Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosIn general, COVID-19 vaccines are safe and effective, but minor adverse effects are common. However, adverse effects have not been measured in several countries including Greece.
ObjectiveTo estimate the prevalence of adverse effects after the first COVID-19 booster dose, and to identify possible risk factors.
Material and methodsWe conducted a cross-sectional study with a convenience sample in Greece during November 2022. We measured several adverse effects after the booster dose, such as fatigue, headaches, fever, chills, nausea, etc. We considered gender, age, chronic disease, self-assessment of health status, COVID-19 diagnóstico, and self-assessment of COVID-19 course as possible predictors of adverse effects.
ResultsIn our sample, 96% developed at least one adverse effect. Half of the participants (50.2%) developed one to five adverse effects, 35.9% developed six to ten adverse effects, and 9.5% developed 11 to 16 adverse effects. Mean number of adverse effects was 5.5. The most frequent adverse effects were pain at the injection site (84.3%), fatigue (70.8%), muscle pain (61%), swelling at the injection site (55.2%), headache (49.8%), fever (42.9%), and chills (41%). Females developed more adverse effects than males (p < 0.001). The prevalence of adverse effects of COVID-19 vaccines was statistically significant and positively associated with the severity of COVID-19 among COVID-recovered individuals (p < 0.05). Moreover, younger age was associated with increased adverse effects (p < 0.001).
ConclusionsAlmost all participants in our study developed minor adverse effects after the booster dose. Female gender, COVID-19 patients with worse clinical course, and younger individuals experienced more often adverse effects.
En general, las vacunas COVID-19 son seguras y eficaces, pero son frecuentes los efectos adversos leves. Sin embargo, los efectos adversos no se han medido en varios países, entre ellos Grecia.
ObjetivoEstimar la prevalencia de efectos adversos tras la primera dosis de refuerzo de COVID-19 e identificar posibles factores de riesgo.
MétodosRealizamos un estudio transversal con una muestra de conveniencia en Grecia durante noviembre de 2022. Se midieron varios efectos adversos tras la dosis de refuerzo, fatiga, dolores de cabeza, fiebre, escalofríos, náuseas, etc. Consideramos el sexo, la edad, la enfermedad crónica, la autoevaluación del estado de salud, el diagnóstico de COVID-19 y la autoevaluación del curso de COVID-19 como posibles predictores de los efectos adversos.
ResultadosEn nuestra muestra, el 96% desarrolló al menos un efecto adverso. La mitad de los participantes (50,2%) desarrollaron de uno a cinco efectos adversos, el 35,9% desarrollaron de seis a diez efectos adversos, y el 9,5% desarrollaron de 11 a 16 efectos adversos. La media de efectos adversos fue de 5,5. Los efectos adversos más frecuentes fueron dolor en el punto de inyección (84,3%), fatiga (70,8%), dolor muscular (61%), hinchazón en el punto de inyección (55,2%), cefalea (49,8%), fiebre (42,9%) y escalofríos (41%). Las mujeres presentaron más efectos adversos que los hombres (p < 0,001). La prevalencia de los efectos adversos de las vacunas COVID-19 fue estadísticamente significativa y se asoció positivamente con la gravedad de COVID-19 entre los individuos recuperados de COVID (p < 0,05). Además, la menor edad se asoció con mayores efectos adversos (p < 0,001).
ConclusionesCasi todos los participantes en nuestro estudio desarrollaron efectos adversos menores tras la dosis de refuerzo. El sexo femenino, los pacientes de COVID-19 con peor evolución clínica y los individuos más jóvenes experimentaron efectos adversos con mayor frecuencia.
