Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosThe moment SARS-CoV-2 seemed to be receding; there was an uncertain emergence of Omicron variant which rapidly spread to all the 6 continents of the globe. The large number of genomic mutations has helped Omicron to evolve and become highly transmissible and escape the natural or vaccine-induced immune response. Until now, the Omicron has evolved into 5 unique lineages namely BA.1, BA.2, BA.3, BA.4, BA.5, and over 1000 sub-lineages. Despite vigorous COVID-19 immunisation programmes, India has been constantly being affected with emergence of new Omicron variants. In contrast to recovered patients following vaccination and breakthrough cases following a second dose against the Omicron variety, our recent research of naive Covishield vaccines showed declining immune response. The finding of this study and other studies with Covaxin depicted less immune response against Omicron post second dose of vaccination. This necessitates the administration of a preventive dose to improve immunity. There was surge in the COVID-19 cases with BA.5, BA.4, BF.7, BQ.1, XBB, and JN.1 infection which has greater transmissibility and vaccine efficacy remarkably dropped. Hence along with administration of booster dose, there is need to tweak the currently available vaccines with these SARS-CoV-2 variants. These types of modified boosters could provide enhances protection against SARS-CoV-2 infection.
En el momento en que el SARS-CoV-2 parecía estar remitiendo, se produjo la incierta aparición de la variante Omicron, que se propagó rápidamente por los seis continentes del planeta. El gran número de mutaciones genómicas ha ayudado al Omicron a evolucionar y convertirse en altamente transmisible y escapar a la respuesta inmunitaria natural o inducida por vacunas. Hasta ahora, el Omicron ha evolucionado en cinco linajes únicos: BA.1, BA.2, BA.3, BA.4, BA.5 y más de 1000 sublinajes. A pesar de los enérgicos programas de inmunización contra el COVID-19, India se ha visto constantemente afectada por la aparición de nuevas variantes del Omicron. En contraste con los pacientes recuperados tras la vacunación y los casos de avance tras una segunda dosis contra la variedad Omicron, nuestra reciente investigación de vacunados ingenuos contra Covishield mostró una respuesta inmune decreciente. El hallazgo de este estudio y otros estudios con Covaxin describieron una menor respuesta inmunitaria contra Omicron tras la segunda dosis de vacunación. Esto hace necesaria la administración de una dosis preventiva para mejorar la inmunidad. Hubo un aumento en los casos de COVID-19 con infección BA.5, BA.4, BF.7, BQ.1, XBB, y JN.1 que tiene mayor transmisibilidad y la eficacia de la vacuna disminuyó notablemente. Por lo tanto, junto con la administración de dosis de refuerzo, es necesario modificar las vacunas actualmente disponibles con estas variantes de SARS-CoV-2. Estos tipos de dosis de refuerzo modificadas podrían proporcionar una mayor protección contra la infección por SARS-CoV-2.
As the world battles the COVID-19 pandemic, a new variant, Omicron, has emerged, causing widespread concern. In November 2021, the Omicron variant was discovered for the first time in Botswana and South Africa1 and has since spread to other countries, including India.2 This variant is highly contagious and has a large number of mutations, which makes it more transmissible3 and potentially more resistant to vaccines.4 The discovery of this new variant has led to renewed efforts to control the spread of the virus, with many countries having imposed travel restrictions and increased their vaccination drives.
