Fibroepithelial tumors of the breast are a heterogeneous group of mixed lesions with an epithelial and a stromal component. The diagnostic spectrum ranges from the benign variant of fibroadenoma to the phyllodes tumor in its benign, borderline, and malignant forms. These lesions are sometimes difficult to diagnose, especially distinguishing between a cellular fibroadenoma and a benign phyllodes tumor. In both phyllodes tumor and fibroadenoma, the differential diagnosis with other breast lesions, benign and malignant, must be established. The treatment surgery and the prognosis, in the case of phyllodes tumors, will depend on their pathological characteristics.
Las lesiones fibroepiteliales de la mama son un grupo hetereogéneo de lesiones mixtas con un componente epitelial y un componente estromal. Su espectro diagnóstico va desde la variante benigna que es el fibroadenoma hasta el tumor filodes en sus formas benigna, borderline y maligna. Son lesiones en ocasiones de difícil diagnóstico sobre todo cuando se trata de distinguir entre un fibroadenoma celular y un tumor filodes benigno. Tanto en el tumor filodes como en el fibroadenoma ha de establecerse el diagnóstico diferencial con otras lesiones mamarias tanto benignas como malignas. En ambas lesiones el tratamiento es quirúrgico y el pronóstico, en el caso de los tumores filodes estará en función de sus características histopatológicas.
Fibroepithelial tumors of the breast are a heterogeneous group of biphasic neoplasms. They show a stromal and an epithelial component. According to their behavior and management they cover a spectrum ranging from fibroadenoma which is the benign variant and its different forms of presentation to the malignant variant of the malignant phyllodes tumor. In the middle there are the benign and borderline phyllodes tumors. Although it is included as a variant of the phyllodes tumor, there is the periductal stromal tumor that in this work we will treat separately.