Development of safe and effective vaccines against COVID-19 have offered protection against SARS-CoV-2 infections and have significantly reduced hospitalizations, intensive care unit admissions, and deaths.1,2 However, the effectiveness and efficiency of COVID-19 vaccines decrease significantly six months following full vaccination.3 For this reason, many countries have adopted booster doses following the initial full vaccination to boost the immune system of the population. Indeed, the effectiveness of the first booster dose has already proven by reducing infections, hospitalizations, and mortality due to COVID-19.4,5 Therefore, reduced immunization against COVID-19 a few months post-vaccination makes booster vaccination necessary, particularly for at-risk populations. However, a meta-analysis found that adverse effects and discomfort experienced following the primary COVID-19 vaccination were the main factors contributing to the decrease in the uptake of the first COVID-19 booster dose.6
Unfortunately, despite the high safety and efficacy of most COVID-19 vaccines, there are also adverse effects. The most common adverse effects include fever, fatigue, muscle aches, pain and swelling at the injection site, menstrual disorders and headache, while more serious adverse effects such as myocarditis, pericarditis and thrombosis are extremely rare.7–10 Other minor adverse effects that occur less frequently are nausea, shortness of breath, and dizziness. According to a systematic review, the most prevalent adverse effects are pain and swelling at the injection site, shooting pain, muscle pain, headaches, chills, fever, swollen lymph nodes, nausea, shortness of breath, and diarrhea.11 Another systematic review investigated menstrual abnormalities and found that more than half of females (52.1%) had some form of a menstrual problem after COVID-19 vaccines (e.g., menorrhagia, metrorrhagia, and polymenorrhea) in a total of 78,138 vaccinated females.12 Younger people and females are more likely to experience adverse effects.13 A systematic review found that 69.8% of females developed adverse effects, while the corresponding percentage for males was 30.2%.11
To date, few studies have investigated adverse effects after the first COVID-19 booster dose focusing only on demographic variables such as gender and age as possible risk factors.14–19 Adverse effects after the first COVID-19 booster dose are similar with those after the primary doses, as analyzed above.
However, to the best of our knowledge, no research has been conducted to evaluate adverse effects following COVID-19 vaccination in Greece. Thus, the aim of our study was to estimate the prevalence of adverse effects after the first COVID-19 booster dose in Greece, and identify possible risk factors.
Material and methodsStudy designWe conducted a cross-sectional study in Greece. Data collection was performed during November 2022. Inclusion criteria were adults over 18 years old, individuals that understand the Greek language, and individuals that had been vaccinated against SARS-CoV-2 with the primary COVID-19 vaccine doses and the first booster dose. We obtained our sample from visitors at a primary health center in Athens. Thus, a convenience sample from the general population was obtained. Response rate was 94.6% (473 out of 500). This health center has provided visitors with the Comirnaty vaccine (Pfizer).
We measured the following adverse effects: pain at the injection site, swelling at the injection site, fatigue, muscle pain, headaches, fever, chills, nausea, itching, diarrhea, shortness of breath, rhinorrhea, swollen lymph nodes, dizziness, sleep disturbances, reduced appetite, and adverse effects on menstrual cycle. Also, we asked participants to add any other possible adverse effect that experienced due to vaccination, but it was not included in our questionnaire. Each adverse effect was measured on a four-point scale: not at all effect, minor effects, moderate effects, and major effects. We considered minor effects as those that last for a few hours, moderate effects as those that last for 24–48 h, and major effects as those that last at least two days. Moreover, we added all adverse effects to calculate a total score of adverse effects.
We considered gender (males or females), age (continuous variable), chronic disease (no or yes), self-assessment of health status (very poor, poor, moderate, good, very good), COVID-19 diagnóstico (no or yes), and self-assessment of COVID-19 clinical course (scale from 0 [very mild] to 10 [extreme severe] as possible predictors of frequency of adverse effects of COVID-19 booster dose. Moreover, we considered a priory that scores of COVID-19 clinical course from 0 to 2 indicate low symptoms of COVID-19, scores from 3 to 7 indicate moderate symptoms, and scores from 8 to 10 indicate extreme symptoms.
Considering that the total number of fully vaccinated adults in Greece was 7.7 million during our research, a 95% confidence level, a 5% margin of error, and a 50% population proportion, the required minimum sample size was 385 participants. We chose to marginally expand our sample to reduce random error.
