Young and late onset patients with paranoid schizophrenia were compared, regarding the initial prodromal and active phases of the disorder, in order to examine the influence of age of onset on the prodromal and active phase symptomatology of the disease.
Materials and methodsWe examined 88 consecutively hospitalized patients with paranoid schizophrenia. Age cutoff points were set at <30 years of age for the young, and ≥35 years of age for the late onset group. Diagnoses were made prospectively, using the Structured Clinical Interview for DSM-IV-Patient Edition for Axis I disorders (SCID-P). Type and severity of psychopathology in the active phase were assessed by applying the Structured Clinical Interview for Positive and Negative Syndrome Scale (PANSS). Patients were retrospectively examined regarding their initial prodromal symptoms by applying the Structured Clinical Interview for DSM-III-R Patient Edition and clinical interviewing for additional symptoms. Comparisons were performed by applying the two-tailed Wilcoxon rank-sum and the chi-square statistical tests.
ResultsThe young onset group was characterized by significantly more negative prodromal symptoms, and heavier negative symptomatology in the active phase, than the late onset group. Differences were more prominently shown in male patients.
ConclusionsOlder age of onset of paranoid schizophrenia appears to be related to a less severe form of the disease, characterized by less severity of negative symptomatology, already demonstrated in the prodromal phase of the disorder.
Se comparó a pacientes con esquizofrenia paranoide de inicio en el joven y de inicio tardío en lo relativo a la fase prodrómica y la fase activa del trastorno, con objeto de examinar la influencia que tenía la edad de inicio en la sintomatología de ambas fases de la enfermedad.
Material y métodosExaminamos a 88 pacientes consecutivos hospitalizados por esquizofrenia paranoide. Los valores de corte de la edad se establecieron en < 30 años para el grupo de inicio en el joven y ≥ 35 años en el grupo de inicio tardío. Los diagnósticos se hicieron de forma prospectiva, con el empleo de la entrevista clínica estructurada Structured Clinical Interview for DSM-IV-Patient Edition para los trastornos del Eje I (SCID-P). Se evaluó el tipo y la gravedad de la psicopatología en la fase activa mediante la aplicación de la entrevista clínica estructurada para la escala Positive and Negative Syndrome Scale (PANSS). Se efectuó un examen retrospectivo de los pacientes respecto a los síntomas prodrómicos iniciales, mediante la aplicación de la Structured Clinical Interview for DSM-III-R Patient Edition y una entrevista clínica respecto a otros síntomas adicionales. Se realizaron comparaciones con el empleo de las pruebas estadísticas de ji2 y de suma de rangos de Wilcoxon bilaterales.
ResultadosEl grupo de inicio en el joven se caracterizaba por la presencia significativamente mayor de síntomas prodrómicos negativos, y por una sintomatología negativa más intensa en la fase activa, en comparación con el grupo de inicio tardío. Las diferencias observadas fueron más prominentes en los pacientes varones.
ConclusionesLa edad de inicio más avanzada de la esquizofrenia paranoide parece estar relacionada con una forma menos grave de la enfermedad, que se caracteriza por una menor intensidad de la sintomatología negativa, que se aprecia ya en la fase prodrómica del trastorno.
Schizophrenia is a severe, lifelong disorder, most often beginning in adolescence or early adulthood.1 Nevertheless, about 23.5% and 12.3% of patients become ill after 40 or 60 years of age, respectively, with age of onset contributing to illness heterogeneity.2
The initial prodromal phase of schizophrenia has received research attention recently, aiming at early intervention.3,4 Prospective studies focus on early detection of young persons at risk of developing the disease.5 The Ultra High Risk criteria require that a young person aged between 14 and 30 years demonstrates attenuated psychotic symptoms, or brief limited intermittent psychotic symptoms, or has either a first degree relative with a psychotic disorder or schizotypal personality disorder, along with significant functional deterioration during the previous year.5 Another prospective approach is by using the “basic symptoms” concept, which are self-experienced subclinical disturbances considered to be close to the core underlying disturbance in schizophrenia.6 The above approaches have been combined recently.7
Retrospective studies, on the other hand, have provided a broader range of prodromal symptoms.8,9 Häfner et al.10,11 have examined the impact of age of onset on the early course of the disorder, finding small differences in symptomatology relating to age of onset and sex, but the initial prodromal period has not been investigated separately. In most cases a prodromal phase precedes the onset of active symptoms, usually beginning with negative and/or non-specific symptoms, while positive prepsychotic symptoms follow, close to the onset of frank psychosis.12
Studies on the active phase of late-onset schizophrenia have reported mixed results. Some have reported small or no differences at all,10,11,13 while others have found less severity of negative symptoms and formal thought disorder14,15 and more severe positive symptoms14,16 in the late onset group. Sato et al.17 studied a sample of first admission patients with a strict diagnosis of schizophrenia and reported less negative symptoms and heavier systematic persecutory delusions in the late onset group.