Its pathologic diagnosis is a challenge, especially in specimens obtained by core biopsy, and the prognosis and therapeutic approach will depend on its correct typing. In several of these lesions there are overlapping morphological findings that often make their distinction very difficult.
In this work, we intend to do a review of the fibroepithelial lesions of the breast with their respective variants and their diagnostic features.
FibroadenomaFibroadenoma is the most frequent fibroepithelial breast tumor, and is the most common benign tumor in this organ.
Its incidence is the highest in women at reproductive age and can be affected by hormonal activity.1 It is presented as a painless and mobile nodule. Unfrequently it can be multiple and bilateral. In older women the lesion is usually asymptomatic and is discovered during a mammographic examination. Fibroadenomas can also be seen in males with gynecomastia.2,3
Gross findingsFibroadenomas are usually less than 3 cm in size and lesions larger than 5 cm are rare. They are well-demarcated, circumscribed white, firm-to-rubbery masses, with a whitish cut surface, and an elastic consistency. Occasionally they can have hard consistency when hyalinized and calcified. Rarely small clefts can be seen.
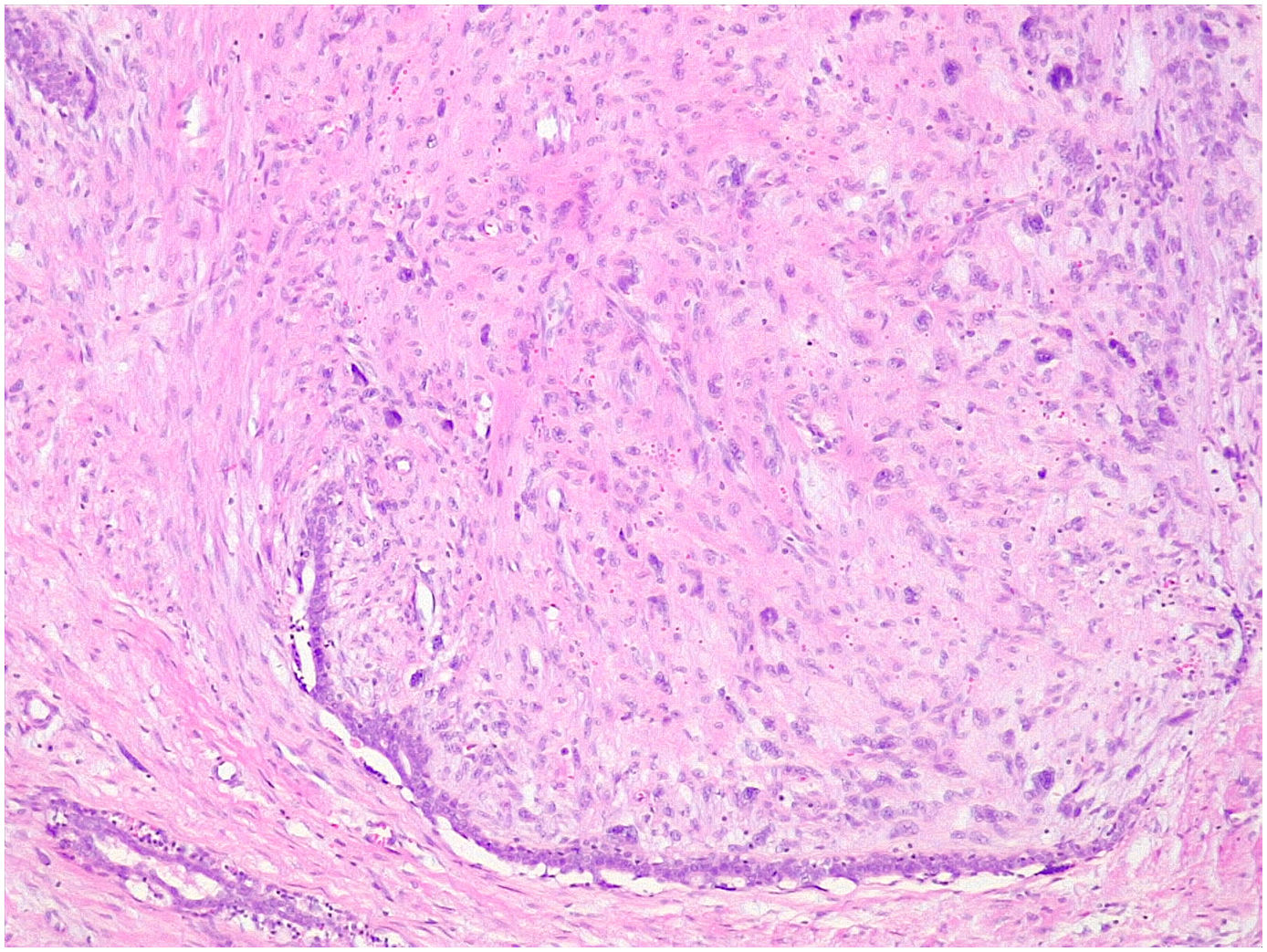
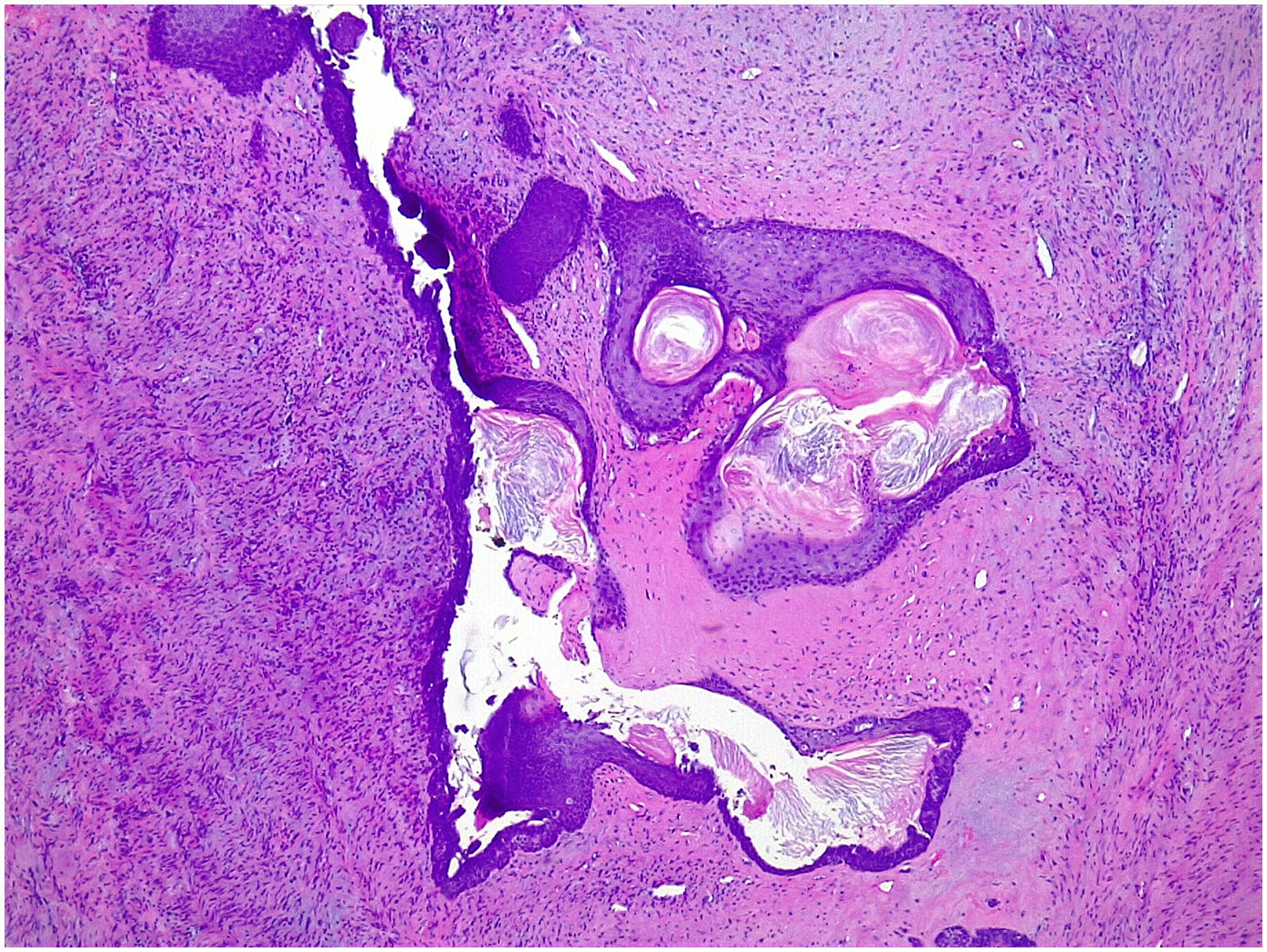
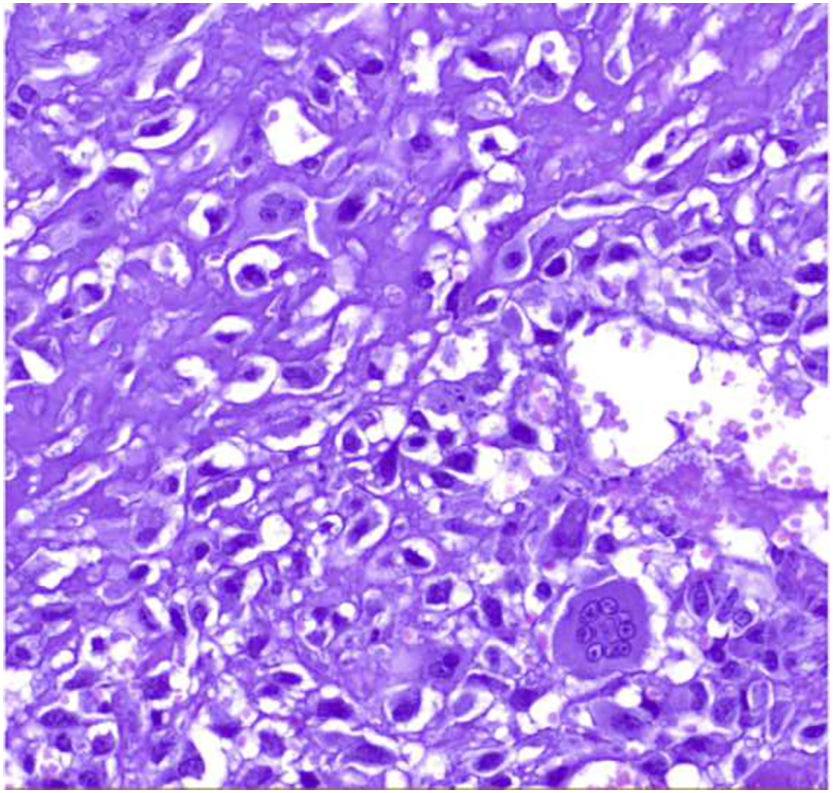
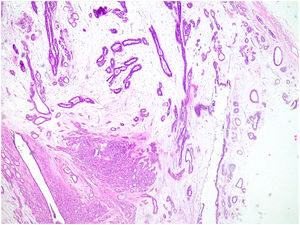
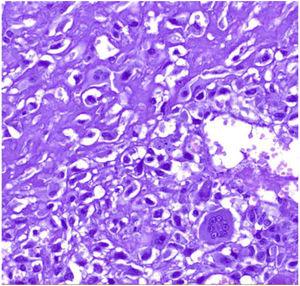