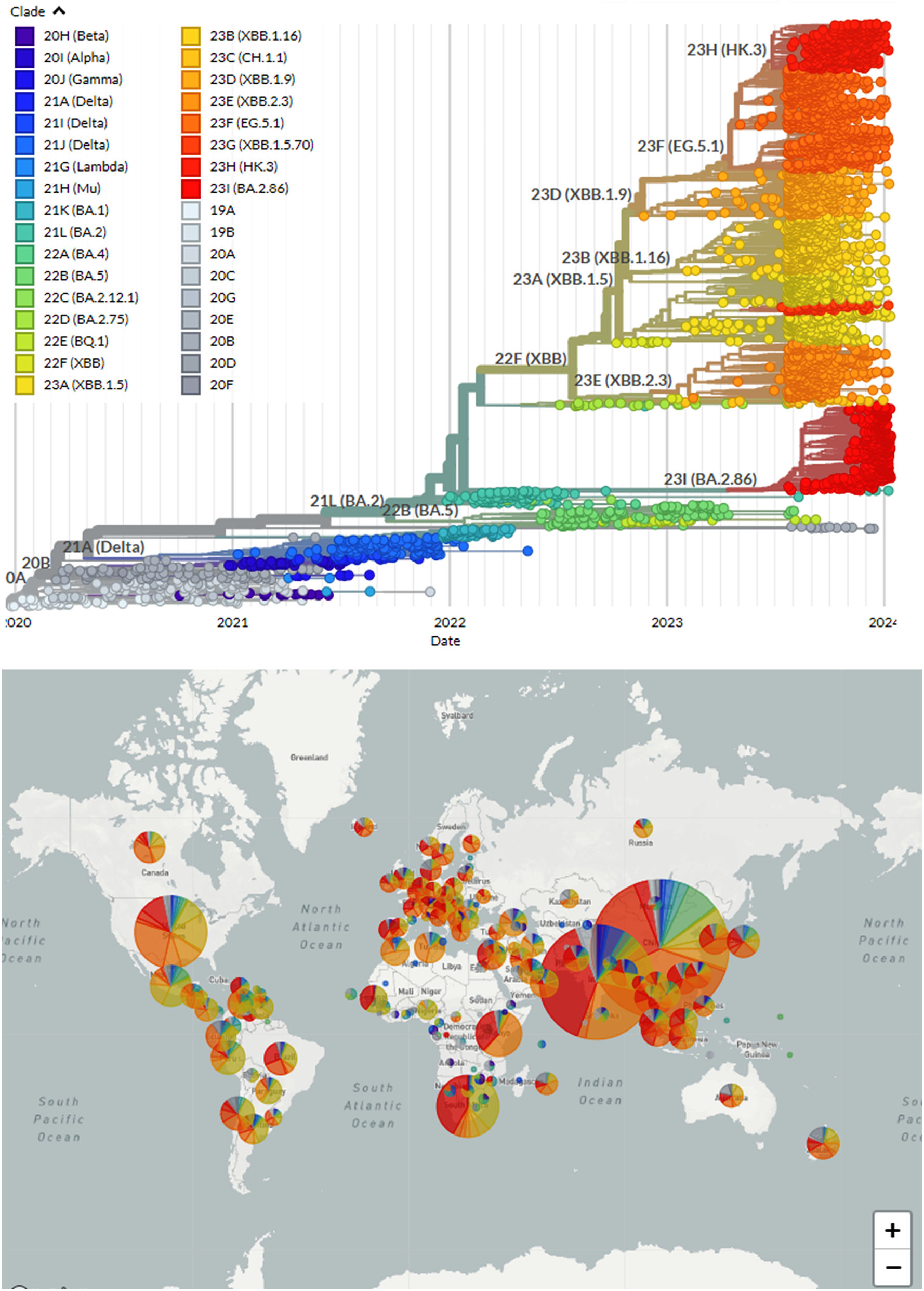
Alpha, Beta, Gamma, and Delta has roughly twice as many amino acids that differ from the wild type strain.5 Among the Omicron lineages, 39 mutations are shared with SARS-CoV-2's ancestral strain, and BA.1 and BA.2 differ by 28 mutations.6,7 The Omicron sub-variants currently include B.1.1.529, BF.7, BA.1, BA.1.1, BA.2, BA.3, BA.4, and BA.5 as well as BQ.1 and XBB and over 1000 sub-lineages.1,2,8–24 Among the sub-variants B.1.1.529, BA.1 and BA.3 are included; BA.1.1 and BA.2 are categorised separately.2,25 While BQ.1 is a sub-variant of BA.5 and has several constituent sub-variants,20 XBB is a recombinant sub-variant of BA.2.10.1 and BA.2.75.21 The recently identified SARS-CoV-2 variant BA.2.86 differs by more than 30 amino acids from BA.2, the dominant Omicron lineage in early 2022. In addition, BA.2.86 differs by around 35 amino acids from the more recently circulated XBB.1.5, which dominated the majority of 2023.26,27 In late 2023, JN.1 (BA.2.86.1.1) the descendant of BA.2.86 has emerged and showed a markedly increased prevalence in Europe, North America, France, and India.28,29 JN.1 harbours hallmark mutation S:L455S which contributed to increased transmissibility and immune escape ability.28 Variants of Omicron are likely to continue to emerge as the pandemic continues. It is more likely that an immunological response from a previous Omicron infection will not protect against a new variation as new sub-variants of Omicron emerges.30
India has already experienced the devastating impact of COVID-19, with over 45 million cases and more than 533 392 deaths reported as on 23 January 2024.31 Since the start of the pandemic, the emergence of VOCs like Alpha, Beta, Gamma, Delta, and the Omicron have been recorded in the country. The Delta variant caused a surge in infections during the year 2021,32 overwhelming healthcare systems and leading to shortages of oxygen and other medical supplies. The Omicron variants emerged at the end of 2021 and were quickly identified as being significantly more transmissible than Delta.1 The impact of Omicron was humongous; while it was less severe than Delta, it still caused a significant number of deaths worldwide.