Age cutoff points for late onset schizophrenia vary among researchers, from 40, to 44, or 50 years of age.13,14,17,18 A consensus has been reached that cases in which onset occurs between ages 40 and 60 should be called late onset schizophrenia.18 However, it has been stated that the cutoff point of 40 years might be too high for the late onset group,18 according to epidemiological data.19 It has been reported that the male distribution of age-at-onset demonstrates two peaks, with modal ages of onset of 21 and 39 years, whereas females exhibit a distribution with three peaks, with modal ages of onset at 22, 37 and 62 years.20 A late-onset group, with age of onset between 35 and 60, has been studied in previous work.8,11,21 On the other hand, it has been found that about 77% of patients with schizophrenia become ill before the age of 30.22 The age of 30 has been used as a cutoff point for a young onset group by other studies.23,24 Regarding paranoid schizophrenia in particular, two peaks have been observed in the distribution of age of onset of the disorder, the first and highest before the age of 30, and the second, mostly represented by women, after the age of 35.25
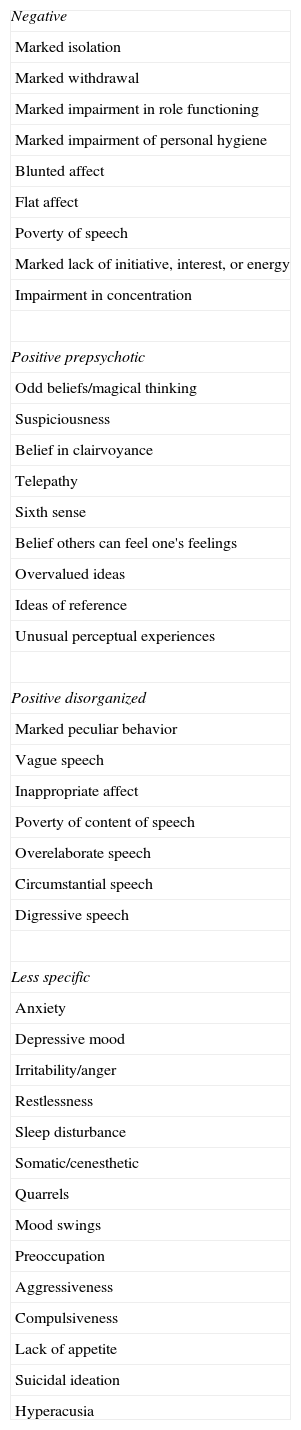
In the present study, two age groups of patients with paranoid schizophrenia are comparatively examined, with illness onset <30 years and ≥35 years, regarding the type and severity of psychopathology during the active phase as well as the symptomatology and duration of the initial prodromal phase, which precedes the first psychotic episode. The prodromal symptoms studied are those listed in DSM-III-R,26 as elaborated by Gourzis et al.9 classified in negative, positive-prepsychotic, positive-disorganized, less-specific (Table 1). The paranoid subtype of the disorder was chosen so as to avoid heterogeneity of the sample, caused by the inclusion of patients with different types of schizophrenia at different proportions in the two age groups.25
Prodromal symptoms in patients with schizophrenia.
| Negative |
| Marked isolation |
| Marked withdrawal |
| Marked impairment in role functioning |
| Marked impairment of personal hygiene |
| Blunted affect |
| Flat affect |
| Poverty of speech |
| Marked lack of initiative, interest, or energy |
| Impairment in concentration |
| Positive prepsychotic |
| Odd beliefs/magical thinking |
| Suspiciousness |
| Belief in clairvoyance |
| Telepathy |
| Sixth sense |
| Belief others can feel one's feelings |
| Overvalued ideas |
| Ideas of reference |
| Unusual perceptual experiences |
| Positive disorganized |
| Marked peculiar behavior |
| Vague speech |
| Inappropriate affect |
| Poverty of content of speech |
| Overelaborate speech |
| Circumstantial speech |
| Digressive speech |
| Less specific |
| Anxiety |
| Depressive mood |
| Irritability/anger |
| Restlessness |
| Sleep disturbance |
| Somatic/cenesthetic |
| Quarrels |
| Mood swings |
| Preoccupation |
| Aggressiveness |
| Compulsiveness |
| Lack of appetite |
| Suicidal ideation |
| Hyperacusia |
Our study was conducted at the Department of Psychiatry of the University Hospital of Patras, an inpatient service admitting mostly new onset cases, in a large administrative area of about 1 million people. Adolescents and adults are admitted in the same ward. Age of onset was defined as the age at which the first prodromal symptom was demonstrated. End of the initial prodromal phase was considered as the time of appearance of the first symptom of the active phase. Subjects with age of onset <12 years and ≥60 years were not included in the study. Cutoff points for the comparison groups were set at <30 years of age, referred to as the young onset group, and ≥35 years of age, referred to as the late onset group in the present work.
