverse events. In this same line, the only patient in our series with positive hepatitis C carrier status showed increased liver cytolysis enzyme levels, while a woman with markers indicating past hepatitis B virus infection showed no increase in cytolytic parameters. These observations justify the assessment of hepatitis C and B before starting MTPiv therapy in all patients with GO.
MTPiv pulse therapy (the sum of two regimens) in our series of patients with moderate GO proved to be effective, with an initial benefit (91%), superior to the reported in earlier studies with MTPiv as monotherapy (60%-77%3,5), although involving a relapse rate of 50% in the course of follow-up in our study. The baseline characteristics of our study population were similar to those described in the literature. The greater percentage of immediate benefit obtained in our series could be due to a less strict definition of the re-evaluation criteria. Earlier studies have reported a greater GC treatment response rate among females and in patients under 50 years of age that could be related to lesser severity of the ophthalmopathy3. These findings could justify the lesser initial response rate with regimen A, which included the four males in our study, although in the present series univariate logistic regression did not demonstrate it. The patients treated with regimen A required fewer additional treatments than those administered regimen B, despite the fact that the cumulative MTPiv dose was higher in the latter treatment group. This observation differs from the findings of other studies comparing intravenous GC regimens with other regimens involving the oral route, where the clinical improvement was related to the dose of GC administered3,5.
The limitations of the present study include a shorter follow-up of the patients of the regimen A than those given regimen B, which may have been a source of bias on interpreting the recurrence or reactivation data. We were also unable to compare the degree of disease activity objectively by means of the clinical activity scale, since the corresponding scores were not systematically documented in the patient case histories at the time of the evaluation cutoff point. Likewise, we had no data on the percentage of smokers nor on the presence of autoimmune hepatitis markers in all of our patients.
In conclusion, the treatment of active moderate-severe GO with MTPiv pulses is safe and effective with cumulative doses of under 8 g. Regimen A appears to involve fewer relapses, with a lesser cumulative corticoid dose (except in very low-weight patients), and it is more suitable to administer.