Microscopic findingsThey are a biphasic tumor of circumscribed and expansive contours consisting of a mixture of epithelial and stromal elements with loss of lobular architecture due to expansion of the stromal component among the epithelial elements. The stroma is low cellular, without atypia and without significant mitotic activity. It may be fibrous, myxoid, or hyalinized. Occasionally heterologous components such as adipose, muscle, cartilage, or bone tissue may be seen, although these conditions are very rare.1 Occasionally there may be multinucleated giant cells that may show a certain degree of atypia4,5 (Fig. 1). Also unfrequently in the stroma, a pseudoangiomatous stromal hyperplasia (PASH) changes can be found.4,5 The epithelial component forms tubules or clefts with an epithelial and myoepithelial lining without atypia. Depending on the distribution of the epithelial component, fibroadenomas have been classified as “intracanalicular” when the stromal component compresses the glandular structures forming clefts and “pericanalicular” in which the glandular structures show their usual round configuration.6
Occasionally, in the epithelial component, changes of usual intraductal hyperplasia, atypical intraductal hyperplasia, atypical lobular hyperplasia, intraductal carcinoma, lobular carcinoma in situ, and infiltrating carcinoma can be seen. The incidence of cancer in fibroadenoma is extremely rare with a prevalence of 0.8%4,5,7 and most are of the lobular type.8,9 The long-term risk of developing cancer in a fibroadenoma without hyperplasia is 1.48–1.7, when there is hyperplasia it is 3.4–3.7 and when there is hyperplasia with atypia it is 6.9–7.29.9
Fibroadenoma variants- •
Cellular fibroadenoma: It is characterized by a stroma with a significant increase in cell density without significant atypia and with preservation of the typical architecture of conventional fibroadenoma that can lead to diagnostic confusion with a benign phyllodes tumor. An increase in the number of mitoses can be seen.10
