Pleomorphic adenoma is considered the most frequent benign tumor found in the salivary glands. Histologically it is characterized by presenting epithelial as well as mesenchymal elements. The present study targets the report of a clinical case of a large size pleomorphic adenoma found in a 45 year old patient. The patient was treated at the Oral and Maxillofacial Surgery Unit of the Eastern General Hospital «Dr. Domingo Luciani». The patient reported onset of the disease approximately 17 years before, with a progressive volume increase in the palate. A surgical Brown type II B maxillectomy was planned, with placement of a shutter plate. At a 10 month post-surgery control, tissue formation was observed in the region of the surgical bed, this formation exhibited similar appearance to the surrounding mucosa, with no signs of recurrence and presence of an oral-nasal fistula measuring approximately 2cm in diameter. Presently, the patient is programmed to receive a fistula closure procedure by means of local flaps as well as subsequent prosthetic rehabilitation.
El adenoma pleomórfico es considerado el tumor benigno más frecuente de las glándulas salivales y se caracteriza histológicamente por presentar tanto elementos epiteliales como mesenquimales. El presente estudio tiene como objetivo reportar un caso clínico de adenoma pleomórfico en paladar de grandes dimensiones de un paciente masculino de 45 años de edad, tratado en la Unidad de Cirugía Buco-Maxilofacial del Hospital General del Este «Dr. Domingo Luciani» quien inicia enfermedad actual, hace 17 años aproximadamente presentando un aumento de volumen progresivo en paladar. Se planificó quirúrgicamente para una maxilectomía de Brown tipo II B y colocación de placa obturadora. En un control postoperatorio de 10 meses se evidenció formación de tejido en la región del lecho quirúrgico de aspecto similar a la mucosa circundante sin señales de recidiva con presencia de fístula oro-nasal de aproximadamente 2cm de diámetro. Actualmente se encuentra en programación de cierre de la misma con colgajos locales y posterior rehabilitación protésica.
Pleomorphic adenoma (PA) is considered the most frequent benign tumor of the salivary glands, representing 60% of all cases.1–3 It is also known as mixed tumor, since it encompasses a wide mix of ductal and myoepithelial elements in one single tumor.4 The term «pleomorphic» refers to the wide variability of the stromal and parenchymal differentiation shown by tumor cells.2
Approximately 80% of all PA develop in the parotid gland,5 generally at the lower pole of the superficial lobe;2,6 10% appear at the submandibular gland, and 10% in minor salivary glands5 in where the palate region represents 60%, followed by 20% in the upper lip and 10% in the oral mucosa.4 The World Health Organization (WHO)5 in 2005 reported that annual incidence of this lesion is from 2.4-3.05 per each 100,000 subjects, who are generally in their fourth and fifth decades of life,1,2 with average age of 46 years.5 From the clinical approach, PA appears as a painless, slow growing tumor, exhibiting firm consistency7 and variable dimensions which can fluctuate from 2 to 6 centimeters in diameter.7,8 This type of tumor is generally associated to the superficial lobe of the parotid gland or the posterior palatal mucosa;2,8 it is less frequently found in nonsalivary glandular tissue of the external auditory meatus, breast