EthicsWe informed participants about the aim and the design of our study, and they gave their informed consent to participate in our study. We did not collect participants' personal data. Thus, participation in our study was anonymous and voluntary.
Guidelines of the Declaration of Helsinki were applied in our study. Also, our study protocol was approved by the Ethics Committee of Faculty of Nursing, National and Kapodistrian University of Athens (reference number; 424, 26-10-2022).
Statistical analysisWe present categorical variables with numbers and percentages and continuous variables with mean and standard deviation (SD). Kolmogorov–Smirnov test and Q-Q plots indicated that continuous variables followed normal distribution. We considered gender, age, chronic disease, self-assessment of health status, COVID-19 diagnóstico, and self-assessment of COVID-19 clinical course as independent variables, and adverse effects of COVID-19 booster dose as dependent variables. We used chi-square test, independent samples t-test, analysis of variance, Pearson's correlation coefficient, and Spearman's correlation coefficient to assess the relationship between independent variables and adverse effects. P-values less than 0.05 were considered as statistically significant. We used the IBM SPSS 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) for the analysis.
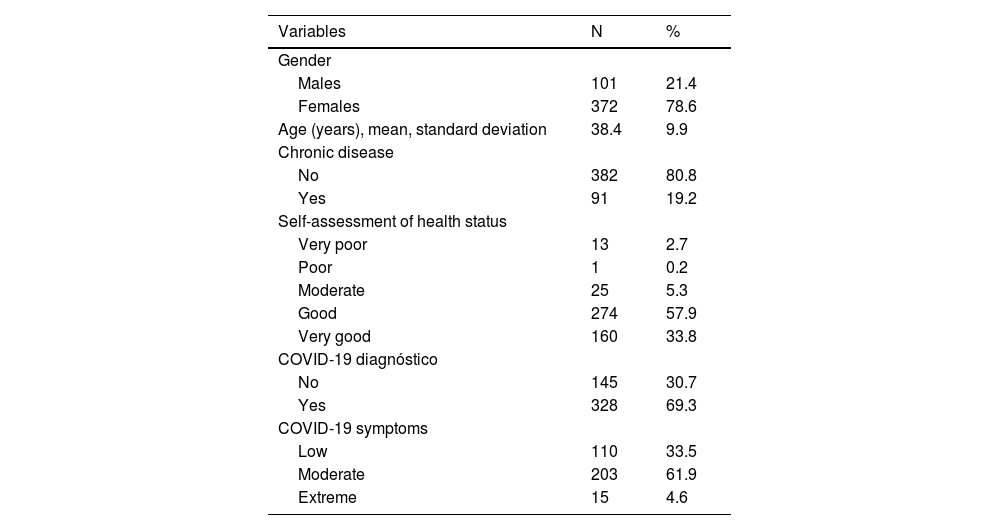
ResultsStudy population included 473 participants. Demographic characteristics of participants are shown in Table 1. Participants' mean age was 38.4 years, while most of them were females (78.6%). Among them, 19.2% had a chronic disease. The majority of the participants reported that their health status was good/very good (91.7%), while 5.3% reported a moderate level of health, and 2.9% a poor/very poor level of health.
Demographic and COVID-19-related variables of participants (N = 473).
| Variables | N | % |
|---|---|---|
| Gender | ||
| Males | 101 | 21.4 |
| Females | 372 | 78.6 |
| Age (years), mean, standard deviation | 38.4 | 9.9 |
| Chronic disease | ||
| No | 382 | 80.8 |
| Yes | 91 | 19.2 |
| Self-assessment of health status | ||
| Very poor | 13 | 2.7 |
| Poor | 1 | 0.2 |
| Moderate | 25 | 5.3 |
| Good | 274 | 57.9 |
| Very good | 160 | 33.8 |
| COVID-19 diagnóstico | ||
| No | 145 | 30.7 |
| Yes | 328 | 69.3 |
| COVID-19 symptoms | ||
| Low | 110 | 33.5 |
| Moderate | 203 | 61.9 |
| Extreme | 15 | 4.6 |
In our sample, 69.3% had been diagnosed with COVID-19 during the pandemic (Table 1). Among them, 61.9% developed moderate symptoms of COVID-19, 33.5% developed low symptoms, and 4.6% developed extreme symptoms.