- •
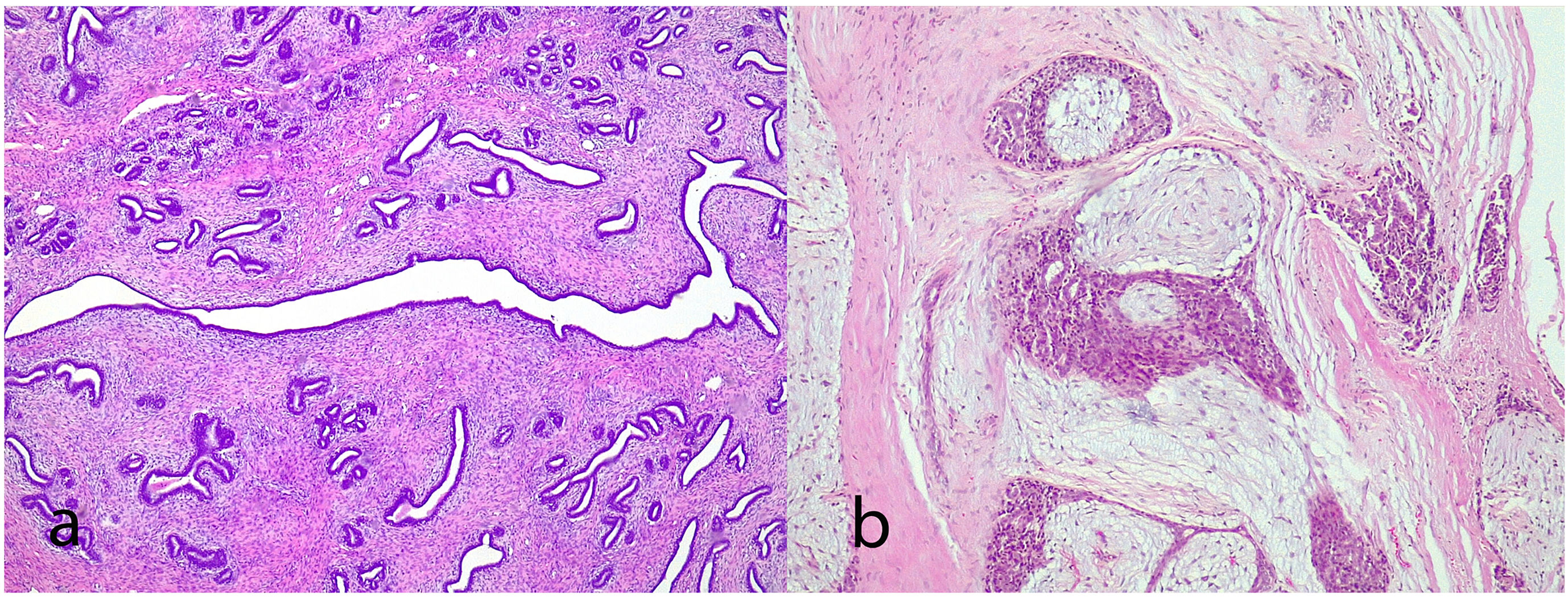
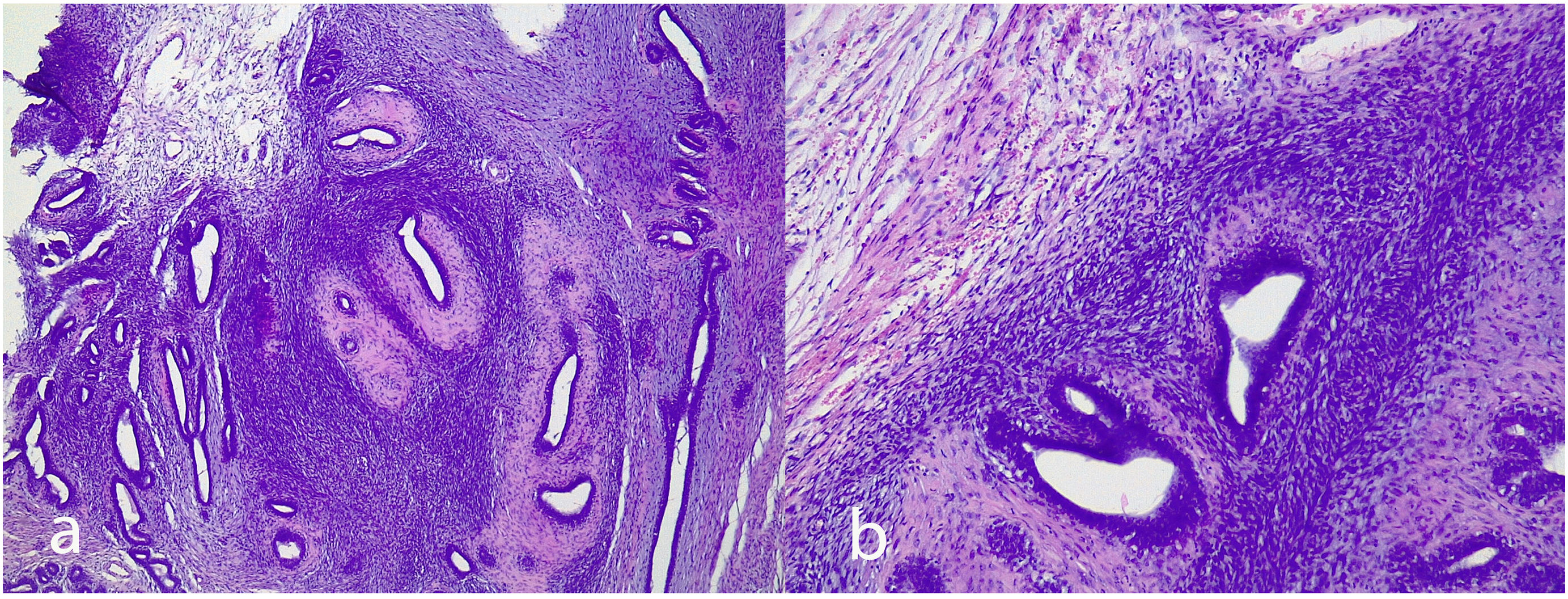
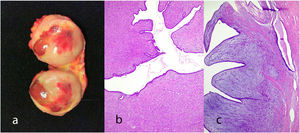
Juvenile fibroadenoma: Has been described in young girls or adolescents and constitute between 7% and 8% of fibroadenomas.10 They can reach a considerable size, exceeding 11 cm.5,11 They show a pericanalicular growth pattern with a slight or moderate increase in cellularity (Fig. 2). It is accompanied by intraductal hyperplasia of gynaecomastoid type and with a mitotic activity in the epithelial component lower than 2 mitoses per 10 HPF.2
- •
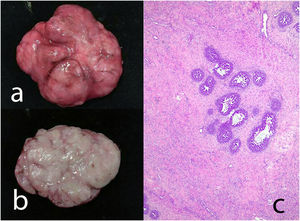
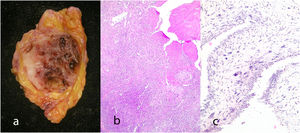
Complex fibroadenoma: These are fibroadenomas in which at least one of the following changes is evident: sclerosing adenosis, papillary apocrine metaplasia, cysts of 3 mm or more and epithelial calcifications (Fig. 3). A three-fold increased risk of cancer has been described in these lesions with respect to the general population.5 They occur in older women and are usually smaller in size than conventional fibroadenomas.4 They seem to have an increased risk of cancer transformation.9
- •
Myxoid fibroadenoma: It shows a stroma with extensive myxoid change that can be confused with a myxoma or a mucinous carcinoma. It is part of the Carney syndrome which is an autosomal dominant entity that presents complex myxomas, spotty skin pigmentation, endocrine hyperactivity, and melanotic psammomatous schwannomas.4
- •
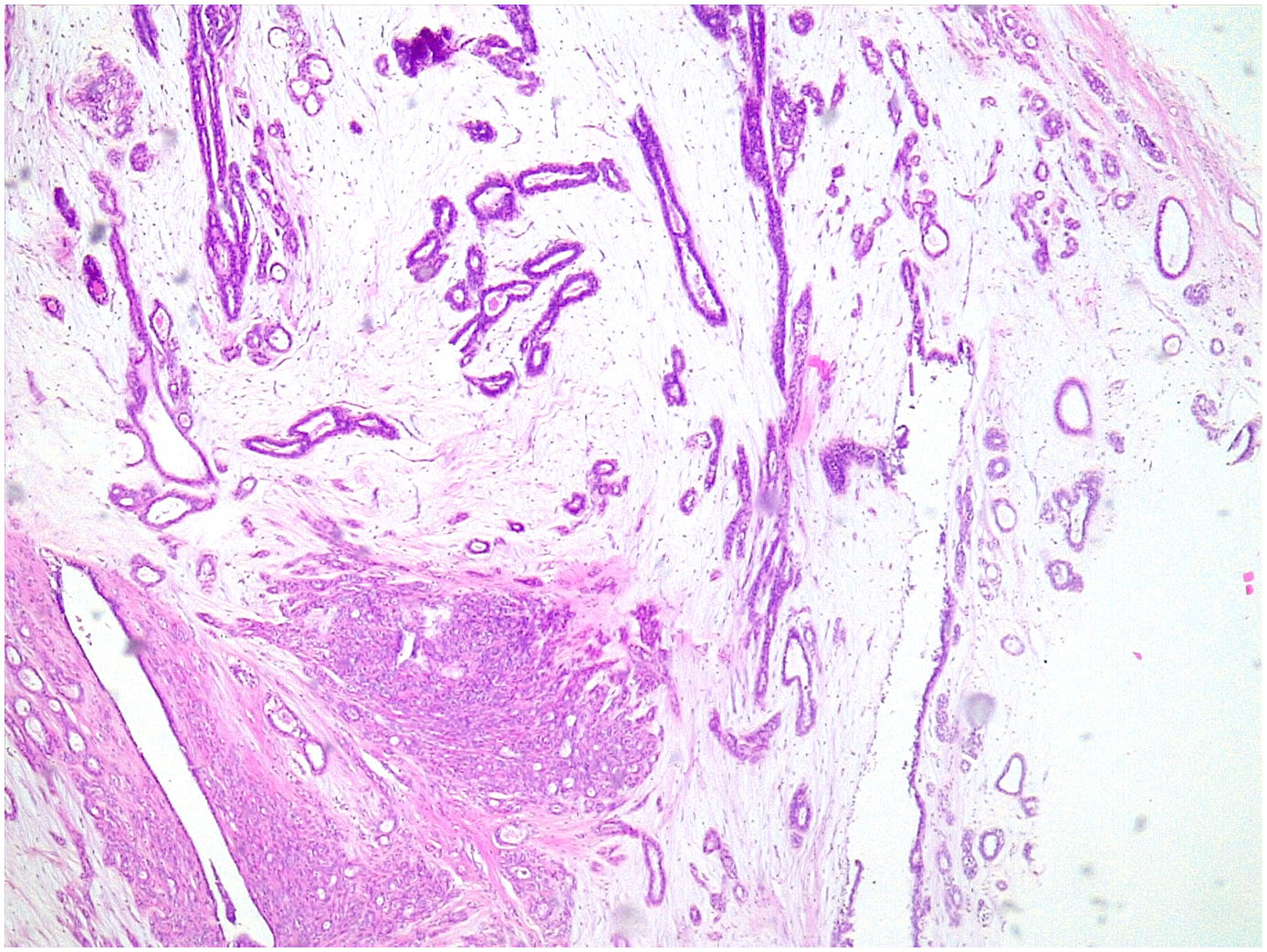
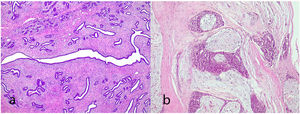
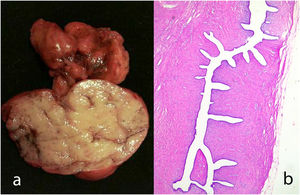
Giant fibroadenoma: These are fibroadenomas of more than 5 cm in diameter10 (Fig. 4).