tissue and tear duct.1 In image studies taken with computerized tomography (CT), it generally appears as a circumscribed image with well-defined margins, with density similar to that of adjacent tissues and lacking homogeneous pattern.1,9 In the parotid gland, it appears as an image with lobulated borders, differing from images taken from submandibular glands and minor salivary glands. The lesion's capsule is extremely difficult to detect as well as the resorption of adjoining bone.9 In a magnetic resonance (MR) procedure, the lesion appears well circumscribed, of variable heterogeneity, being T1 or T2 depending on weight. The contrast between the lesion and surrounding tissue tends to be high in T1 (67%) and T2 (90%),1,9 therefore, the capsule is very easy to detect in T2 weighing (87-90%) and hard to detect in T1 weighing (33%).9 The lesion borders appear lobulated in the parotid gland and in the submandibular gland tumors, differing from tumors in the palate where smooth borders are normally found.9
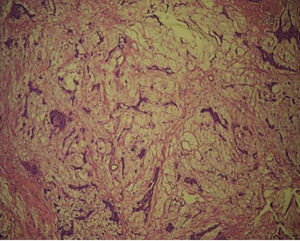
With respect to microscopic characteristics, there is a wide spectrum of histological findings, due to the expression of variable epithelial and mesenchymal characteristics ; therefore giving rise to the term «pleomorphic».1,4,5,10 PA appears as an encapsulated lesion when it develops in major salivary glands, differing from where it appears in minor salivary glands, where, normally, there is an incomplete capsule,1,4,5 in most cases there are structures similar to a glove's finger which extend inward forming satellite nodules which are linked to the tumor by means of an isthmus.5 Cells of epithelial origin result in ductal structures and are closely mixed with mesenchymal elements which might develop mixoid,7 hyaline, cartilaginous or osseous changes.1,5,10 Myoepithelial cells frequently represent a great percentage of tumor cells: they possess varied morphology, sometimes they appear in an angular or fusiform shape, in other instances they exhibit rounded shapes and show an eccentric nucleus with hyalinized eosinophilic cytoplasm resembling plasmatic cells.4 These characteristic myoepithelial cells are predominant in tumors arising in minor salivary glands.4
Treatment of this type of lesions depends on their size and location, therefore, surgical excision is the preferred treatment.1,3,4,5,7 Several authors2,4,11 describe partial parotidectomy with identification and preservation of the facial nerve when treating PA located in the superficial lobe of the parotid gland. Conversely, total parotidectomy is necessary in tumors found in the deep lobe.3,4
Tumors of the hard palate are generally excised, including periosteum and adjacent mucosa.3,4,7
CLINICAL CASE PRESENTATIONA Caracas-born 45 year old male patient, attended the Oral and Maxillofacial Surgery Unit of the «Dr. Domingo Luciani» Eastern Hospital, complaining of a progressive volume increase in the palate; the lesion exhibited a 17 year evolution and the patient informed of dysphagia and dyspnea.
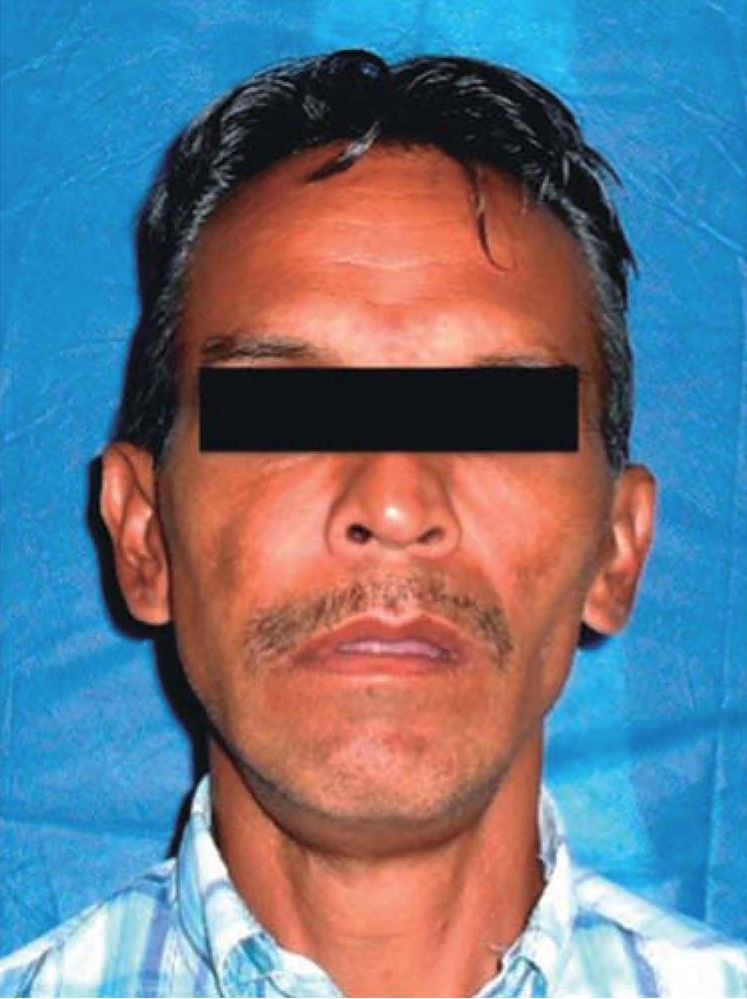
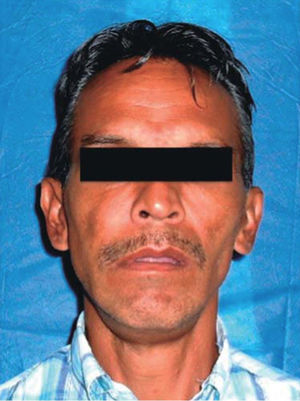
Physical examination revealed facial asymmetry at the expense of the facial middle third; with predominance in the right half of the face and presence of labial incompetence (Figure 1). Endonasal exploration revealed total obstruction of both nasal fossae (nostrils) due to the presence of the tumor lesion.