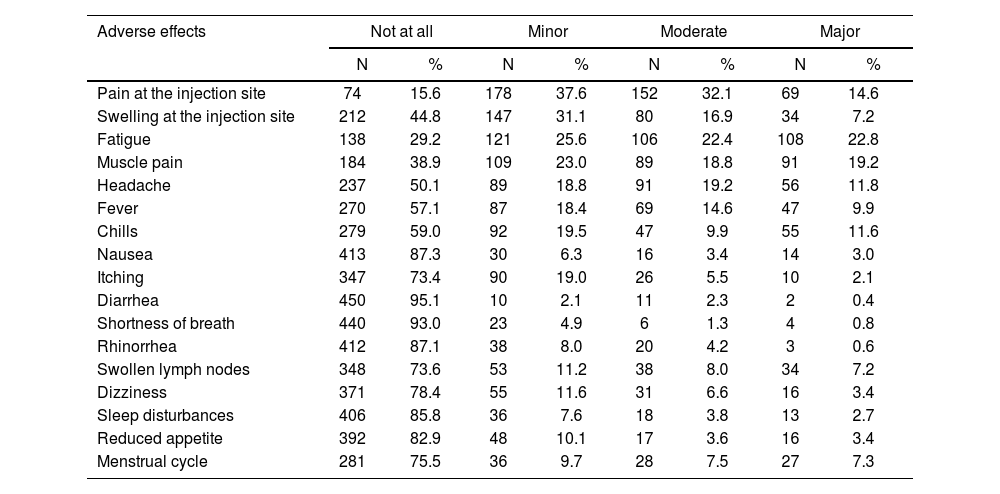
Adverse effects of COVID-19 booster dose in participants are shown in detail in Table 2. In our sample, 96% (n = 454) had at least one adverse effect. Half of the participants (50.2%, n = 239) had one to five adverse effects, 35.9% (n = 170) had six to ten adverse effects, and 9.5% (n = 45) had 11 to 16 adverse effects. Mean number of adverse effects was 5.5 (standard deviation = 3.5) with a median value of 5, a minimum value of 0 and a maximum value of 16.
Adverse effects of COVID-19 booster dose in participants.
| Adverse effects | Not at all | Minor | Moderate | Major | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Pain at the injection site | 74 | 15.6 | 178 | 37.6 | 152 | 32.1 | 69 | 14.6 |
| Swelling at the injection site | 212 | 44.8 | 147 | 31.1 | 80 | 16.9 | 34 | 7.2 |
| Fatigue | 138 | 29.2 | 121 | 25.6 | 106 | 22.4 | 108 | 22.8 |
| Muscle pain | 184 | 38.9 | 109 | 23.0 | 89 | 18.8 | 91 | 19.2 |
| Headache | 237 | 50.1 | 89 | 18.8 | 91 | 19.2 | 56 | 11.8 |
| Fever | 270 | 57.1 | 87 | 18.4 | 69 | 14.6 | 47 | 9.9 |
| Chills | 279 | 59.0 | 92 | 19.5 | 47 | 9.9 | 55 | 11.6 |
| Nausea | 413 | 87.3 | 30 | 6.3 | 16 | 3.4 | 14 | 3.0 |
| Itching | 347 | 73.4 | 90 | 19.0 | 26 | 5.5 | 10 | 2.1 |
| Diarrhea | 450 | 95.1 | 10 | 2.1 | 11 | 2.3 | 2 | 0.4 |
| Shortness of breath | 440 | 93.0 | 23 | 4.9 | 6 | 1.3 | 4 | 0.8 |
| Rhinorrhea | 412 | 87.1 | 38 | 8.0 | 20 | 4.2 | 3 | 0.6 |
| Swollen lymph nodes | 348 | 73.6 | 53 | 11.2 | 38 | 8.0 | 34 | 7.2 |
| Dizziness | 371 | 78.4 | 55 | 11.6 | 31 | 6.6 | 16 | 3.4 |
| Sleep disturbances | 406 | 85.8 | 36 | 7.6 | 18 | 3.8 | 13 | 2.7 |
| Reduced appetite | 392 | 82.9 | 48 | 10.1 | 17 | 3.6 | 16 | 3.4 |
| Menstrual cycle | 281 | 75.5 | 36 | 9.7 | 28 | 7.5 | 27 | 7.3 |
The most frequent adverse effects were pain at the injection site (84.3%), fatigue (70.8%), muscle pain (61%), swelling at the injection site (55.2%), headache (49.8%), fever (42.9%), and chills (41%). Itching (26.6%), swollen lymph nodes (26.4%), adverse effects on menstrual period (24.5%), dizziness (21.6%), and reduced appetite (17.1%) were frequent adverse effects. Moreover, participants experienced less often sleep disturbances (14.1%), rhinorrhea (12.8%), nausea (12.7%), shortness of breath (7%), and diarrhea (4.8%).