- •
Fibroadenoma in pediatric age: Constitute around 91% of solid masses in patients under 19 years of age.5 They may show irregular contours and small leaf-like clefts, although they are focal. Mitotic activity is mild.1
- •
Hyalinized fibroadenoma. Occurs in older women. They show a poorly cellular stroma with extensive hyalinization and coarse calcifications. The epithelium is atrophic.10
- •
Fibroadenoma phyllodes: It is a fibroadenoma in which leaf-like clefts are identified without stromal overgrowth.12
The phyllodes tumor is a biphasic tumor with an exaggerated intracanalicular component that forms structures of leaf-like cleft morphology lined by a double cellular layer of epithelium and myoepithelium. They represent between 0.3% and 1% of breast tumors and account for 2.5% of fibroepithelial lesions of the breast.1,4,6,13 They seem to originate in the intralobular and periductal stroma with stromal epithelial–stromal interactions contributing to their pathogenesis.2,14
In 1938, Johannes Muller suggested the denomination of cystosarcoma phyllodes derived from the Greek word “Phyllodium” which means leaf-like, despite the fact that the cystic appearance is not the form of presentation of the lesion.15–17
It has been associated with paraneoplastic syndromes such as hypocalcemia secondary to insulin-like growth factor II secretion and hypertrophic osteopathy.2,4
The age of presentation is generally later than the age of diagnosis of fibroadenomas and they are usually present for a long period of time before they become clinically evident. They are usually presented as a lesion with a recent history of rapid growth, although this growth is not necessarily associated with malignancy.17 Phyllodes tumors have been occasionally described in males with gynecomastia,3,17,18 in pediatric patients,19 in pregnant or lactating women,15,20 and also in ectopic breast tissue.12,21
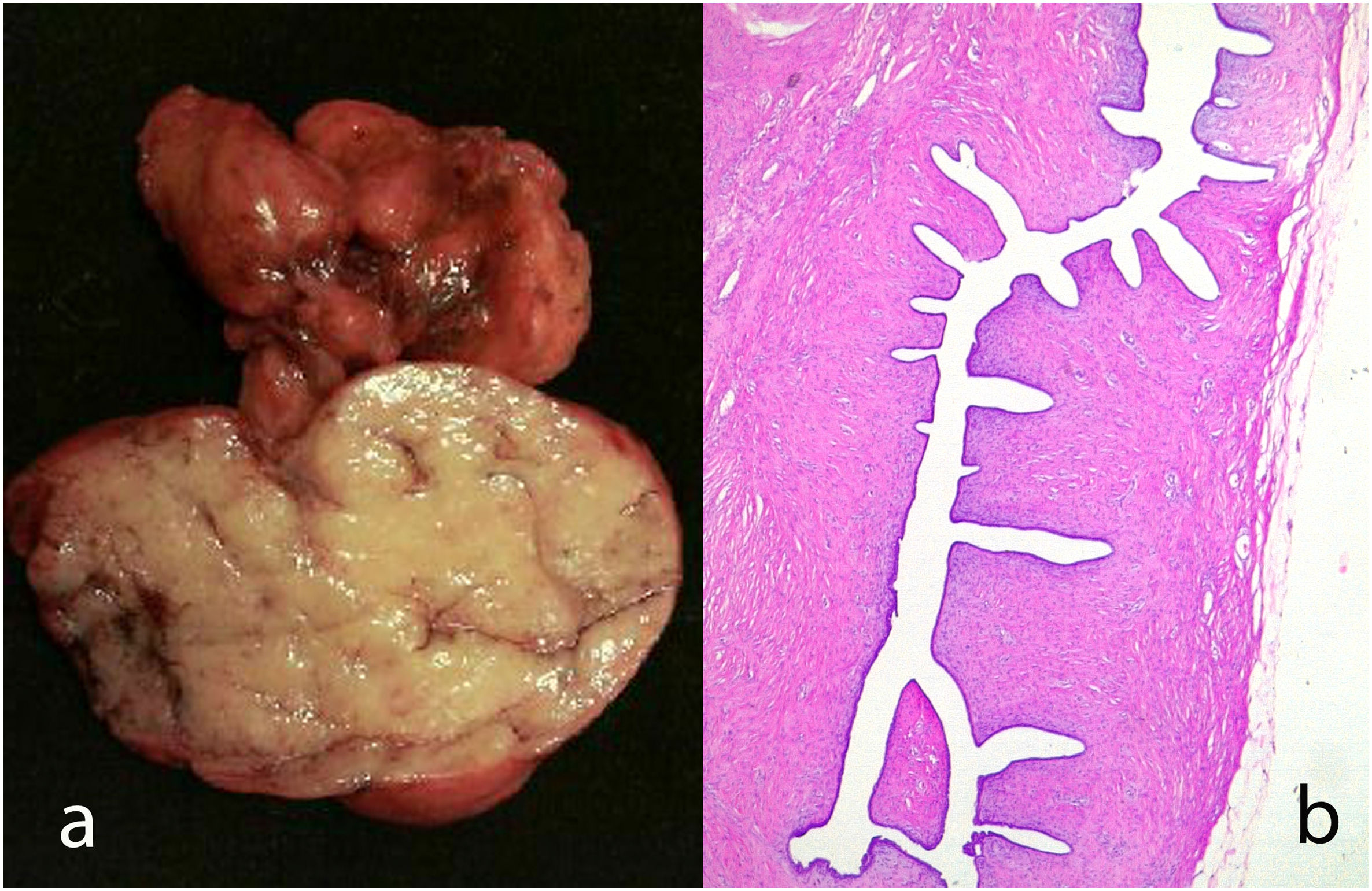
Gross findingsThey are generally circumscribed, unilateral lesions with a size ranging between 4 and 7 cm, although sometimes they can exceed 10 cm in diameter and exceptionally 40 cm.22–24 When are large lesions they can ulcerate of the skin due to ischemia, which does not necessarily imply malignant behavior.1,2,17,25 The margins are usually well defined and that makes their surgical enucleation easy.
The cut surface is grayish and mucoid in appearance with cystic changes or small clefts distributed in the tumor. There may be necrosis and hemorrhage.8,16
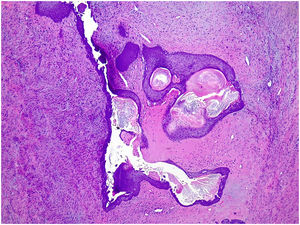
Microscopic findingsIt is a fibroepithelial tumor with a cellular stroma and intranalicular epithelial growth pattern with leaf-like clefts or small cystic spaces. The epithelial component is formed by a double cell lining of epithelial and myoepithelial cells.7 The epithelial component in rare occasions can show hyperplasia of the usual type and squamous or apocrine metaplasia (Fig. 5). Malignant transformation has been described.7,25 It seems is due to the friction of the stromal protrusions in the epithelial clefts with metaplastic change, particularly the squamous one.1 Malignant transformation of the epithelium can be in the form of carcinoma in situ or infiltrating.26 The myoepithelial component is attenuated in high-grade lesions.7 The stromal component shows increased condensation around the epithelial component.25 Depending on the characteristics of the stromal component, phyllodes tumors are subclassified into benign phyllodes tumors, borderline phyllodes tumors, and malignant phyllodes tumors (Table 1).2,5,6
Histological features for grading phyllodes tumors.