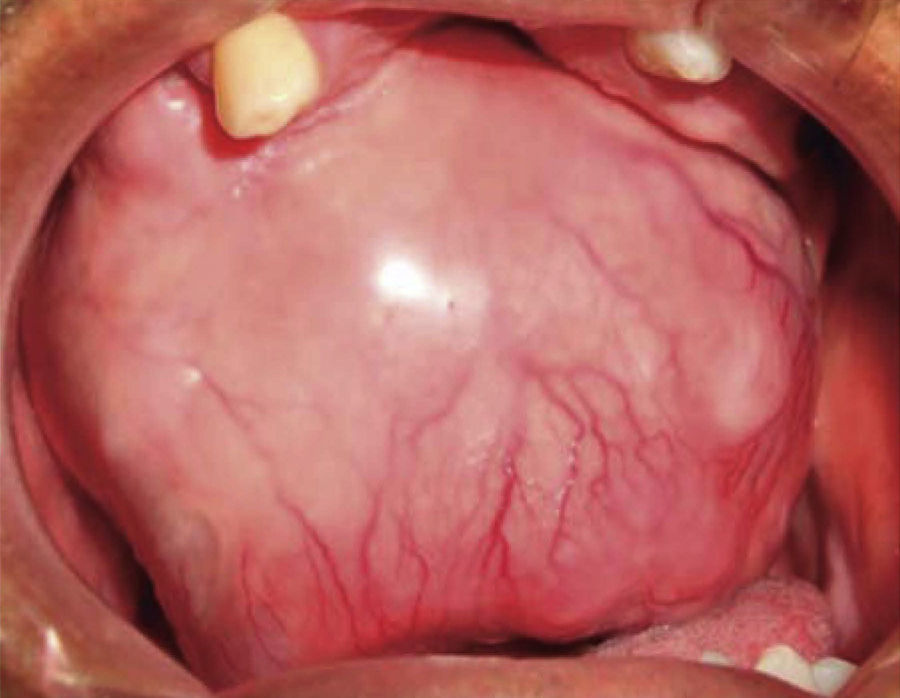
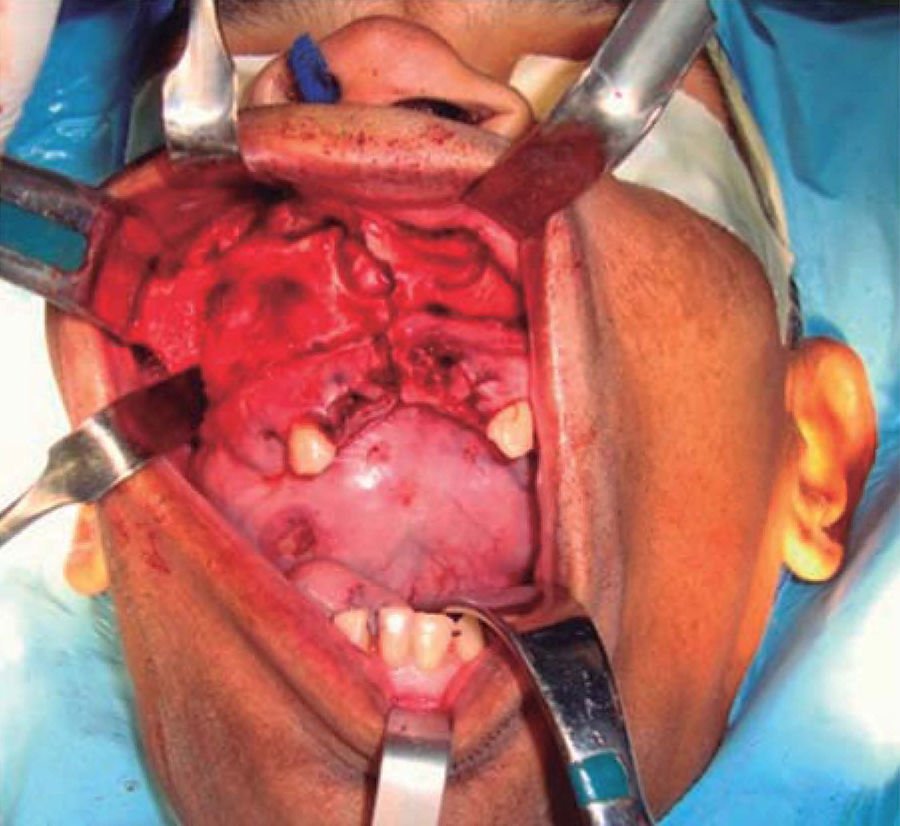
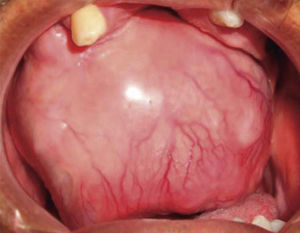
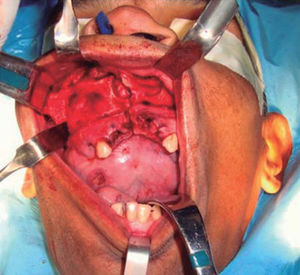
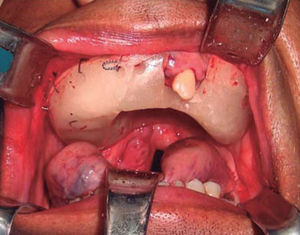
Intraoral examination revealed bi-maxillary partial edentulism, poor oral hygiene with presence of multiple root remnants, and a tumor lesion measuring approximately 9 × 9cm long, which appeared coated with a mucosa similar to the oral mucosa. The lesion exhibited smooth surface with vascular framework all over the