India has experienced third wave of infection with Omicron variant. In December 2021, the first cases of Omicron variant were identified in Karnataka State. In India, there was no clear evidence of transmissibility, immune evasion, or severity at first. In January 2022, Omicron community transmission occurred and was primarily dominant in multiple metro cities, with BA.2 being the dominant lineage. While most of the Omicron cases have been asymptomatic or mild, hospitalisations and ICU admissions have increased in the current wave. As the situation rapidly evolved with community spread, the threat level remained high and required constant vigilance. Numerous previously existing BA.2 sequences were reclassified into the newly identified BA.2 sub-lineages BA.2.10 and BA.2.12. So far, these sub-lineages have not been connected to increasing disease severity. SARS-CoV-2 cases with BA.4 and BA.5 infection were reported from India in May 2021, but there was no surge in cases, and BA.2 remained dominant. The most prevalent sub-lineages in recent weeks seem to be the Omicron sub-variants BA.2 and BA.2.38. Additionally, the number of patients with the BA.5 variant infection has increased. However, there has been no increase in hospitalisations or reports of disease severity. BA.2, BA.2.10, BA.2.38, BA.5, BA.2.75, and BA.2.76 variants were discovered in July and August. During this time period, however, there was no increase in hospitalisations or disease severity.33
In September 2022, the SARS-CoV-2 infection rate has decreased across India. The most prevalent lineage BA.2.75 is the first, then BA.2.38 which declined since the previous week. BA.2, BA.2.X, and BA.5, have been also detected in small fraction. During this time period, there was no increase in hospitalisations or disease severity. In November, the overall infection rate was less than 500 per day. The most commonly circulating Omicron sub-lineage was discovered to be BA.2.75, BA.2.10, XBB, and its sub-lineages. In January, the presence of the BQ.1 sub-lineage was observed in Western and Southern India. Overall, XBB is the most common omicron variant sub-lineage (Fig. 1). Over the last week, some BF.7 sub-lineage occurrences have been observed in Eastern and Northern India. During this time, there has been no increase in disease severity or hospitalisation.33 Since December 2022, XBB sub-lineage was found to be the most prevalent omicron variant. The percentages of recombinant lineages around the world have been steadily rising. The recombinant variety XBB and its offspring lineages are increasingly common, which defines the global variant landscape.33
During the year 2023, the Omicron and its sub-lineages found to be the dominant variants in India. In January, the dominant variants were mainly XBB, BA.2.75, and BA.2.10, with the presence of BQ.1 sub-lineage was observed in various parts of India. No disease severity or hospitalisation was observed during this period. In March, a rise in infection was noted, especially in Western, Southern, and Northern India, with the emergence of a newly recombined variant, XBB.1.16, which accounted for 38.2% of the infections. In April, the upward thrust was observed in terms of infection, with XBB.1.16 accounting for 68.7% of the infections. The situation remained stable with no disease severity and hospitalisation. In May, June, and July, the dominance of XBB.1.16 continued. In August, the prevalence of XBB.1.16 reduced to 25.0%, with other XBB sub-lineages accounting for 75.0% of the infections. In September, the dominance of XBB and XBB.1.16 continued, with the identification of 4 cases of the EG.5.1 variation. In October, XBB remained the most common variant, and no rise in disease severity or hospitalisation was observed. Overall, the dominance of the recombinant variation XBB.1.16 was observed from 38.2% in March to nearly 70.0% in July, and lowering to 25.0% in August 2023. The emergence of different sub-lineages of Omicron, i.e., BQ.1, BA.2.75, BA.2.10, and EG.5.1 was observed during this period.33
The new Omicron sub variant BA.2.86 (Pirola) emerged in Denmark during August 2020 and now being reported from at least 33 countries across the globe.34 In late 2023, the descendant of BA.2.86, JN.1 (BA.2.86.1.1) is emerged. JN.1 has showed a markedly increased prevalence in Europe and North America, especially in France. Recently in November 2023, cases of JN.1 has been reported from India and found to be dominant variant. JN.1 harbours hallmark mutation S:L455S and 3 mutations in non-S proteins. The mutation in S:L455F had contributed to increased transmissibility and immune escape ability. This mutation enables JN.1 to evade Class 1 neutralising antibodies, leading to humoral immune evasion.28,29
Until now, India has detected the presence of over hundreds of Omicron sub-lineages in the community.30 The infection with Omicron sub-variants generally causes less severe disease than other prior variants. Regardless of the sub-variants, the clinical presentation seems to be similar to the original omicron lineage, which is mild and includes mild fever, sore throat, running nose, dry cough, body pain, vomiting, and mild diarrhoea. Only small percentage of the cases with no vaccination, co-morbidity and old age could develop severe illness and needs hospitalisation. This is why it becomes necessary to take proper precautions to avoid the infection.