| Histologic feature | Phyllodes tumors | ||
|---|---|---|---|
| Benign | Borderline | Malignant | |
| Tumor border | Well-defined | Well-defined, may be focally permeative | Peremeative |
| Stromal cellularity | Cellular, usually mild, may be non-uniform or diffuse | Cellular, usually moderate, may be non-uniform or diffuse | Cellular, usually marked and diffuse |
| Stromal atypia | Mild or none | Mild or moderate | marked |
| Mitotic activity | Usually low: <2.5 mitosis/mm2 (<5 per 10 HPFs) | 2.5 to <5 mitosis/mm2 (5–9 per 10 HPFs) | Usually abundant ≥5 mitosis/mm2 (≥10 per 10 HPFs) |
| Stromal overgrowth | Absent | Absent or very focal | Often present |
| Malignant heterologous elements | Absent | Absent | May be present |
| Distribution relative to all breast tumors | Uncommon | Rare | Rare |
| Relative proportion of all phyllodes tumors | 60–75% | 15–26% | 8–20% |
Based on the degree of stromal cellularity, atypia, number of mitoses, stromal overgrowth, presence of malignant heterologous elements, and the characteristics of the lesion contour, phyllodes tumors are classified into1,2,5,7,13,14,17,25,27:
- •
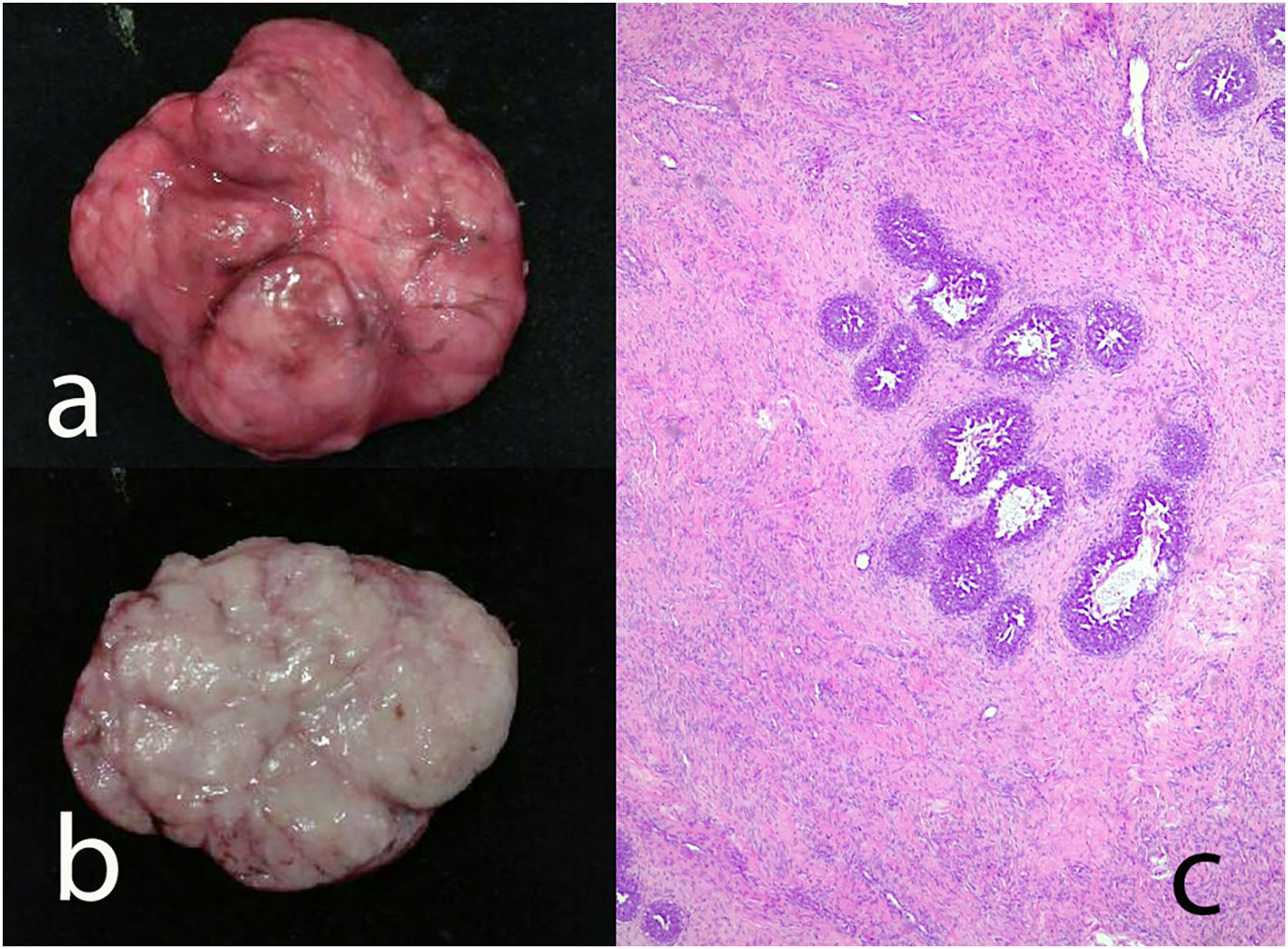
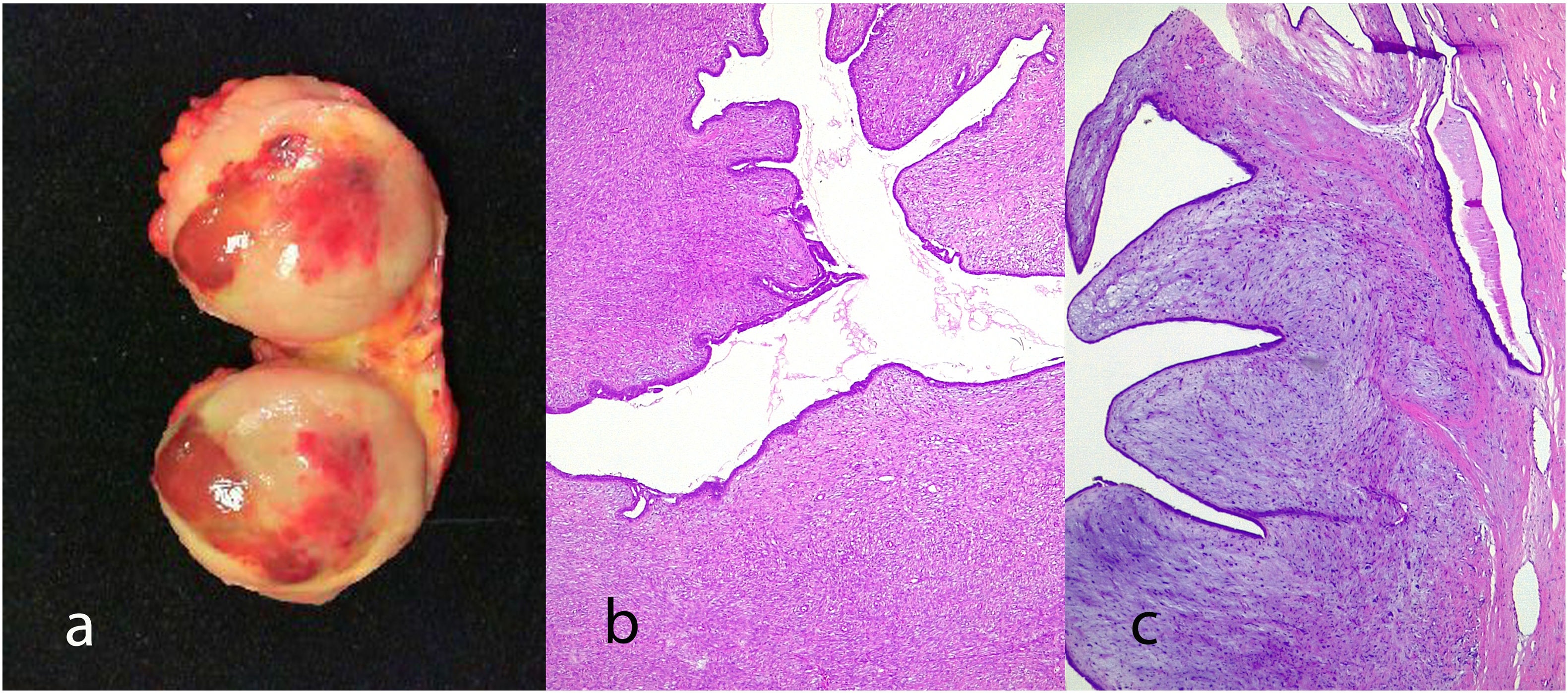
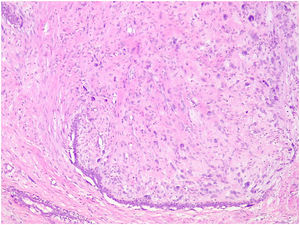
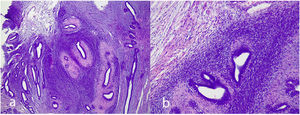
Benign phyllodes tumor: They account for 60–75% of phyllodes tumors. They show a circumscribed contour with slight increase in stromal cell density, with absent or mild atypia, with few mitosis figures (less than 2.5 mitoses/mm2 or less than 5 per 10 HPF), without stromal overgrowth or heterologous element. They may show slight peri-epithelial stromal condensation (Fig. 6).