We studied 88 consecutively hospitalized patients, aged 17–65 years at the time of entry in the study, during a three-year period, from 15 March 2005 to 7 May 2008. Patients with paranoid schizophrenia and a history of no more than three psychotic episodes were included in the study, provided that they accepted to participate. Subjects with a history of organic brain disease, or psychotic disorder due to substance use or general medical condition were excluded. Of the patients, 21 had disease onset at age ≥35 years and 60 had disease onset at age <30 years. Seven patients had their first prodromal symptom between 30 and 35 years of age; this group was not further studied, because it was too small to make any assumptions.
ProcedureThe diagnosis of paranoid schizophrenia was made prospectively, during the patients’ hospitalization, by the author MS, according to DSM-IV-TR,27 using the Structured Clinical Interview for DSM-IV-Patient Edition for Axis I disorders.28 Current diagnoses of alcohol abuse or dependence, and cannabis abuse or dependence, were made according to DSM-IV-TR criteria. Also, during their active psychotic phase and within 5 days from admission at the Psychiatric Department, all patients were evaluated by the author by applying the structured clinical interview for Positive and Negative Syndrome Scale (PANSS),29 validated for the Greek population.30
After remission of the active phase, patients were retrospectively assessed for the presence and duration of initial prodromal symptomatology. Additional information was obtained by their family members. This was not possible in one patient, due to fast discharge. The prodromal symptoms investigated were those reported previously9 (Table 1), and were obtained by applying the Structured Clinical Interview for DSM-III-R Patient Edition31 and clinical interviewing for additional symptoms of each patient and at least one of significant others.
In order to assess inter-rater agreement, twenty of the patients were re-evaluated by applying SCID-P, PANSS, and assessed for prodromal symptoms and duration of prodromal period, by the author IA, who was unaware of the results of the evaluation of the previous interviewer.
All patients participating in the study provided written informed consent. The study was approved by the ethics and deontology committee of the University Hospital of Patras Medical School.
Statistical analysisA non-parametric statistical test for unpaired data (two-tailed Wilcoxon rank-sum test) was used for comparing differences between the groups for single items and each category of positive, negative and general psychopathology of the PANSS, as well as for the number of prodromal symptoms studied and duration of initial prodromal period.
The chi-square statistical test was used for comparing differences between the groups for all negative, positive-prepsychotic, positive-disorganized and non-specific prodromal symptoms, as well as for the number of psychotic episodes and rates of alcohol and cannabis abuse or dependence. For large samples chi-square test was corrected for continuity (i.e. 12≤years<30 vs 35≤years≤59, young vs late with first episode). For small samples, chi-square test (in case where all expected frequencies were more than 5), or Fisher's exact test was used (i.e. young vs late males with first episode, young males vs young females with first episode).
Comparisons were made between the young onset and late onset groups, separately for male and female patients (young onset vs late onset), and also between the two sexes (male vs female patients), within each age-of-onset group. A subanalysis of first episode patients was also conducted. However, the examination of young onset (No.=6) vs late onset (No.=8) females, and late onset males (No.=5) vs late onset females (No.=8) was not possible due to very small sample sizes.
In general, differences were considered statistically significant if p-values were lower than 0.05. To compensate for multiple comparisons, Bonferroni correction was applied in order to derive the level of significance in single items of positive and negative psychopathology (i.e. 0.05/7=0.007) and in single items of general psychopathology (i.e. 0.05/16=0.003) of the PANSS. Derived values of p<0.007 and p<0.003, respectively, indicate statistically significant difference.
Statistical analysis was performed, using a statistical software package tool (NCSS Statistical software 2007, Kaysville, Utah, USA).
Inter-rater agreementThe inter-rater agreement between the two examiners was studied by means of the unweighted kappa (κ) statistical test. There was complete agreement between the two diagnosticians for the diagnosis of paranoid schizophrenia (κ=1.0). Inter-rater reliability for all items of the PANSS studied was substantial to perfect, with 0.71≤κ≤0.84. Inter-rater reliability for each prodromal symptom studied was also substantial to perfect, with 0.76≤κ≤1.0. There was perfect agreement for the duration of the prodromal period (Spearman rank order correlation, rs=0.99). In all cases, the agreement observed was statistically significant (0.00001<p<0.0478).