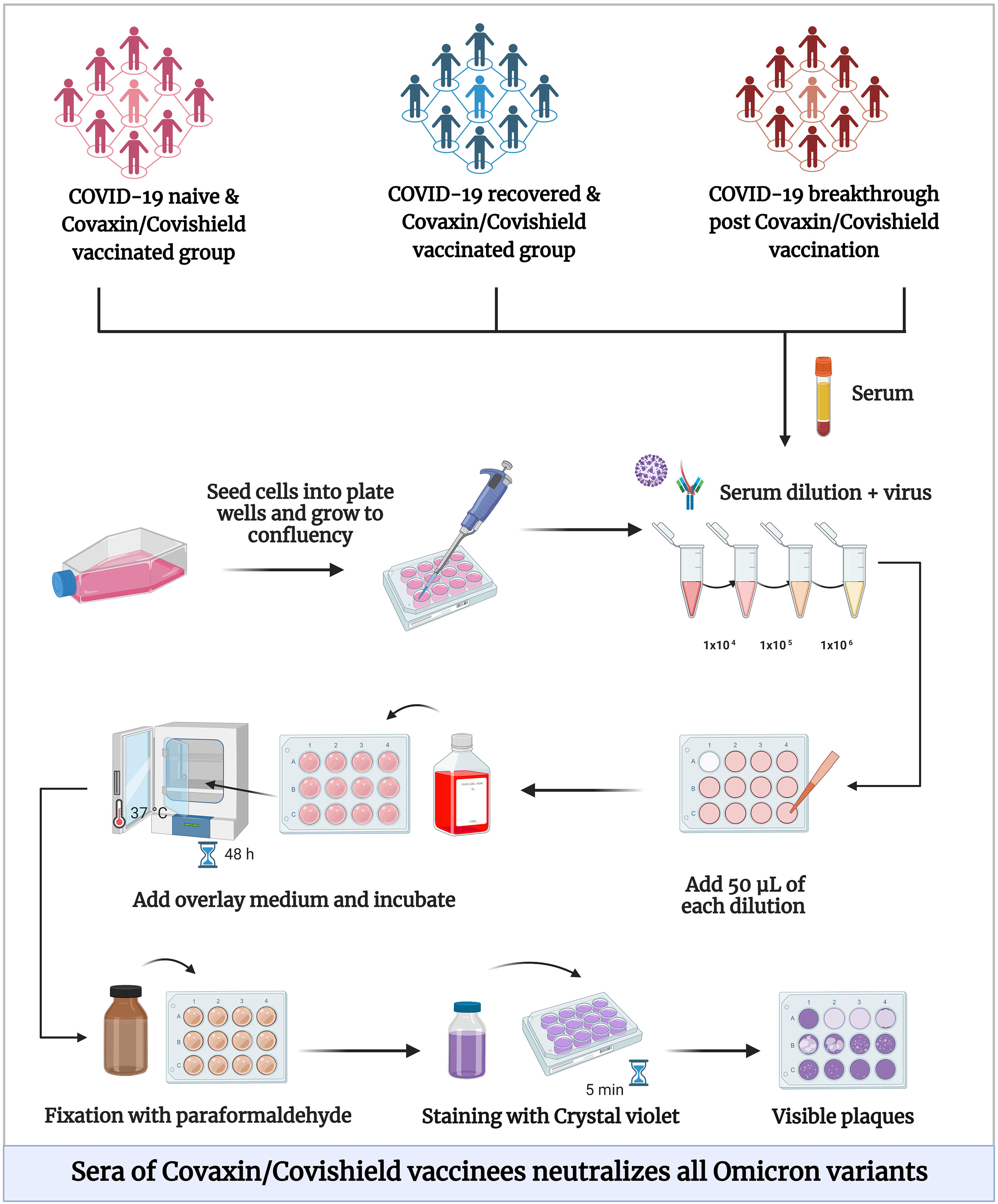
Although vaccines are effective against severe COVID-19, there was significant incidence of breakthrough and re-infection cases. The emergence of novel Omicron sub-lineages highlights the need for continued vigilance and a renewed focus on vaccination. In this review, we have discussed about the waning immune response post natural and vaccine-induced immunity, neutralisation potential against Omicron (Fig. 2) and the need for administration of booster vaccine dose.