- •
Borderline phyllodes tumors: They represent 15–26% of phyllodes tumors. They are tumors with well-circumscribed contours although they can present focal permeation. There is an increase in stromal cellularity that can be moderate and non-uniform or diffuse. Stromal atypia is mild to moderate, and mitoses are between 2.5 and 5 per mm2 or between 5 and 9 per 10HPF. Stromal overgrowth is absent, or it is focal and no malignant heterologous elements are observed (Fig. 7).
- •
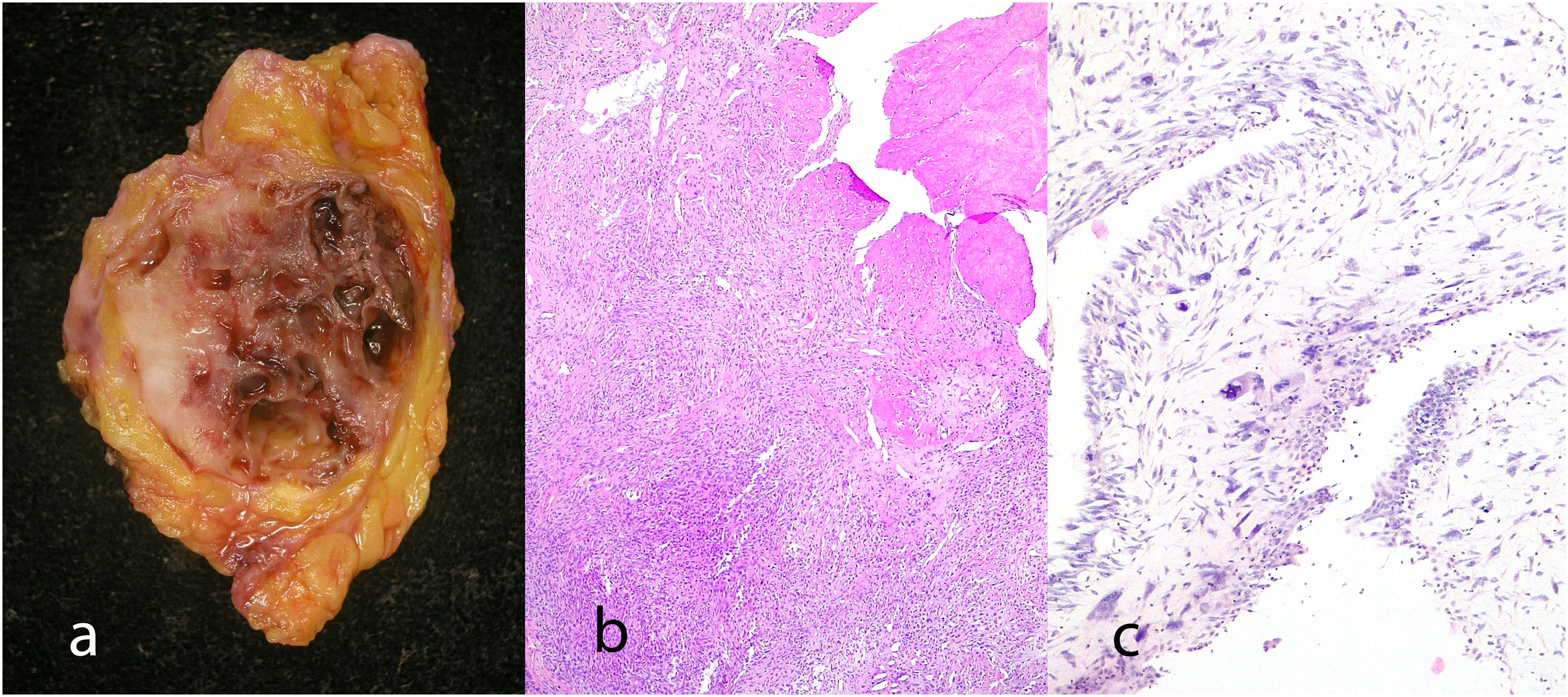
Malignant phyllodes tumors: They represent 8–20% of phyllodes tumors. They present permeative borders with a marked and diffuse increase in stromal cellularity, marked stromal atypia with increased mitotic activity (5 or more mitoses/mm2 or 10 or more mitoses per 10HP) (Fig. 8), with areas of stromal overgrowth in which no epithelial elements are identified and presence of malignant heterologous elements such as chondrosarcoma, osteosarcoma, rhabdomyosarcoma, and leiomyosarcoma (Fig. 9). The presence of a malignant heterologous component is a sufficient criteria for the diagnosis of malignant phyllodes tumor with the exception of a component of the well-differentiated liposarcoma type which is not sufficient since it has been seen that the metastatic potential of this is very low when it is a single heterologous component.2,5,6
The intratumoral heterogeneity that these lesions exhibit often makes it necessary to look for the area with the highest degree of atypia in order to accurately classify the lesion.1,4,24
Immunohistochemical findings- •
Hormone receptors: Benign phyllodes tumors can express estrogen and progesterone receptors in the epithelial component but not in the stroma.1,28
- •
β-Catenin: Positivity for β-Catenin has been described in stromal cells of benign and borderline phyllodes tumors.8,29
- •
Insulin-like growth factor (IGF): Overexpression of IGF 1 and 2 has been seen, especially in areas of higher stromal cell density although it is weak in malignant phyllodes tumors.8
- •
Ki67: Ki67 expression is useful for the diagnosis of fibroepithelial lesions but there is no threshold determined for Ki67 value to categorize lesions.8
- •
CD117 (c-Kit): Although increased CD117 expression has been described in phyllodes tumors, there is no relationship between this expression and tumor grade.6,8,27,30
- •
P53: Malignant phyllodes tumors show a strong nuclear expression of p53 and this expression is associated with increased Ki67 expression.6,8,30
- •
CD10: Borderline and malignant phyllodes tumors express CD10, whereas fibroadenomas and benign phyllodes tumors do not. CD10 expression correlates with tumor grade.8,27,30
- •
CD34: Its expression is more frequent in benign phyllodes tumors.6,25
- •
Cytokeratins: They are always negative in the stromal component of the phyllodes tumor, which differentiates them from metaplastic carcinoma.8
- •
EGFR: A correlation has been demonstrated between EGFR expression and tumor grade, stromal cellularity, mitotic activity, nuclear pleomorphism, and stromal overgrowth in phyllodes tumors, so that malignant phyllodes tumors express more EGFR than benign ones.8,27
- •
IMP3: Malignant phyllodes tumors express IMP3 but neither borderline nor benign ones do, nor does the surrounding mammary parenchyma.8
It is considered a variant of the phyllodes tumor that has been previously labeled as periductal stromal sarcoma although the term periductal stromal tumor is currently preferred.4 It is characterized by a stromal proliferation surrounding an epithelial component that does not show the leaf-like clefts deformation of the phyllodes tumor. It grows as coalescent nodules that can form large masses. By definition it shows moderate stromal atypia with at least 3 mitosis figures per 10 HPFs and an infiltrative boundary1,2,4,25 (Fig. 10). Sometimes it shows a heterologous component of liposarcomatous type.4 It has also been described in pediatric age and in males.4
Differential diagnosisFibroadenoma- •
Fibroadenomatoid change: Are those changes that resemble those of a fibroadenoma but without showing a nodular configuration.10
- •
Tubular adenoma: It is an exaggerated pericanalicular fibroadenoma with little stromal component.31
- •
Nodular stromal pseudoangiomatous stromal hyperplasia: It is a lesion with small stromal spaces that show positivity for CD34 and are negative for CD31 and factor VIII and that do not distort the normal glandular component of the parenchyma in which the lesion is located.32
- •
Mammary hamartoma: Has a more disordered distribution of the epithelial component than fibroadenoma and also very often shows mature adipose tissue.33
- •
Fibroadenoma: The differential diagnosis is basically established by the stromal cellularity which is higher in the phyllodes tumor. The leaf-like clef pattern of the epithelial component is also characteristic of the phyllodes tumor although it has been described focally in fibroadenoma.1,6,25,34 The phyllodes tumor shows an infiltrative contour and the number of mitoses is higher and nuclear pleomorphism is much more pronounced in the phyllodes tumor.6 Multinucleated giant cells are typical of fibroadenoma, however, they can also be found in phyllodes tumors.1,5,14,35 Phyllodes tumors can display areas of fibroadenoma, that makes an extensive sampling of phyllodes essential for their correct diagnosis.1,4,6 The expression of Ki67 and P53 can be useful for the diagnosis.5,14
Differential diagnosis can be difficult as has been shown in a study with 10 pathologists with expertize in breast pathology who were given 21 cellular fibroepithelial lesions to study. There was diagnostic consensus in only 2 of the lesions.36
The distinction between benign phyllodes tumor and fibroadenoma can be particularly difficult in core needle biopsy specimens. The majority of benign phyllodes tumors diagnosed by core needle biopsy have been found to be fibroadenomas in the surgically resected specimen. This is due to the lack of evaluable diagnostic criteria in the core needle biopsy such as margin, and sampling defect.4,13,24
The WHO has recommended the term benign fibroepithelial neoplasia to designate those lesions of difficult differential diagnosis in order to avoid overtreatment.1,2,11,24,37