lesion, and upon palpation was firm and painless. It encompassed a large section of the oral cavity and prevented the exercise of mastication, deglutition, breathing and phonation functions (Figure 2).
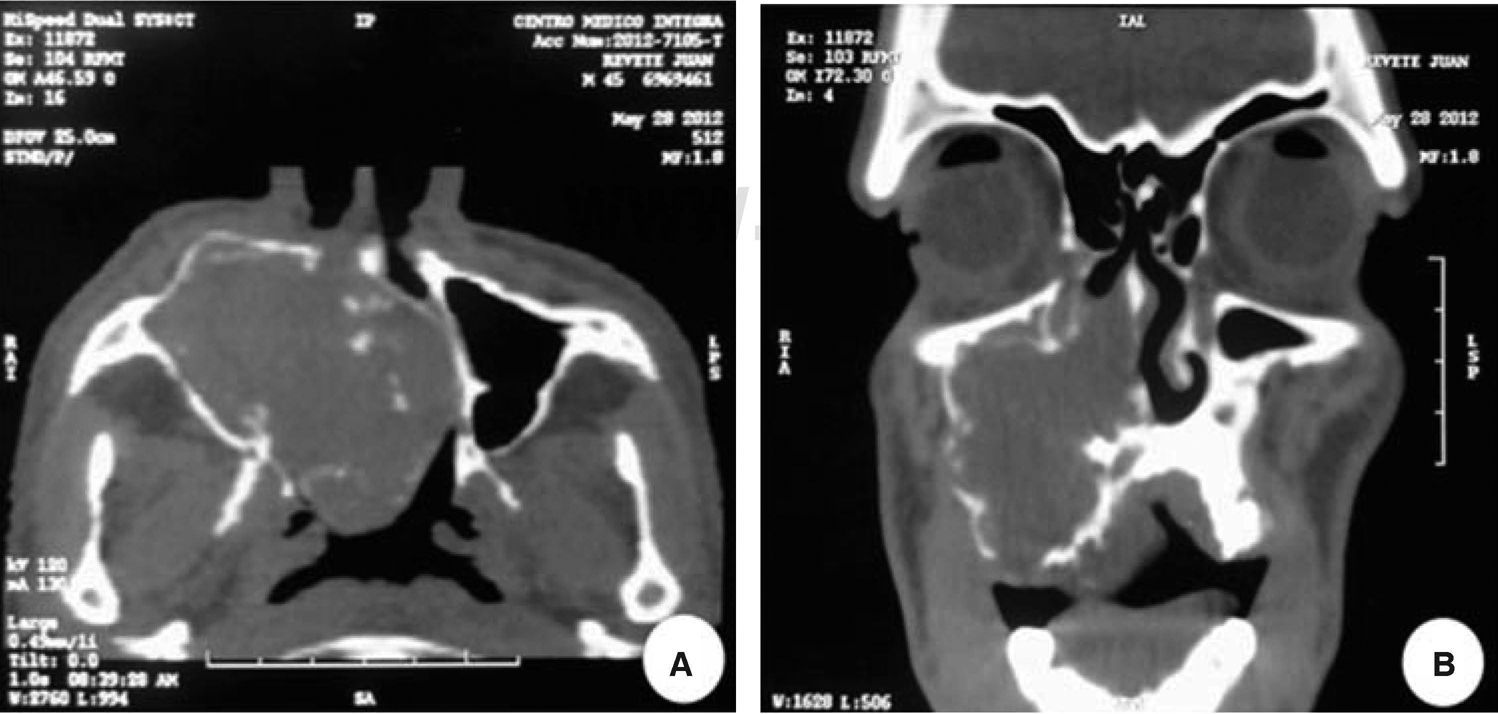
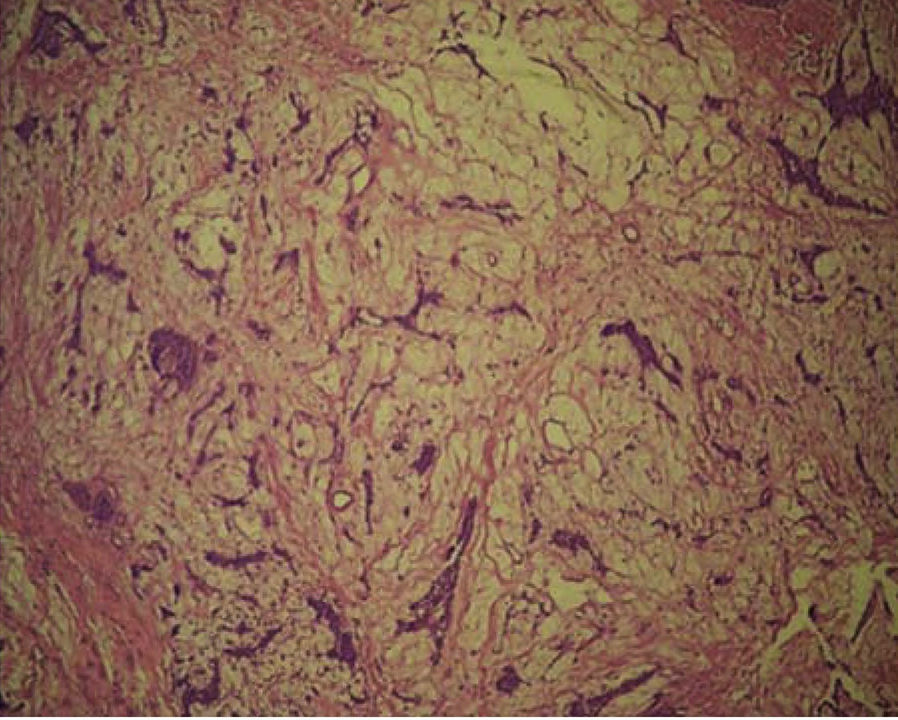
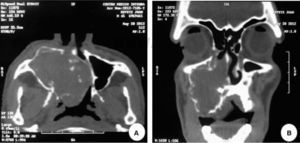
A computed tomography was requested; in an axial section it revealed a lesion filling the space. The lesion appeared isodense with respect to adjacent soft tissues, was located at the right maxillary mid-arch, involving the maxillary antrum, nasal turbinates, septum and nasal-pharyngeal region (Figure 3A). A coronary section revealed an isodense lesion with hyperdense borders located in the right maxillary antrum, invading nostrils and obstructing airways, but nevertheless ipsilaterally preserving the floor of the orbit (Figure 3B). An incisional biopsy under local anesthesia was decided upon and the harvested sample was sent to undertake histopathological study. Study report was «pleomorphic adenoma» (Figure 4).