National COVID-19 vaccination programme and its successBeginning on 16 January 2021, India's National COVID-19 immunisation Program—the largest immunisation campaign in the world—had the primary goal of quickly vaccinating the nation's adult population. The initiative was expanded to include everyone 12 years of age and older, as well as everyone 18 years of age and older for the precautionary dose.35
The introduction of COVID-19 vaccines entailed a number of challenges, including the need to ensure the provision of all necessary health services as well as the scaling up of the vaccination programme. Additional challenges included the need to train more than 2.6 lakh vaccine administers and 4.8 lakh other vaccination team members and the introduction of difficult-to-reach populations. Additionally, logistical issues such the decentralised storage and distribution of vaccinations across 29 000 cold chain locations, the expansion of the cold chain's capacity, and the creation of an IT platform for beneficiary registration and vaccine service delivery were noted. The programme was able to get through these obstacles and accomplish its objectives quickly.35
The highest level of political and administrative ownership, commitment, and support was necessary for the COVID-19 immunisation programme to be implemented successfully. The Ministry of Health and Family Welfare, India founded as a professional body called as “National Expert Group on Vaccine Administration for COVID-19 (NEGVAC)” to provide advice and guidance related to COVID-19 vaccination. An online system “COVID-19 Vaccine Intelligence Network (Co-WIN)” technology was used to track the recipients of the COVID-19 vaccines in real time. Only pre-registered beneficiaries received vaccinations at the vaccination location in accordance with the prioritisation. The success of the COVID-19 vaccine rollout was largely dependant on the calibre of training provided to enumerators for beneficiary listing, health functionaries for vaccination activities, social mobilisers for all mobilisation activities, and communication training for all workers involved in the vaccination process.36 Extending on the success of India's National COVID-19 Vaccination Programme, more than 220 crore doses of the COVID vaccine dose could be administered in India.35
Waning immunity against viral vaccinesThe mankind has benefited tremendously with more than 70 licenced vaccines which saved human population from infections or lessen the severity against about 30 critical diseases. Even though a lifelong, durable immunity is the intention of vaccination, it is now known that host protective immunity brought on by means of vaccination step by step deteriorates over the years for some of infectious diseases.37
The influenza virus has the ability to adapt and evade vaccination-induced protection. Genetic drift, or HA protein mutations made viable by the way of DNA polymerase, leads to the continuous manufacturing of versions which might be proof against neutralisation. It is essential to reformulate the vaccine with the use of the influenza virus strains which might be projected to be the most common in the imminent season. The reason that circulating influenza viruses go through sufficient genetic and serological changes emphasises re-administration of the vaccines post 1–2 years. The immune response to influenza virus found to decline post 6–18 months of vaccination, depending on the vaccine used, the study population, and the method used to assess immune response.38–40 The research should explore the vaccines and vaccination strategies required to generate long-lasting B cells, T cells memories, and plasma cells. Adjuvants can be used to efficiently appeal to the specified practical cells and release a powerful and long-lasting immune reaction in the host. These methods have the capability to offer lengthy-lasting immunity with progressed safety towards influenza virus infection, in conjunction with new candidates for influenza vaccines.41
Similarly, the relationship between the dosage of the MMR vaccine, the onset of an infection and period of time between vaccination and infection suggests a gradual decline in the immunity provided by the mumps vaccine. According to studies, the mumps virus-unique Ig and maternally received Abs supply protection. Seronegative people are inversely correlated with amount of neutralising Abs and safety is mediated by using Abs. There's no connection among the type of the CMI and protection, notwithstanding the fact that virus-precise CMI endures for more than 20 years after infection and T cellular help is obviously required for Ab responses. Consistent with information from outbreak investigations, the hazard of contracting the mumps rose with the aid of 10%–27% for every 12 months after vaccination, pointing to declining immunity as the reason of those outbreaks.42,43 The studies have confirmed that neutralising Ab titers wanes notably with time post vaccination.44,45 A heterologous top-boost routine to induce virus-unique memory B cells and extra avid Ab are more strategies being researched to extend the length of the immune response after immunisation. The virus-specific memory B cells and the avidity of Abs are decrease against the mumps virus.46,47