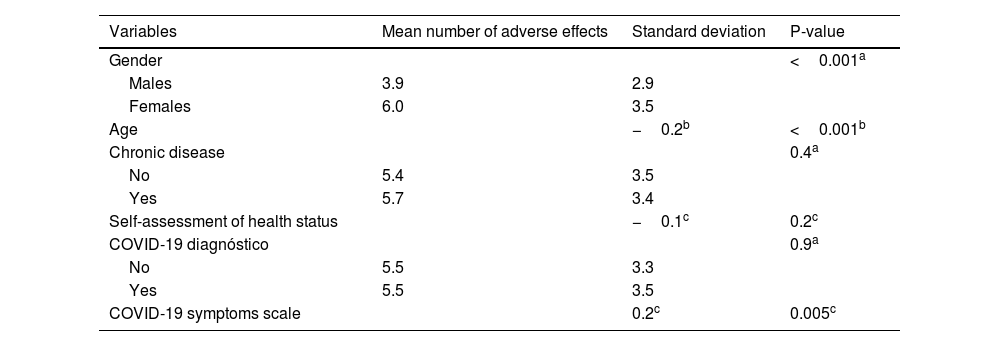
Relationships between demographic and COVID-19-related variables and total number of adverse effects of COVID-19 booster dose are shown in Table 3. Females developed more adverse effects than males (p < 0.001). Also, we found a positive relationship between severity of COVID-19 symptoms and adverse effects of COVID-19 booster dose (p = 0.005). Moreover, younger adults experienced more often adverse effects (p < 0.001). In particular, mean number of adverse effects among individuals <45 years was 5.8 (SD = 3.4), while among those aged from 45 to 60 was 4.8 (SD = 3.5), and among those >60 years was 3.3 (SD = 3.1), (p = 0.002).
Relationships between demographic and COVID-19-related variables and total number of adverse effects of COVID-19 booster dose.