ResultsThe number of psychotic episodes was not significantly different among the groups studied. Of young onset males (No.=46), 25 (54%) were in their first, 15 (33%) were in their second and 6 (13%) were in their third psychotic episode, compared with 5 (63%), 2 (25%) and 1 (13%) of late onset males (No.=8), respectively. Regarding females, the number of patients in their first, second, or third psychotic episode were 6 (43%), 5 (36%), 3 (21%) and 8 (62%), 4 (31%), 1 (8%) for the young onset (No.=14) and late onset group (No.=13), respectively.
Mean time intervals±SD between the end of the prodromal period and entry in the study were 40.50±38.56 months (median: 33 months) and 45.56±43.66 months (median: 30 months) for the young and the late onset group respectively, with no significant difference among the groups. In the first episode sample, mean time intervals±SD were 20.84±34.93 months (median: 6 months) and 31.46±39.91 (median: 24 months) for young onset and late onset patients, respectively (p=0.12, not significant).
Demographic featuresThe mean age±standard deviation (SD) of the patients at admission was 30.71±8.68, with a range from 17 to 59 years for males, and 36.47±10.59, with a range from 21 to 65 years for females. The educational level of the patients was primary level in 23.43%, high school level in 45.7%, and college or university level in 30.9%, in the whole sample studied (No.=81), with no significant differences among the groups.
Alcohol and cannabis useCurrent abuse and dependence of alcohol was observed in 18.5% and 3.7% of the whole sample, respectively, whereas 7.4% and 2.5% of the whole sample received current diagnoses of cannabis abuse and dependence, respectively, without any significant differences among the groups studied.
In the first episode sample, current alcohol abuse and dependence were observed in 15.9% and 0%, and current cannabis abuse and dependence in 9.1% and 2.3% of the sample, respectively, with no significant differences among groups.
Age of onsetMean ages of onset±SD were 20.89±4.66 years for young onset males (range: 12–29 years), 23.86±3.86 years for young onset females (range: 19–29 years), 38.75±4.00 years for late onset males (range: 35–45 years) and 40.46±6.25 years for late onset females (range: 35–57 years).
Young onset females become ill on average 3 years later than young onset males, a difference which is statistically significant (p=0.032). The age of onset in the late onset group is not significantly different between the two sexes (p=0.533). The male-female difference in the young onset group is also observed in the first episode sample, with mean ages of onset±SD 20.56±4.98 years and 24.33±4.17 years, for males (No.=25) and females (No.=6), respectively, though marginally not significant (p=0.071).
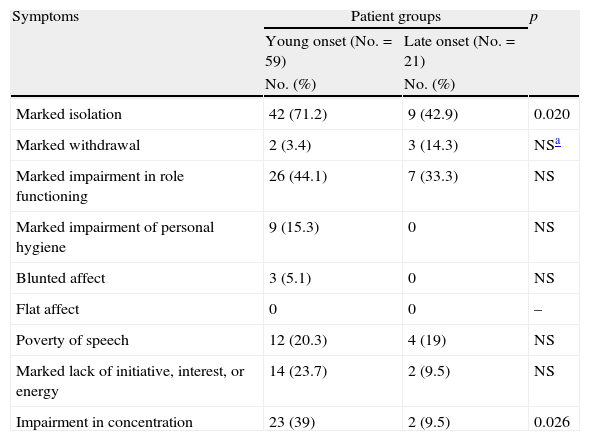
Prodromal periodYoung onset patients had a significantly greater number of negative prodromal symptoms than the late onset group (mean±SD: 2.22±1.48 and 1.28±1.18 respectively, p=0.008), as well as significantly higher frequencies in marked isolation and impairment of concentration (Table 2). No difference was found in the duration of the prodromal period for the young-onset and late-onset groups (mean±SD: 34.88±39.70 months and 37.95±41.50 months, respectively). Significantly more negative prodromal symptoms were also observed in young onset than in late onset men (mean±SD: 2.39±1.46 and 1.12±1.00, respectively, p=0.018), however, notwithstanding higher frequencies of each negative prodromal symptom in young onset males, none reached significance level. In the female sample, no significant differences were observed between the two age groups. Young onset males demonstrated significantly longer initial prodromal periods than young onset females (mean±SD: 41.1±42.39 months and 12.8±14.17 months, respectively, p=0.006).