In 1913, William Hanna said that 93% of humans immunised against smallpox had been ailment-unfastened for over 50 years, at the same time as immunity to smallpox dwindled over the years. Current studies have also confirmed that key immunological markers persists following smallpox vaccination. El-advert and co-workers observed that, regardless of a decline within the first 3 years after vaccination, antibody levels stays for as a minimum 30 years.48 T-cell immunity has also been shown to be steady for many years, whilst B-cell response suggested to remain for 50–90 years. These findings strongly imply that T cells and antibodies each make contributions drastically to long-term protection against smallpox.49–51
Vaccine breakthrough and re-infectionAlthough the COVID-19 vaccines are effective, some vaccine recipients might get COVID-19 infection with the exposure to the SARS-CoV-2. They are referred to as “vaccine breakthrough cases.” Vaccine breakthrough after primary series but no bivalent booster is defined as a probable or confirmed SARS-CoV-2 infection occurs 14 days or more post the last dose of a COVID-19 monovalent vaccine (primary series or booster dose) in a person who hasn't received a bivalent booster but has at least had a primary series. While, vaccine breakthrough after a bivalent booster is defined as a probable or confirmed SARS-CoV-2 infection that develops 14 days or more post a booster dose of a bivalent COVID-19 vaccination that has obtained FDA authorization or approval.52
SARS-CoV-2 re-infection denotes a case of infection, recovery, and re-infection. Repeated re-infections of one person are possible. Two SARS-CoV-2 RT-PCR tests that are positive with a Ct less than 35 (or virus isolation using in vitro methods or the presence of subgenomic RNA) at 2 different times are considered to be a true re-infection. In addition to this, the infection should be by 2 different SARS-CoV-2 strains confirmed by sequencing method.53 It is unknown if most infected people establish sufficient and durable immunological memory responses including neutralising Abs and and memory T cells. The titers of specific IgG antibodies against SARS-CoV and MERS-CoV decreased with time, according to long-term investigations that looked at the durability of these antibodies more than a year after infection. Nevertheless, the presence of antibodies does not always signify a protective immunity, especially if the antibodies' neutralising action is unidentified, weak, or non-existent.54
An antibody response waned, primarily after 90 days, according to earlier trials. However, a further prolonged follow-up investigation discovered that SARS-CoV-2 antibody titers lasted even over 4 months.55 Several SARS-CoV-2 clades and subclades have been identified with thousands of distinct and diverse variations with over 400 mutations in the spike protein. The immunological pressure of these novel new strains is capable of evading immune responses to prior infections leading to re-infections. It is unclear whether re-infection will be more severe or milder. The antibody-dependent augmentation, or the strain-specific virulence can lead to more severe disease.56 The role of adaptive immunity, leading to prior priming could help in reducing the re-infection severity.57
Waning immune response and SARS-CoV-2 re-infections with OmicronHere, we studied COVID-19 naive participants (n=4) with 2 dose Covishield vaccine administered 1 month apart. Two cases of breakthrough infection (one each of the Kappa and Delta variants) were asymptomatic; the other 2 cases (one each of the Kappa and Delta variants) presented with symptoms such fever, sore throat, cough, headache, loss of appetite, myalgia, weakness, loss of smell, and taste. After a mean of 275 days, these cases got re-infection with BA.2 sub-lineage. The breakthrough cases had the median neutralising antibody (NAb) titers of 4621, 1211, and 58.5 against B.1, Delta, and BA.1, respectively.58 This highlights the significance of the booster vaccine dose. Regardless of immunity induced by natural infections or vaccinations, breakthrough and re-infection cases were documented across the globe.