| Variables | Mean number of adverse effects | Standard deviation | P-value |
|---|---|---|---|
| Gender | <0.001a | ||
| Males | 3.9 | 2.9 | |
| Females | 6.0 | 3.5 | |
| Age | −0.2b | <0.001b | |
| Chronic disease | 0.4a | ||
| No | 5.4 | 3.5 | |
| Yes | 5.7 | 3.4 | |
| Self-assessment of health status | −0.1c | 0.2c | |
| COVID-19 diagnóstico | 0.9a | ||
| No | 5.5 | 3.3 | |
| Yes | 5.5 | 3.5 | |
| COVID-19 symptoms scale | 0.2c | 0.005c |
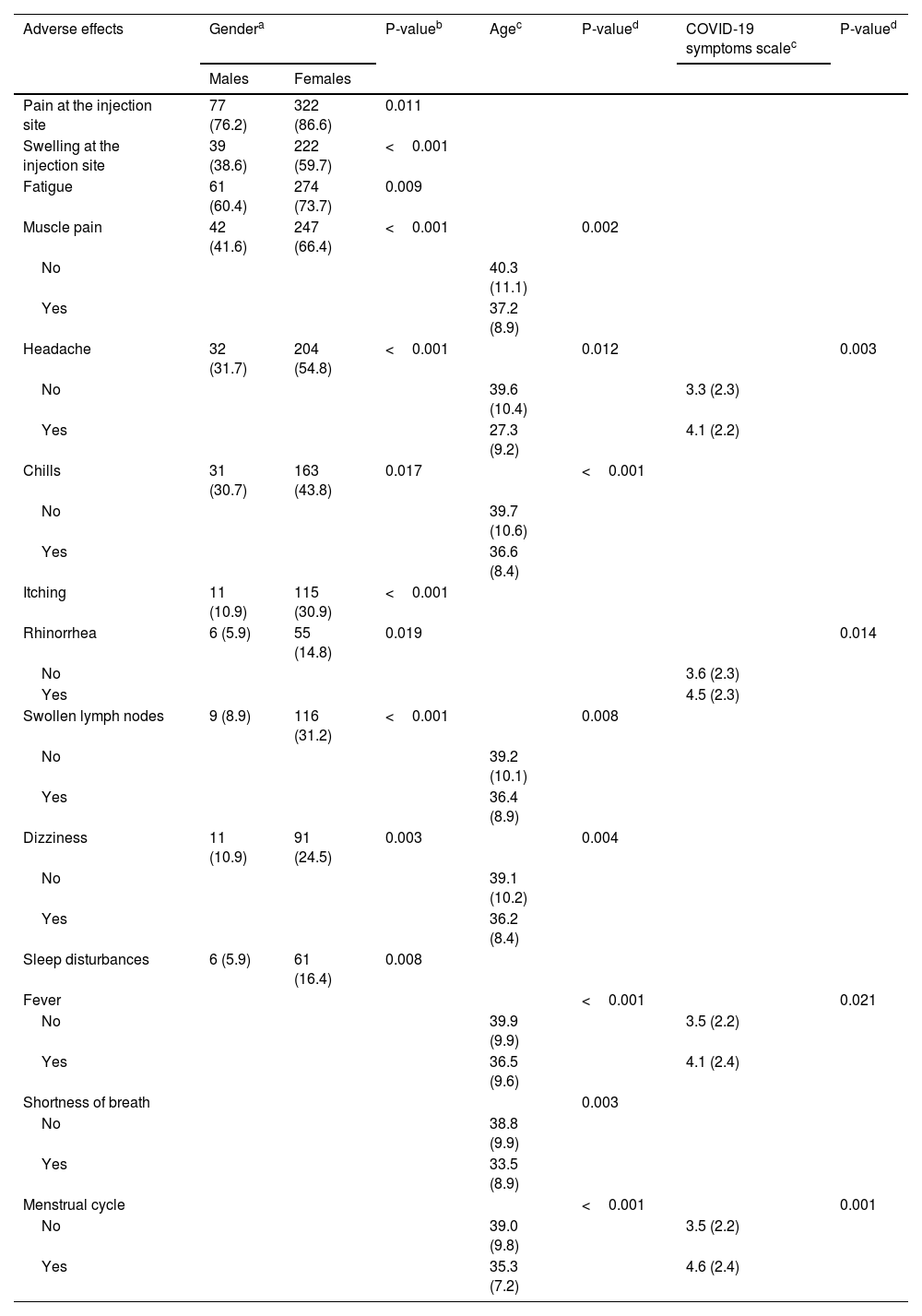
We present statistically significant relationships between demographic and COVID-19-related variables and adverse effects separately in Table 4. Females developed more often pain at the injection site (p = 0.011), swelling at the injection site (p < 0.001), fatigue (p = 0.009), muscle pain (p < 0.001), headache (p < 0.001), chills (p = 0.017), itching (p < 0.001), rhinorrhea (p = 0.019), swollen lymph nodes (p < 0.001), dizziness (p = 0.003), and sleep disturbances (p = 0.008). Younger participants developed more often muscle pain (p = 0.002), headache (p = 0.012), fever (p < 0.001), chills (p < 0.001), shortness of breath (p = 0.003), swollen lymph nodes (p = 0.008), dizziness (p = 0.004), and adverse effects on menstrual cycle (p < 0.001). Participants with worse COVID-19 clinical course had more often headache (p = 0.003), fever (p = 0.021), rhinorrhea (p = 0.014), and adverse effects on menstrual cycle (p = 0.001).