Negative prodromal symptoms in the young onset and late onset groups.
| Symptoms | Patient groups | p | |
| Young onset (No.=59) | Late onset (No.=21) | ||
| No. (%) | No. (%) | ||
| Marked isolation | 42 (71.2) | 9 (42.9) | 0.020 |
| Marked withdrawal | 2 (3.4) | 3 (14.3) | NSa |
| Marked impairment in role functioning | 26 (44.1) | 7 (33.3) | NS |
| Marked impairment of personal hygiene | 9 (15.3) | 0 | NS |
| Blunted affect | 3 (5.1) | 0 | NS |
| Flat affect | 0 | 0 | – |
| Poverty of speech | 12 (20.3) | 4 (19) | NS |
| Marked lack of initiative, interest, or energy | 14 (23.7) | 2 (9.5) | NS |
| Impairment in concentration | 23 (39) | 2 (9.5) | 0.026 |
Similarly, in first onset patients, there was a greater number of negative prodromal symptoms in the young onset group (No.=31) compared with the late onset one (No.=13) but the difference was marginally not significant (mean±SD: 2.42±1.50 vs 1.62±1.26, respectively, p=0.07). Mean duration of prodromal period±SD was not significantly different between young (42.77±47.88 months) and late onset patients (53.30±45.49 months). A higher frequency of impairment of concentration was also observed in young onsets at a marginally not significant level (38.7% vs 7.69%, p=0.090). In the male sample no significant results were observed. In the young onset group, the difference in the prodromal duration between males (No.=25) and females (No.=6) was again found (mean±SD: 49.32±50.80 months vs 15.50±16.20 months, respectively, p=0.058, marginally not significant).
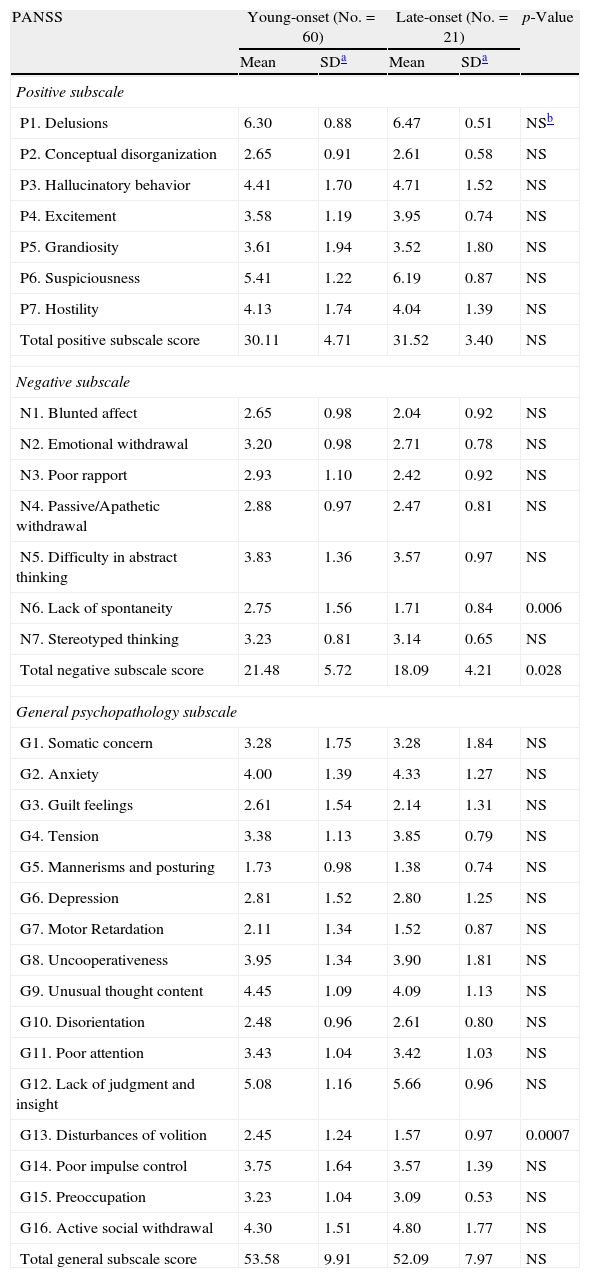
Active phaseYoung onset patients were characterized by significantly higher total negative subscale scores than late onset ones (p=0.028), as well as significantly more severe lack of spontaneity and disturbances of volition (p=0.006 and p=0.0007, respectively). There was not any significant difference at the total positive or general psychopathology subscale scores (Table 3). In the male sample, young onset patients had more severe blunted affect (mean±SD:2.63±0.92 vs 1.50±0.53, p=0.002), lack of spontaneity (mean±SD: 2.63±1.45 vs 1.25±0.46, p=0.003), and higher total negative subscale scores (mean±SD:21.21±5.33 vs 16.37±3.29, p=0.011) than late onset men. Regarding females, no significant differences were found at positive, negative and general psychopathology subscales of the PANSS. Disturbances of volition were heavier in young onset vs late onset males (mean±SD: 2.26±1.08 and 1.25±0.46, respectively, p=0.008) and females (3.07±1.54 and 1.76±1.16, respectively, p=0.004), but the differences were marginally not significant.