Then, from March 2020 to October 2021, when the first wave of SARS-CoV-2 infections occurred, we also looked into 6 people who had this original infection. After 2 months of second dose vaccination, serum samples revealed 2.8-fold increases in IgG and NAb titres fold increase was observed against B.1 (6.8), Delta (24.5), and Omicron (47.5), compared to the initial infection. A genomic examination of clinical samples revealed that the SARS-CoV-2 infecting variant belongs to BA.1 and BA.2 sub-lineages. These sub-lineages' capacity to escape the immune system is correlated with frequent changes in BA.1 and BA.2. Re-infection incidences were found 7 months following the second vaccination, and it was discovered that this hybrid immunity had deteriorated to a very low level.59
The neutralisation of Omicron with sera of Covaxin vaccineesThe participants in this study were grouped into COVID-19 naive persons with 2 dosages of Covaxin vaccination (n=52), SARS-CoV-2 recovered patients with 2 dosages of Covaxin (n=31), and breakthrough cases following 3 months of 2 dose Covaxin vaccination (n=40). Neutralising antibody Geometric mean titres (GMT) against B.1, Beta, Delta, and Omicron were determined in sera from all the participants. In all 3 groups, it was discovered that the GMT values decreased with Delta, then Beta, and finally the Omicron version. The neutralising antibody titre was also reduced in recovered and breakthrough cases' sera against Omicron (7.98, 8.84), Delta (1.60, 1.53), and Beta (2.54, 3.55), respectively, compared to B.1 strain. The immune response after infection was significantly increased in breakthrough instances, which had the strongest neutralising activity against all types. Evidently, the naive subjects' extremely low neutralising titres showed that their immunity had begun to decline 3 months after receiving the second dose.60
Studies on neutralisation of Omicron with sera of Covishield vaccinesIn this study, the serum samples were taken from individuals who had received 2 dosages of Covishield after being immunised against COVID-19 (n=24; 6 months after second dose), from people who had recovered from COVID-19 after receiving 2 dosages of Covishield (n=17; 6 months after second dose), and from people who had developed SARS-CoV-2 after receiving 2 dosages of Covishield (n=46; 14–30 days after infection). The wild-type B.1 variant was detected in retrieved sequences (n=17). In comparison to recovered cases (112.1) and breakthrough infections (594.1), a similar trend of declining Ab titres was seen in COVID-19 naive vaccinated individuals with N protein ELISA (77.2). The sera of subjects from all 3 groups neutralise B.1, Beta, and Delta more effectively than Omicron. Sera from unvaccinated individuals had a GMT titre of NAb for Omicron that was lower (0.11) than those from recovered cases (11.28) and breakthrough cases (26.25). Although Omicron variant showed the highest fold-reduction among breakthrough cases (46.86%), it had the maximum NAb titre compared to recovered cases and unvaccinated individuals.61 These findings demonstrate that naive vaccinees had the lowest IgG and NAb response compared to other groups. The diminishing immune response in naive vaccine recipients after the second dosage is also highlighted, which supports the administration of a preventive dose to strengthen protection.
Immune response in Omicron infected breakthrough and unvaccinated individualsThe study explored Omicron positive foreign returnees (28) and their high-risk contacts (11). The subjects were divided into 3 groups: people who had not had vaccinations (n=6), people who developed illnesses after receiving 2 doses of the vaccines [Covishield (n=25), and BNT162b2 mRNA (n=8)]. The immune response in these individuals was determined with S1-RBD, N protein and whole virion-based IgG ELISA and NAb responses using plaque reduction neutralisation test. A reduction in GMTs of 2.5–3.6 times was observed with N protein and whole virion IgG ELISA in the sera of breakthrough cases vaccinated with BNT162b2 mRNA vaccine than the Covishield. There was a fold-reduction in the neutralising antibodies of breakthrough cases vaccinated with Covishield and BNT162b2 mRNA vaccine and unvaccinated individuals against Alpha (3.23, 1.16, 9.08), Beta (2.38, 1.35, 0.3), Delta (3.23, 1.52, 0.49), and Omicron (4.31, 7.41, 0.22) variants, respectively, compared to B.1. It demonstrated significant fold reduction in NAb titres against Omicron with sera of BNT162b2 mRNA and Covishield breakthrough cases than unvaccinated individuals.62 This suggests that the Omicron-induced immune response could effectively neutralise Delta variant, which emphasises the need for tweaked vaccine.
Immune response against Omicron post-heterologous prime-boost vaccineIn a previous work, we evaluated the safety and immunogenicity of heterologous vaccination of Covishield followed by Covaxin. This was further compared with 2 cohorts who have received 2 dosages of homologous vaccination of Covishield or Covaxin.63 Subsequently, 6 months post the second dose, and we were able to follow up with 17 of 18 individuals in the heterologous group from the previous study. The results of the humoral and neutralising antibody responses of all the participants against Alpha, Beta, Delta variants were compared to B.1 at 1 and 6 months and post 6 months against Omicron. There were 21 men and 15 women in the homologous Covishield/Covishield group with a median age of 65.5 years. Even though the GMT of the heterologous group had been significantly reduced, their NAbs were elevated than those of homologous group.64 Discussions on creating novel vaccination tactics have been triggered by the slow transition from Delta to Delta sub-lineages and Omicron to Omicron sub-lineages as well as the observed decrease of immunity 6 months post vaccination.