Statistically significant relationships between demographic and COVID-19-related variables and adverse effects of COVID-19 booster dose.
| Adverse effects | Gendera | P-valueb | Agec | P-valued | COVID-19 symptoms scalec | P-valued | |
|---|---|---|---|---|---|---|---|
| Males | Females | ||||||
| Pain at the injection site | 77 (76.2) | 322 (86.6) | 0.011 | ||||
| Swelling at the injection site | 39 (38.6) | 222 (59.7) | <0.001 | ||||
| Fatigue | 61 (60.4) | 274 (73.7) | 0.009 | ||||
| Muscle pain | 42 (41.6) | 247 (66.4) | <0.001 | 0.002 | |||
| No | 40.3 (11.1) | ||||||
| Yes | 37.2 (8.9) | ||||||
| Headache | 32 (31.7) | 204 (54.8) | <0.001 | 0.012 | 0.003 | ||
| No | 39.6 (10.4) | 3.3 (2.3) | |||||
| Yes | 27.3 (9.2) | 4.1 (2.2) | |||||
| Chills | 31 (30.7) | 163 (43.8) | 0.017 | <0.001 | |||
| No | 39.7 (10.6) | ||||||
| Yes | 36.6 (8.4) | ||||||
| Itching | 11 (10.9) | 115 (30.9) | <0.001 | ||||
| Rhinorrhea | 6 (5.9) | 55 (14.8) | 0.019 | 0.014 | |||
| No | 3.6 (2.3) | ||||||
| Yes | 4.5 (2.3) | ||||||
| Swollen lymph nodes | 9 (8.9) | 116 (31.2) | <0.001 | 0.008 | |||
| No | 39.2 (10.1) | ||||||
| Yes | 36.4 (8.9) | ||||||
| Dizziness | 11 (10.9) | 91 (24.5) | 0.003 | 0.004 | |||
| No | 39.1 (10.2) | ||||||
| Yes | 36.2 (8.4) | ||||||
| Sleep disturbances | 6 (5.9) | 61 (16.4) | 0.008 | ||||
| Fever | <0.001 | 0.021 | |||||
| No | 39.9 (9.9) | 3.5 (2.2) | |||||
| Yes | 36.5 (9.6) | 4.1 (2.4) | |||||
| Shortness of breath | 0.003 | ||||||
| No | 38.8 (9.9) | ||||||
| Yes | 33.5 (8.9) | ||||||
| Menstrual cycle | <0.001 | 0.001 | |||||
| No | 39.0 (9.8) | 3.5 (2.2) | |||||
| Yes | 35.3 (7.2) | 4.6 (2.4) | |||||
We conducted a cross-sectional study in Greece to assess the prevalence of adverse effects after COVID-19 vaccines and investigate possible risk factors. To the best of our knowledge this is the first study that estimates adverse effects after COVID-19 vaccination in Greece.
We found that almost all participants (96%) developed at least one adverse effect after the first booster dose. Literature supports this finding since two other studies found that all participants had adverse effects after the first booster dose.20,21 Other studies found high prevalence of adverse effect after the first booster dose as well, ranging from 74.7% to 89%.14–17,22,23 Slight differences in prevalence of adverse effects among studies could be attributed to different study designs, populations, and definitions.