PANSS scores in the young onset and late onset groups.
| PANSS | Young-onset (No.=60) | Late-onset (No.=21) | p-Value | ||
| Mean | SDa | Mean | SDa | ||
| Positive subscale | |||||
| P1. Delusions | 6.30 | 0.88 | 6.47 | 0.51 | NSb |
| P2. Conceptual disorganization | 2.65 | 0.91 | 2.61 | 0.58 | NS |
| P3. Hallucinatory behavior | 4.41 | 1.70 | 4.71 | 1.52 | NS |
| P4. Excitement | 3.58 | 1.19 | 3.95 | 0.74 | NS |
| P5. Grandiosity | 3.61 | 1.94 | 3.52 | 1.80 | NS |
| P6. Suspiciousness | 5.41 | 1.22 | 6.19 | 0.87 | NS |
| P7. Hostility | 4.13 | 1.74 | 4.04 | 1.39 | NS |
| Total positive subscale score | 30.11 | 4.71 | 31.52 | 3.40 | NS |
| Negative subscale | |||||
| N1. Blunted affect | 2.65 | 0.98 | 2.04 | 0.92 | NS |
| N2. Emotional withdrawal | 3.20 | 0.98 | 2.71 | 0.78 | NS |
| N3. Poor rapport | 2.93 | 1.10 | 2.42 | 0.92 | NS |
| N4. Passive/Apathetic withdrawal | 2.88 | 0.97 | 2.47 | 0.81 | NS |
| N5. Difficulty in abstract thinking | 3.83 | 1.36 | 3.57 | 0.97 | NS |
| N6. Lack of spontaneity | 2.75 | 1.56 | 1.71 | 0.84 | 0.006 |
| N7. Stereotyped thinking | 3.23 | 0.81 | 3.14 | 0.65 | NS |
| Total negative subscale score | 21.48 | 5.72 | 18.09 | 4.21 | 0.028 |
| General psychopathology subscale | |||||
| G1. Somatic concern | 3.28 | 1.75 | 3.28 | 1.84 | NS |
| G2. Anxiety | 4.00 | 1.39 | 4.33 | 1.27 | NS |
| G3. Guilt feelings | 2.61 | 1.54 | 2.14 | 1.31 | NS |
| G4. Tension | 3.38 | 1.13 | 3.85 | 0.79 | NS |
| G5. Mannerisms and posturing | 1.73 | 0.98 | 1.38 | 0.74 | NS |
| G6. Depression | 2.81 | 1.52 | 2.80 | 1.25 | NS |
| G7. Motor Retardation | 2.11 | 1.34 | 1.52 | 0.87 | NS |
| G8. Uncooperativeness | 3.95 | 1.34 | 3.90 | 1.81 | NS |
| G9. Unusual thought content | 4.45 | 1.09 | 4.09 | 1.13 | NS |
| G10. Disorientation | 2.48 | 0.96 | 2.61 | 0.80 | NS |
| G11. Poor attention | 3.43 | 1.04 | 3.42 | 1.03 | NS |
| G12. Lack of judgment and insight | 5.08 | 1.16 | 5.66 | 0.96 | NS |
| G13. Disturbances of volition | 2.45 | 1.24 | 1.57 | 0.97 | 0.0007 |
| G14. Poor impulse control | 3.75 | 1.64 | 3.57 | 1.39 | NS |
| G15. Preoccupation | 3.23 | 1.04 | 3.09 | 0.53 | NS |
| G16. Active social withdrawal | 4.30 | 1.51 | 4.80 | 1.77 | NS |
| Total general subscale score | 53.58 | 9.91 | 52.09 | 7.97 | NS |
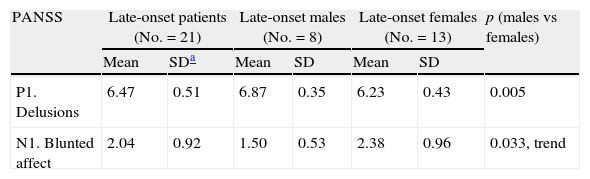
Within each age group, no significant differences were found between the two sexes, with the exception of delusions, which was heavier in late onset men compared with late onset women. Late onset women tended to suffer from heavier blunted affect than late onset men (Table 4).
Sex differences in PANSS scores in the late onset group. Only significant results are demonstrated.
