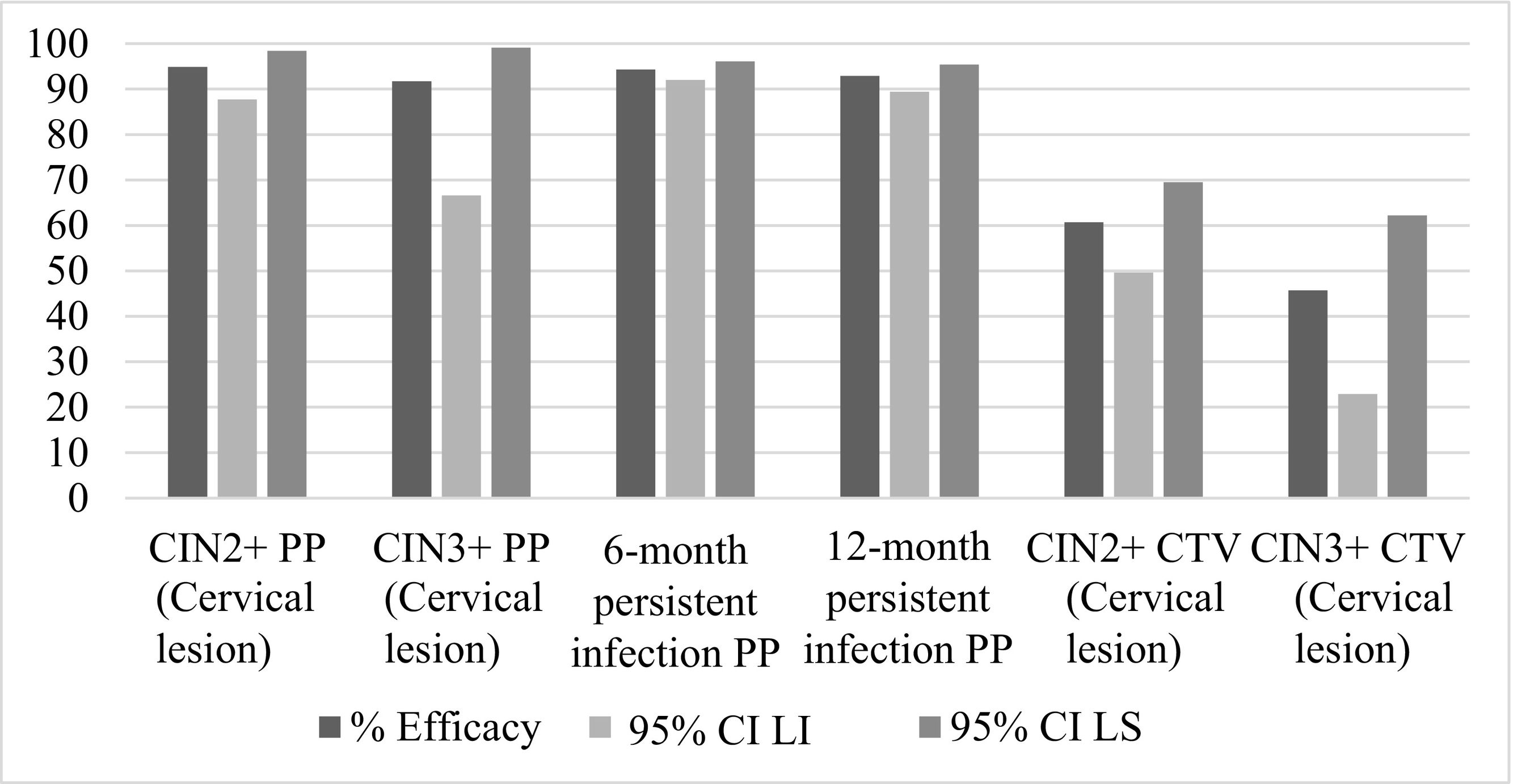
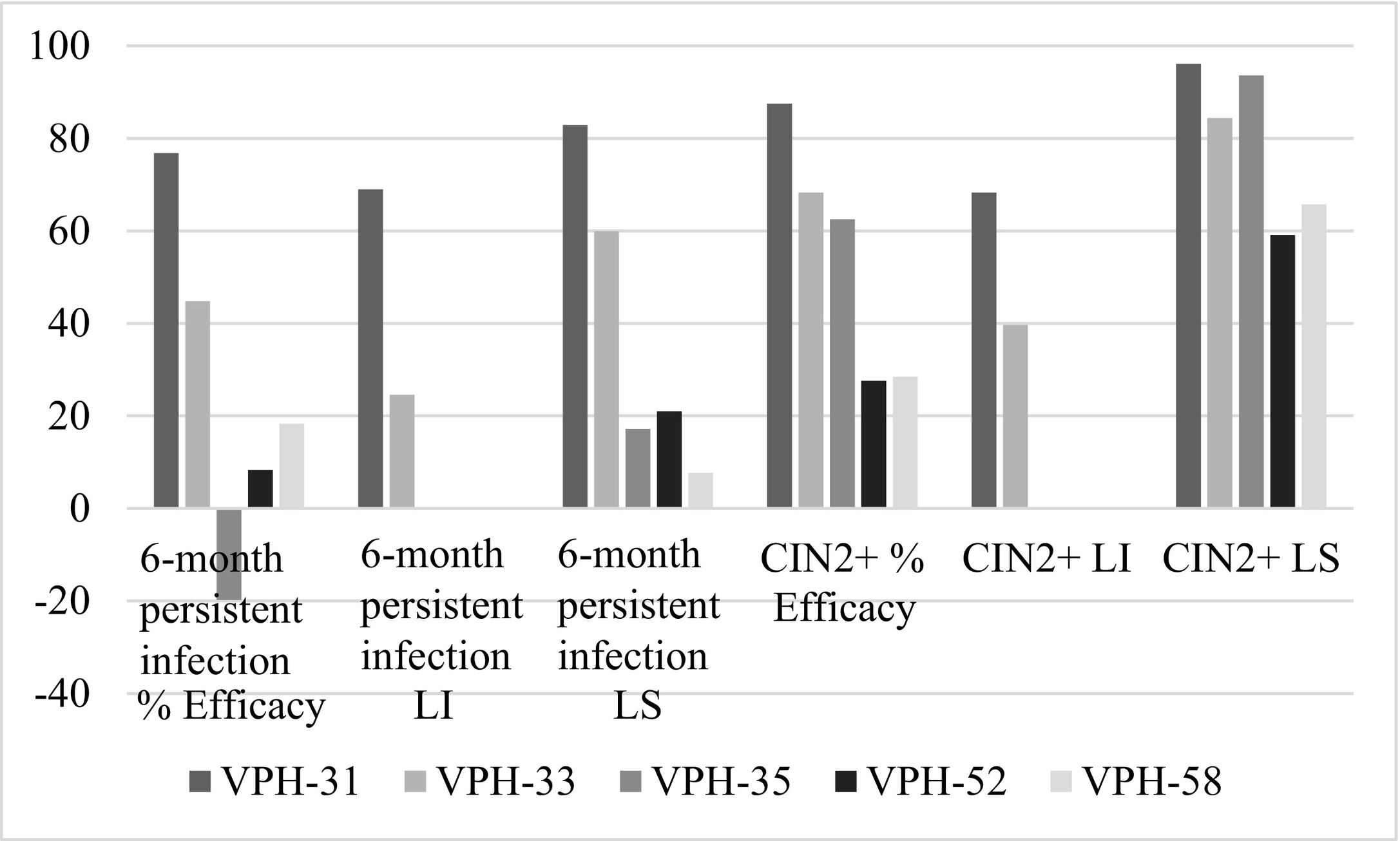
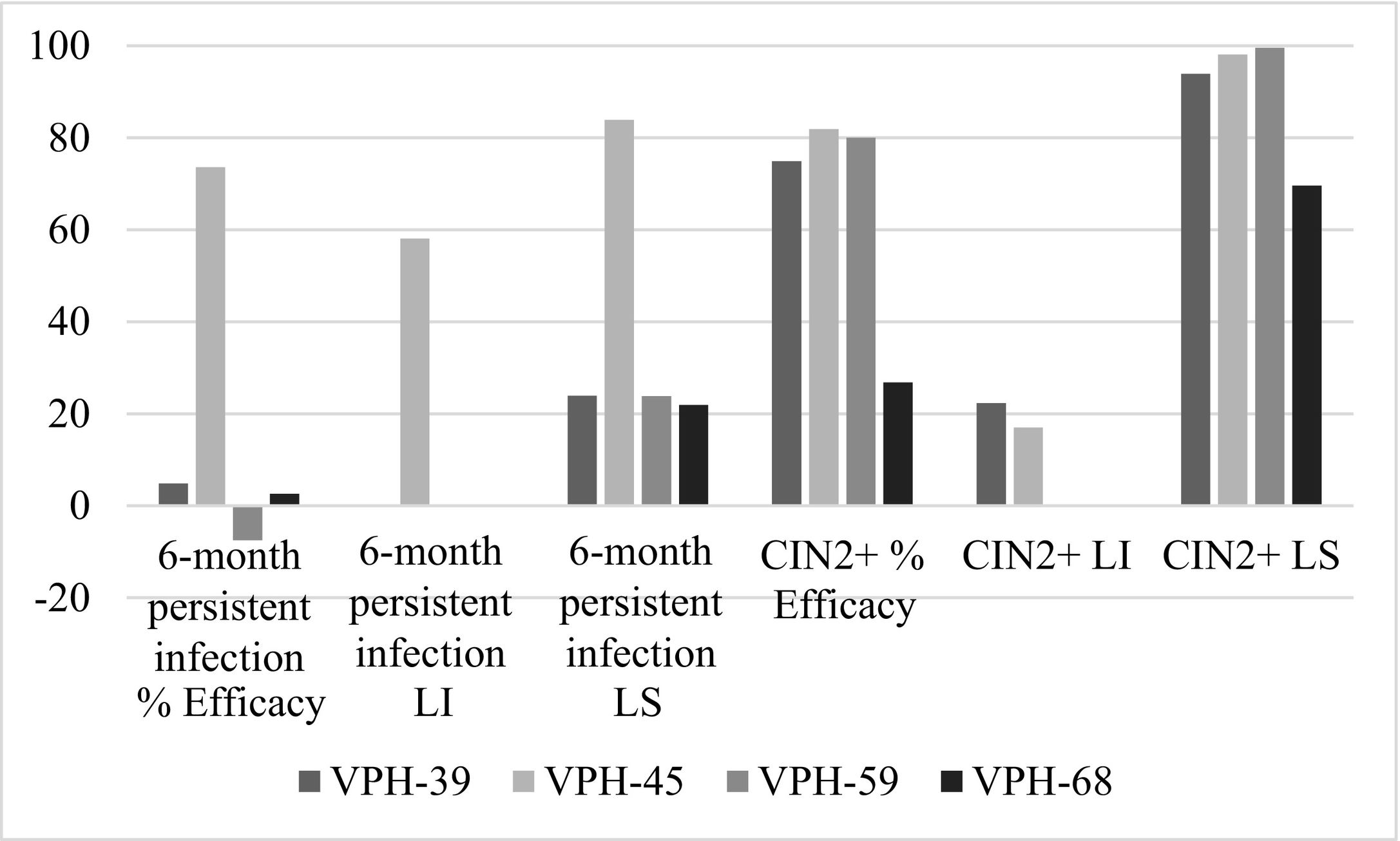
The inferential analysis of the pharmacological properties of the Cervarix® vaccine against papillomavirus reduces the risk of HPV-related cancers and high-grade precursor lesions, specifically cervical. The study methodology is quantitative, through different measurement-based procedures and is non-experimental. Using the Hope-Maximization Cluster, we seek to explain the efficacy of the Cervarix® vaccine in terms of entropy. The results show that the efficacy of Cervarix® vaccines to reduce the risk of CIN2+ and CIN 3+ was over 87.5% for HPV-31 and 81.9% against HPV-45, being in these typologies those with the highest efficacy at the end of the study control. The efficacy of vaccination was estimated at 91.7% and 94.9% associated with HPV-16/18 in CIN2+ and CIN3+ cervical lesions. Meanwhile, the efficacy is between 94.3% and 92.9% for persistent infections at 6 months and 