- •
Fibromatosis: They are usually poorly circumscribed lesions with a softer cellularity and abundant extracellular collagen deposition. They are lesions in which the epithelial component is not a characteristic finding and if present it is trapped as part of the lesion.4,5 Fibromatosis cells express β-Catenin which can also be expressed by benign and borderline phyllodes tumors.8 The expression of CD34 appears in phyllodes tumors and not in fibromatosis.5
- •
Metaplastic carcinoma: These are high-grade spindle cell tumors in which epithelial components with glandular or squamous differentiation are also identified.1,6,7,25 In addition, metaplastic carcinoma is often accompanied by carcinoma in situ or conventional invasive carcinoma.5,14,17 Metaplastic carcinoma cells diffusely express P53.14
- •
Periductal stromal tumor: It lacks the foliaceous structure of the epithelial component.1,2,4,5,25
- •
Sarcoma: Primary sarcomas of the breast are extremely rare and are usually angiosarcomas with a history of breast irradiation. The absence of epithelial component and immunohistochemical findings can help in the differential diagnosis.1,4,6,14,25
- •
Periductal stromal tumor: It lacks the foliaceous structure of the epithelial component.1,2,4,5,25
- •
Malignant melanoma: Sometimes it can show spindle cell morphology and raise the differential diagnosis with a tumor phyllodes.
Immunohistochemical positivity for HMB45, Melan-A, and S-100 protein will be diagnostic of melanoma.4
In the case of fibroadenomas, surgical excision is curative, although some recurrences have been described.37
In the phyllodes tumor the therapeutic approach is surgical excision with a wide safety margin.4,5,25 The recommended distance between the tumor and the margin is 1 cm.1,23,28,38,39 In some cases of benign phyllodes tumor, a follow-up conduct has been postulated if there are affected surgical margins.14,40 In cases of large lesions, mastectomy may be necessary.28,39
The low incidence of axillary metastases makes axillary lymphadenectomy unnecessary.6,14,23,28
There are no randomized studies that demonstrate a beneficial effect of adjuvant therapy either hormonal, radio, or chemotherapy.14,28,38,39
PrognosisThe rate of recurrence of the phyllodes tumor varies according to its grade. Local recurrences have been described in 10–17% of benign phyllodes tumors, in 14–25% of borderline phyllodes tumors, and in 23–30% of malignant phyllodes tumors.2,4,13,23,24,30 In incompletely excised fibroadenomas, some recurrences ranging from 15 to 17% have been described which are very similar to those described for benign phyllodes tumor.37 Recurrence of benign phyllodes tumor is usually benign (56%), but 35% recur as borderline phyllodes tumor and 8% as malignant phyllodes tumor. The recurrence of borderline phyllodes tumor is in the form of benign phyllodes tumor in 25%, in the form of borderline phyllodes tumor in 62.5%, and in the form of malignant phyllodes tumor in 12.5%. Finally, the malignant phyllodes tumor always recurs in malignant form.5
The metastasis rate is 0.13% for benign phyllodes tumors, 1.62% for borderline phyllodes tumors, and 16.7% for malignant phyllodes tumors.2,5,41 Metastases are almost exclusively from the stromal component or heterologous components.4,39 Isolated cases of epithelial and stromal component metastases have been described.14,42 They usually occur in the lung, pleura, and bone, although they have also been reported in the pancreas,43,44 orbit,45 tonsil,46 heart,47 brain,48 and ovary.49 Axillary metastases are extremely rare.6,50
The possibility of recurrence and the risk of metastasis are related to the status of the tumor margin, the grade of the tumor, and the presence of heterologous components.1,2,4,6,50
In conclusion, fibroepithelial tumors of the breast constitute a heterogeneous group of mixed epithelial and stromal lesions, which depending on the stromal component show benign or malignant behavior. It is important to establish the differential diagnosis between cellular fibroadenoma and benign phyllodes tumor and the grading of phyllodes tumor into benign, borderline, and malignant is very important to establish the prognosis of the lesion.
Take home messages- •
Fibroepithelial tumors of the breast are mixed lesions with an epithelial and a stromal component. The prognosis depends on the characteristics of the stromal component.
- •
The category of fibroepithelial tumors includes: fibroadenoma, benign, borderline, or malignant phyllodes tumor and periductal stromal tumor, although the latter has been included in the category of phyllodes tumor.
- •
Fibroadenoma is a benign lesion, very frequent in women of reproductive age. Depending on its histological features there are different varieties: cellular, juvenile, complex, myxoid, giant, pediatric, hyalinized, and phyllodes.
- •
Phyllodes tumor is a rare lesion in the breast and depending on its histological features it is classified as benign, borderline, or malignant. This classification is related to its behavior in terms of locoregional recurrence and its ability to metastasize.
- •
The main differential diagnosis of fibroepithelial tumors is to distinguish a cellular fibroadenoma from a benign phyllodes tumor. The differential diagnosis is established based on the morphologic features of the epithelial component.
- •
In rare occasions the epithelial component of both fibroadenoma and phyllodes tumor may became malignant, either in the form of carcinoma in situ or infiltrating carcinoma.
- •
The treatment of both fibroadenoma and phyllodes tumor is surgical removal. In the case of phyllodes tumor with a security margin of 1 cm or more.
No funding has been used for this review.
Ethical comitee aprovalThe characteristics of the review exempt it from ethics committee approval.
Conflict of interestF. Tresserra is Co-Managing Editor of Revista de Senologia y Patologia Mamaria. The other authors declare no conflict of interest.
This study has been done under the auspices of the Càtedra d'Investigació en Obstetrícia i Ginecologia de la Universitat Autònoma de Barcelona.