| PANSS | Late-onset patients (No.=21) | Late-onset males (No.=8) | Late-onset females (No.=13) | p (males vs females) | |||
| Mean | SDa | Mean | SD | Mean | SD | ||
| P1. Delusions | 6.47 | 0.51 | 6.87 | 0.35 | 6.23 | 0.43 | 0.005 |
| N1. Blunted affect | 2.04 | 0.92 | 1.50 | 0.53 | 2.38 | 0.96 | 0.033, trend |
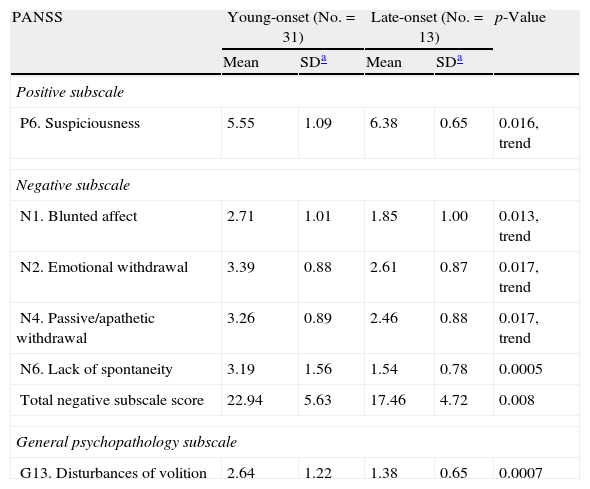
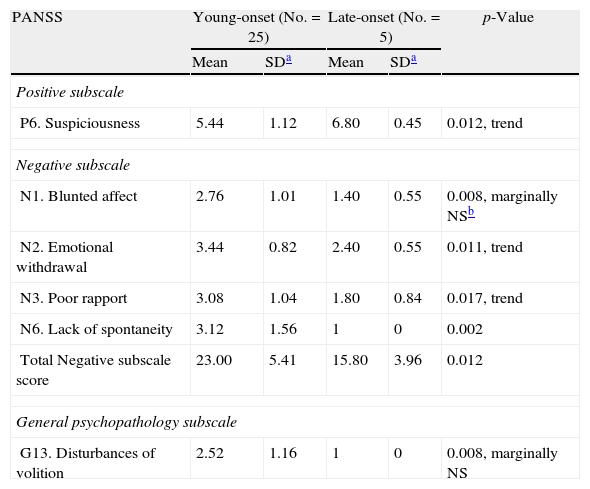
Similar or even more robust results were found in the first onset sample, regarding negative symptoms. Moreover, there was a trend for heavier suspiciousness in the late onset group (Tables 5 and 6).
PANSS scores in first episode young onset and late onset patients. Only significant results are presented.
| PANSS | Young-onset (No.=31) | Late-onset (No.=13) | p-Value | ||
| Mean | SDa | Mean | SDa | ||
| Positive subscale | |||||
| P6. Suspiciousness | 5.55 | 1.09 | 6.38 | 0.65 | 0.016, trend |
| Negative subscale | |||||
| N1. Blunted affect | 2.71 | 1.01 | 1.85 | 1.00 | 0.013, trend |
| N2. Emotional withdrawal | 3.39 | 0.88 | 2.61 | 0.87 | 0.017, trend |
| N4. Passive/apathetic withdrawal | 3.26 | 0.89 | 2.46 | 0.88 | 0.017, trend |
| N6. Lack of spontaneity | 3.19 | 1.56 | 1.54 | 0.78 | 0.0005 |
| Total negative subscale score | 22.94 | 5.63 | 17.46 | 4.72 | 0.008 |
| General psychopathology subscale | |||||
| G13. Disturbances of volition | 2.64 | 1.22 | 1.38 | 0.65 | 0.0007 |
PANSS scores in first episode young onset and late onset men. Only significant results are presented.
| PANSS | Young-onset (No.=25) | Late-onset (No.=5) | p-Value | ||
| Mean | SDa | Mean | SDa | ||
| Positive subscale | |||||
| P6. Suspiciousness | 5.44 | 1.12 | 6.80 | 0.45 | 0.012, trend |
| Negative subscale | |||||
| N1. Blunted affect | 2.76 | 1.01 | 1.40 | 0.55 | 0.008, marginally NSb |
| N2. Emotional withdrawal | 3.44 | 0.82 | 2.40 | 0.55 | 0.011, trend |
| N3. Poor rapport | 3.08 | 1.04 | 1.80 | 0.84 | 0.017, trend |
| N6. Lack of spontaneity | 3.12 | 1.56 | 1 | 0 | 0.002 |
| Total Negative subscale score | 23.00 | 5.41 | 15.80 | 3.96 | 0.012 |
| General psychopathology subscale | |||||
| G13. Disturbances of volition | 2.52 | 1.16 | 1 | 0 | 0.008, marginally NS |
In the present study, differences were observed between young and late onset patients with paranoid schizophrenia, mainly regarding negative symptoms, in the prodromal and the active phase of the disorder. Young onset patients, particularly males, exhibited more severe negative symptoms than the late onset group.