Our study showed that the most frequent adverse effects were pain at the injection site, fatigue, muscle pain, swelling at the injection site, headache, fever and chills. These findings are in accordance with the literature regarding the adverse effects of the first booster dose.14–18,22,23 Moreover, a recent systematic review investigated the prevalence of adverse effects after the primary COVID-19 vaccine doses and found that the most frequent adverse effects among 10,632 participants were pain at the injection site (77.3%), muscle pain (39.7%), swelling at the injection site (34%), headache (33.3%), chills (18.3%), fever (18%), swollen lymph nodes (8%), nausea (7.9%), and shortness of breath (7.6%).11 Fortunately, although adverse effects after COVID-19 vaccination are common, they are usually mild and self-limited.11
Also, we found a positive relationship between severity of COVID-19 symptoms and adverse effects of COVID-19 booster dose. To our knowledge, our study investigated for the first time the relationship between severity of COVID-19 symptoms and adverse effects, making a direct comparison with other studies not feasible. However, it is well known that adverse effects are more common among individuals with a history of COVID-19 or/and past exposure to SARS-CoV-2 not only after the primary vaccination24–26 but also after the first booster dose.16,20 Repeated COVID-19 vaccine doses can induce more adverse effects due to cytokine production especially in people who had previously been infected with SARS-CoV-2.27 Moreover, humoral immunity in people with a history of COVID-19 and a single dose of the COVID-19 vaccine is equal to or stronger than in uninfected individuals with two doses.28
Our findings indicate that females developed more adverse effects than males. Several studies support the observation that females are more likely to encounter adverse effects following the first booster dose.14,16,17,20 Several theories could explain this gender-based difference in post COVID-19 vaccination adverse effects. Firstly, in general, females typically exhibit a greater response to vaccines than males due to differences in antibody, inflammatory, and cell-mediated immune responses.29,30 Additionally, genetic, hormonal, and behavioral factors might also contribute to the explanation of the higher frequency of adverse events in females.30–33 In particular, the innate and adaptive immune response in males has been depressed by testosterone.34,35
Moreover, we found a negative relationship between age and incidence of adverse effects after the first booster dose. Several studies confirm our finding since they revealed that younger adults were more likely to develop adverse effects after COVID-19 vaccination compared to older adults.36–38 Adverse effects after COVID-19 vaccination are a by-product of the exuberant production of type-I interferon that happens to trigger an effective immune response against the SARS-CoV-2.39 Since the generation of type-I interferon is more potent in younger adults, this mechanism may account for the higher prevalence of adverse effects in this age group.39,40
Our study had several limitations. First, although we achieved the minimum required sample size, we obtained a convenience sample from a single center. Therefore, our sample may not accurately represent the vaccinated adult population in Greece. In particular, our participants were predominantly young females. Future research using larger and more representative samples could add valuable insights. Second, our study collected data through self-reported questionnaires, so information bias may be present due to the lack of clinical confirmation. For example, we assessed COVID-19 clinical course through self-assessment since we did not have access to participants' clinical data. Also, in that case we used a self-assessment scale from 0 to 10 to measure clinical course but we cannot validate this scale since participants' clinical data were unknown. Additionally, a recall bias is possible as participants received the booster dose at least six months before our study. Timely clinical evaluation of individuals after COVID-19 vaccination in future studies could minimize information and recall bias. Third, we performed a cross-sectional study and therefore, we cannot determine causality between risk factors and adverse effect incidence. Fourth, we investigated several risk factors of adverse effects but future research should expand our knowledge on this field. Fifth, since we performed our study in a single center all participants received the same COVID-19 vaccine, preventing comparisons between different vaccines. Sixth, we only measured short-term adverse effects without assessing the duration of these effects. Finally, we did not measure participants' medical status (e.g. comorbidities) and the way this could have influenced the booster dose's adverse effects.
ConclusionsAlmost all participants in our study developed adverse effects after the first booster dose. However, these adverse effects were minor and not life-threatening. Pain and swelling at the injection site, fatigue, headache, muscle pain, and fever were the most common adverse effects. Moreover, our results showed that females, young individuals and those in worse clinical course of COVID-19 experienced an increased number of adverse effects. Further studies with bigger and more representative samples should be conducted worldwide in order to get more valid results.
FundingNone.