12 months. Entropy decreasingly ranks the efficacy of Cervarix® in HPV-16/18 type lesions as follows: CIN3+ PP, CIN2+ PP, 12-month persistent infection PP, CIN3+ CTV, 6-month persistent infection PP, and CIN2+ CTV. Finally, patterns within the observations of vaccine efficacy against non-vaccine oncogenic HPV types; are explained by entropy in decreasing order: VPH-68/58/52/59/45/31/39/35/33. However, the best cluster accuracy is for HPV-31 and HPV-45 with 100%, followed by 83.33% for HPV-33.
El análisis inferencial de las propiedades farmacológicas en la vacuna Cervarix® contra el papilomavirus, reduce el riesgo de cánceres relacionado con el VPH y lesiones precursoras de alto grado, específicamente cervicales. La metodología de estudio es de tipo cuantitativo, a través de diferentes procedimientos basados en la medición y es no experimental. Empleando el Clúster de Esperanza-Maximización, se busca explicar la eficacia de la vacuna Cervarix® en términos de entropía. Los resultados muestran que la eficacia de las vacunas de Cervarix® para reducir el riesgo de CIN2+ y CIN 3+ fue de más del 87,5% para VPH-31 y 81,9% frente al VPH-45, siendo en estas tipologías las de mayor eficacia al finalizar el control de estudio. La eficacia de la vacunación se estimó en un 91,7% y 94,9% asociadas con VPH-16/18 en lesiones cervicales CIN2+ y CIN3+. En tanto, la eficacia está entre 94,3% y 92,9% para las infecciones persistentes a 6 meses y 12 meses. La entropía ordena en forma decreciente la eficacia de Cervarix® en las lesiones tipo para VPH-16/18, quedando así: CIN3+ PP, CIN2+ PP, Infección persistente a 12 meses PP, CIN3+ CTV, Infección persistente a 6 meses PP y CIN2+ CTV. Finalmente, los patrones dentro de las observaciones de eficacia de la vacuna frente a tipos oncogénicos no vacunales de VPH; son explicados por la entropía en el orden decreciente: VPH-68/58/52/59/45/31/39/35/33. Sin embargo, la mejor precisión del clúster es para VPH-31 y VPH-45 con el 100%, seguido por 83.33% para VPH-33.