Enhanced immune response 6 months post 2 doses of Covaxin in COVID-19 recovered individualsIn 2 adult populations, COVID-19 naive vaccine recipients (n=118) and COVID-19 recovered recipients (n=128), we assessed the immunogenicity of 2 dosages of Covaxin. The immune response in the study group was evaluated at 3 points: 1 month after first dose, 1 and 6 months after second dose. The GMTs of S1-RBD IgG increased among subjects in both groups 1 month after 2 doses compared to GMTs 1 month after first dose. The humoral response was found to be marginally higher in naive vaccinees (446.3–685.9), but slightly lowered in COVID-19 recovered vaccinees (1127–839.8). Taking into account the recovered population's hybrid immunity, the NAbs were elevated than naïve vaccinees at each follow-up against B.1, Delta, Omicron BA.1, and BA.2 with lowest NAb titres against BA.1. At various time intervals, the humoral and neutralising antibody response was found to be persistent for 6 months post vaccination.65 The research emphasises the importance of a administration of booster dose post 6 months of vaccination.
Persistence of neutralising antibodies against omicron post 2 dose and booster dose of Covishield and CovaxinWe measured the neutralising antibody responses in sera collected from individuals 6 months after receiving 2 doses (N=88) of Covishield (n=44) or Covaxin (n=44), as well as from those who received 3 doses (N=102) of Covishield (n=46) or Covaxin (n=56), including a booster dose. These responses were tested against the prototype B.1 strain and the Omicron sub-variants XBB.1, BQ.1, BA.5.2, and BF.7. The sera from individuals in both the 2- and 3-dose (post booster dose) groups exhibited neutralising activity against all variants. The level of neutralising antibodies (NAbs) was highest against the prototype B.1 strain, followed by BA5.2 (5–6 fold lower), BF.7 (11–12 fold lower), BQ.1 (12 fold lower), and XBB.1 (18–22 fold lower). We observed comparable persistence of NAb responses in individuals with 2 and 3 doses 6 months after vaccination. Among the Omicron sub-variants, XBB.1 displayed significant neutralisation escape, suggesting a potential immune evasion that could lead to increased infections. Further research is needed to assess the full impact of boosters and hybrid immunity on protection against new variants (Unpublished data).
The importance of vaccination against OmicronVaccination is the most effective way to protect against COVID-19, and this remains true for the Omicron variant. While there is still much to learn about the effectiveness of existing vaccines against Omicron, early data suggest that booster shots may be necessary to provide adequate protection. This is because the Omicron and its sub-lineages have a large number of mutations, which may enable it to evade the immune response generated by previous vaccinations.
The benefits of booster dose and addressing vaccine hesitancy and misinformationBooster shots are additional doses of a vaccine that are given after the initial doses to provide continued protection against a disease. In the case of COVID-19, booster shots are being recommended to help maintain immunity and protect against new variants, such as Omicron and sub-lineages. Booster doses are typically given several months after the initial vaccine regimen, and the timing may vary depending on the type of vaccine. Receiving a third vaccine dose can provide several benefits in the context of the Omicron surge. First, it can help boost immunity and provide additional protection against the new variant. Second, it can help reduce the spread of the virus by reducing the likelihood of transmission. Finally, it can help prevent severe illness and hospitalisation, which can help alleviate the burden on healthcare systems.
The Indian government has recommended that individuals who are immune-compromised or over the age of 60 receive a booster shot. Additionally, frontline workers, such as healthcare workers and police personnel, are also being prioritised for booster shots. However, as more data become available about the effectiveness of vaccines against Omicron, it is possible that booster shots may be recommended for a wider population.
Despite the proven effectiveness of vaccines in controlling the spread of COVID-19, there is still a significant amount of vaccine hesitancy and misinformation in India. Many things can be attributed for this, including a loss of faith in the government and healthcare system, false information on social media, and worries about side effects. It is important to address these concerns and provide accurate information about the safety and efficacy of vaccines.
ConclusionsIn conclusion, the emergence of the Omicron and its sub-lineages highlights the need for continued vigilance and a renewed focus on vaccination in India. While there is still much to learn about these newly emerging Omicron sub-lineages, early data suggest that booster shots may be necessary to provide adequate protection. Receiving a third vaccine dose can help boost immunity, reduce the spread of the virus, and prevent severe illness and hospitalisation. It is important to address vaccine hesitancy and misinformation, and to continue following COVID-19 safety measures, to ensure that India can navigate the Omicron surge and protect the health and well-being of its citizens.
While vaccination is an important tool in controlling the spread of COVID-19, it is not a panacea. It is important to continue to follow COVID-19 safety measures which can help reduce the spread of the virus and prevent the emergence of new variants.
FundingThe financial support for this study was provided by the Indian Council of Medical Research-National Institute of Virology, Pune.