A-B. Computed tomography: A) Axial cut displaying a lesion filling the space located in the right maxillary mid-arch. Lesion involves the maxillary antrum, nasal turbinates, septum as well as pharyngeal nasal region. B) Coronal cut displaying isodense region located in the right maxillary antrum, invading ipsilateral nasal fossa (nostril).
Surgical planning for the case consisted on guaranteeing airway by means of a tracheostomy performed under local anesthesia and sedation, since nasal or tracheal intubation was impossible due to the obstruction generated by the lesion.
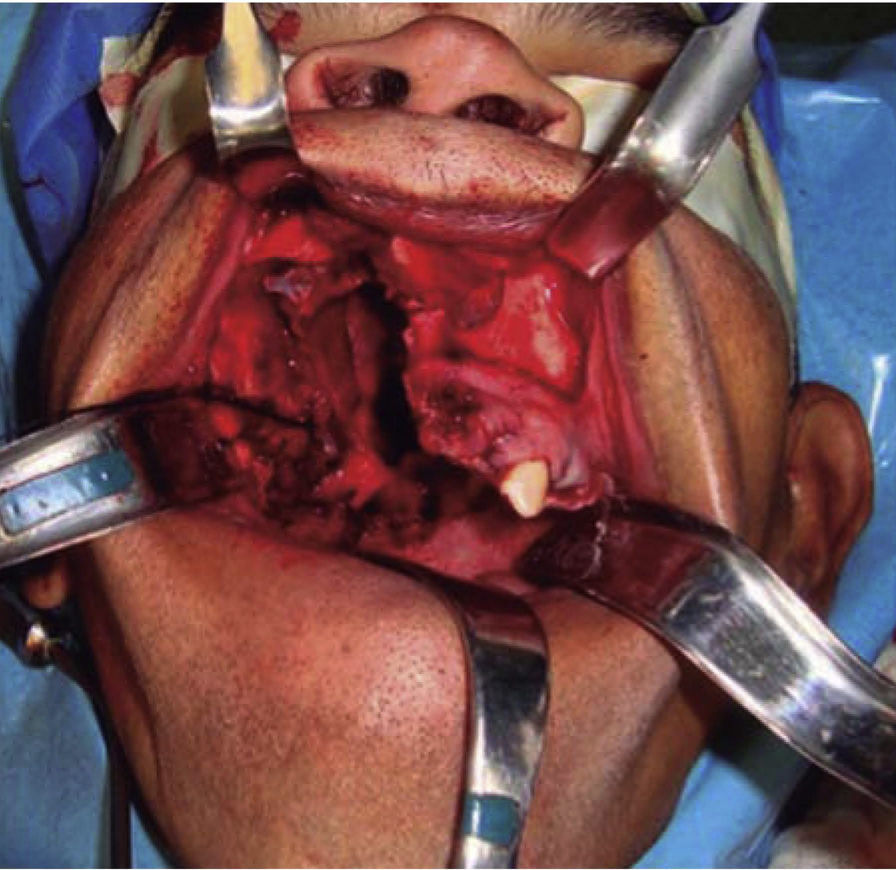
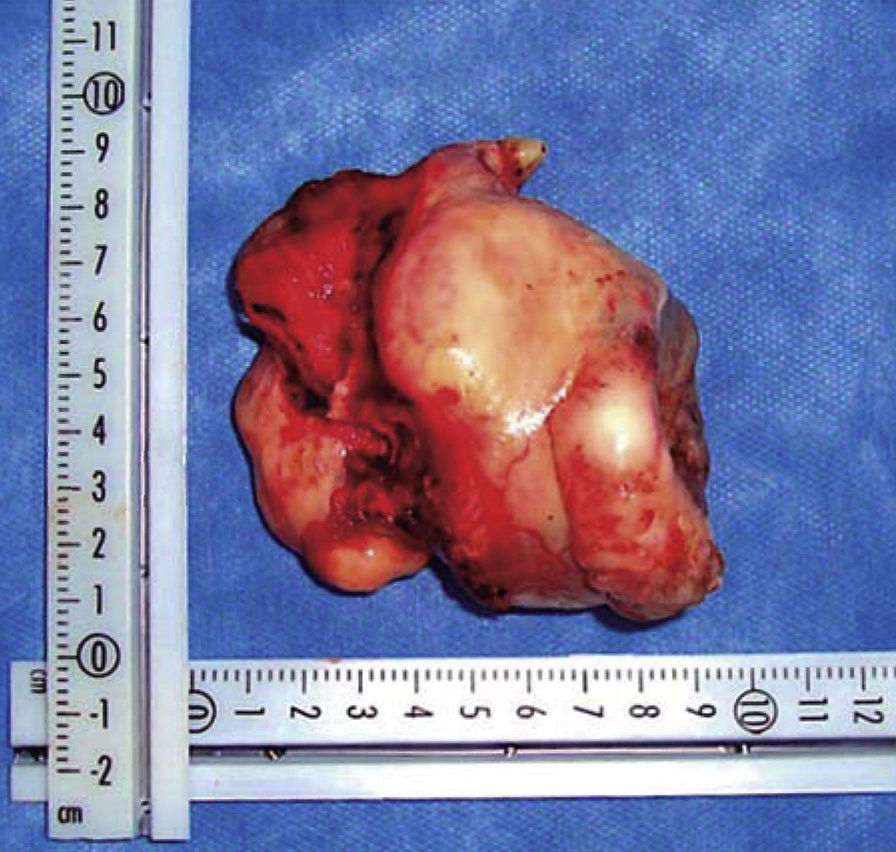
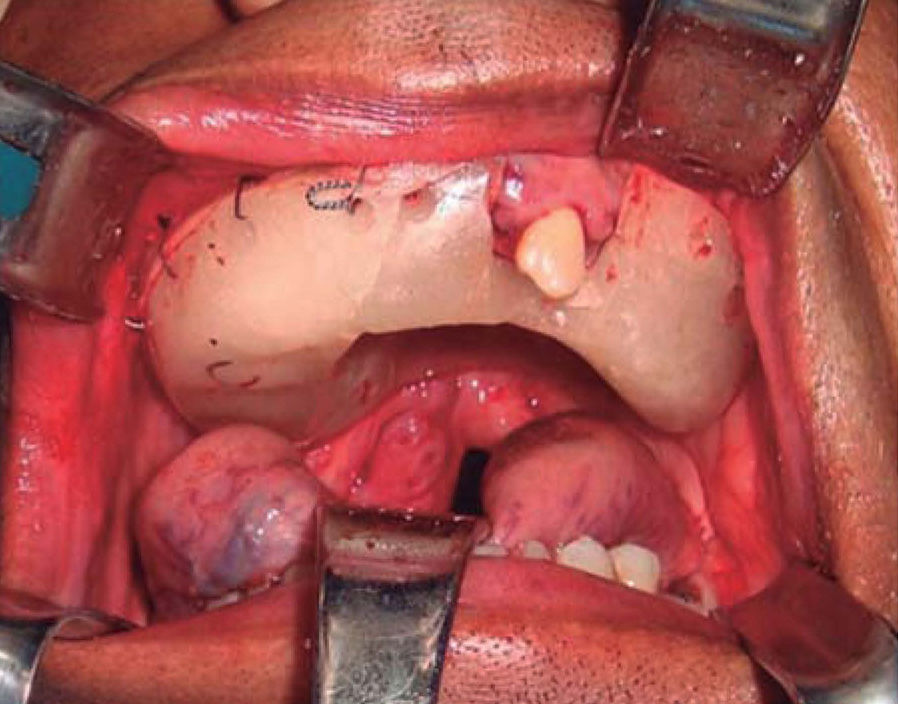
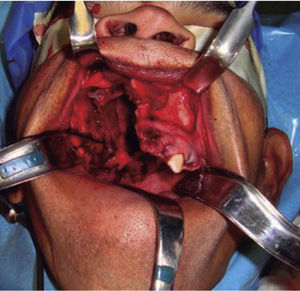
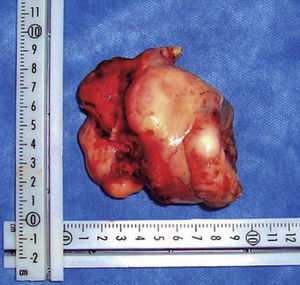
Once the airway was secured, under balanced general anesthesia, a muco-periosteal flap was raised by means of an intra-oral approach conducted with an upper circumvestibular incision; this procedure exposed the lateral and anterior wall of the right maxillary sinus. A Brown type II B maxillectomy (Figure 5) was marked with the help of a 702 burr, the osteotomy was completed with hammer and chisel (Figure 6). A 9 × 9 × 10cm sample was obtained (Figure 7) which generated oral-nasal-antral communication. The maxillary shutting plate was then suspended with 0.6mm gauge wire so as to avoid collapse of soft tissues and to guide the healing of possible oral-nasal fistulae during the first week of treatment (Figure 8).