Heavier negative symptomatology was found in the prodromal phase of the disease in young onset patients. Of the negative prodromal symptoms, marked isolation and impaired concentration were found at a higher frequency in young onset patients. More negative prodromal symptoms were also found in the male, but not in the female sample. The subanalysis of first episode patients actually revealed similar results, regarding number of negative prodromal symptoms and impaired concentration, though at a marginally not significant level. These observations could be of relevance to prevention strategies, possibly targeted at an older age group than currently under concern.5
Heavier loading of negative symptoms is also observed in the active phase of young onset patients, in accordance with previous studies,14,15,17 that is higher total negative subscale scores and heavier lack of spontaneity. Higher scores on total negative subscale, blunted effect and lack of spontaneity were observed in the young onset group of the male sample as well, but not in females. The finding of heavier disturbances of volition in the young onset group, possibly points to a greater degree of disorganization, related to earlier age of onset, in agreement with most,14,15,23 but not all,13 studies. Similar results were found in the first-onset sample, with the additional finding of heavier suspiciousness, though marginally not significant, in late onset patients, and in late onset males, in accordance with previous work.14,16,17
Häfner et al.8 have observed that, in females, late onset schizophrenia can be characterized by negative symptoms of even greater severity than young onset schizophrenia. Male sex has been reported to be related to heavier negative symptoms,32 but this could be due to the earlier onset age of the disease in men. Besides, the fact that fewer differences have been observed between the two sexes points to age of onset as a stronger contributor to the heterogeneity of the illness than gender, in agreement with Sato et al.17
Research and clinical practice have recently shifted toward a dimensional approach, as a way of reducing syndrome heterogeneity, with negative symptom pathology at the forefront of heterogeneity reduction for schizophrenia.33 Our findings suggest a modulating effect of age of onset and sex on symptom dimensions of paranoid schizophrenia. Younger age of onset seems to be related with heavier negative and disorganized symptoms. Male sex and older age of onset seem to be related to a positive dimension, while female sex seems to be related to a negative symptom dimension.
Concerning distributions of onset age of paranoid schizophrenia, it seems that men are more vulnerable than women in late adolescence or early adulthood, while women are almost equally prone to develop schizophrenia in their late teens, early twenties, or later,25 which is in accordance with our findings. It has been postulated that female gonadal hormones might play a protective role.8,34 Nevertheless, late adolescence proves not to be the only window for schizophrenia to evolve. Maturation and remodeling of the brain continues throughout the life span.35 It has been found that a great decline in gray matter density occurs normally between 7 and 60 years of age.36 Myelination of the white matter, beginning from the first years of life, continues into adulthood, in frontal and associative areas.37 Frontal and temporal white matter volumes continuously increase in normal men, and reach their peak around age 47.37 Females, on the other hand, have a greater amount of myelin in temporal lobes in the first three decades of life, compared with males, possibly because of the stimulatory effect of female gonadal hormones on myelination.38 White matter myelination may be crucial for the brains’ functional synchrony, providing adequate speed of neural transmission, which may be especially important for prefrontal functions.37 It has been suggested that myelin-related dysfunction, interacting with gray matter changes, could play an important role in the pathogenesis of schizophrenia.39
As late adolescence is a much more vulnerable period for men rather than women for the development of paranoid schizophrenia, most men of a certain trajectory have disease onset at this point in life. Later on, aberrations in the ongoing processes of brain maturation, resulting in brain disconnectivity, might lead a person to develop schizophrenia at an older age, over 35, if not already got ill by late adolescence. This could be triggered by hormonal reasons in females, or by other reasons, perhaps by dysregulation of the changes occurring in gray and white matter, both in males and females.
It has been speculated that a more severe impairment of the anterior cingulate circuit function, which is normally involved in motivational processes, is related to the heavier negative symptomatology of the young onset group.14 Lesions of this circuit result in impaired motivation, as expressed by marked apathy, reduced creativity, poverty of spontaneous speech, indifference to pain and poor response inhibition.40 Our study confirmed heavier negative symptoms in the active and prodromal phase of the disorder in the young onset group, particularly in males, and specifically of blunted effect and lack of spontaneity. It is possible that an insult later in life, on a brain that has passed through more developmental stages and thus, is myelinated at a greater extend, cannot have the detrimental effects on brain circuitry that could have had if it were to act earlier in development, and therefore can lead to a disease phenotype with less negative symptoms.
Limitations of our study are that it was based on a sample of hospitalized patients, excluding patients who do not enter the health system; the retrospective examination of the prodromal period, which could be subject to recall bias, and also the fact that no scale was used; finally, the small female sample.
Concluding, we believe that the present study contributes to a better understanding of the phenomenology of the initial prodromal and active phases of paranoid schizophrenia, in relation to age of onset and sex, and may help research on the illness heterogeneity.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Skokou M, et al. Sintomatología de las fases activa y prodrómica de la esquizofrenia paranoide de inicio en el joven y de inicio tardío. Rev Psiquiatr Salud Ment (Barc.). 2012;5:150–9.