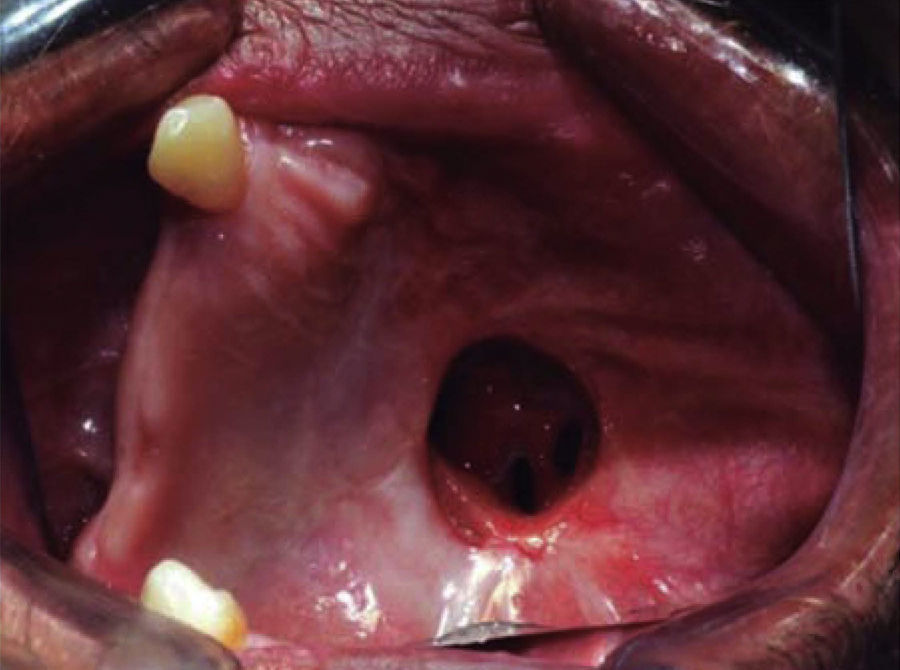
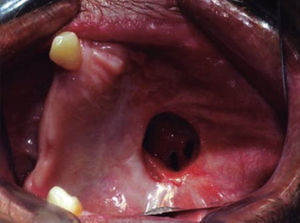
The shutting plate was removed at the onemonth post-operative control, the following was observed: healing oral mucosa similar to adjacent mucosa, with presence of oral-nasal-antral fistula measuring approximately 5cm diameter, the remaining contralateral maxillary mid-arch exhibited tissue formation in the region of the surgical bed, with presence of oral-nasal-antral fistula measuring approximately 2cm diameter (Figure 9).
DISSCUSSIONPleomorphic adenoma is considered the most common benign condition of the salivary glands, it represents 45 to 74% of all salivary gland diseases.8 When it appears in minor salivary glands, it is most frequently located in the palate region.4 Depending on its location, PA can vary in size, it generally appears as an asymptomatic tumor seldom exceeding 6cm diameter.8 Due to the lesion’ s slow growth, if not treated in its initial stages, it can reach large proportions4,8 even to the point of compromising vital functions such as breathing, feeding and phonetics; this was similarly evidenced in our case, where the tumor reached a size of 9 × 9 × 10cm.
Managing these patients’ airway is crucial to reach success in treatment. Authors such as Haspel et al,12 in a retrospective study, described tracheostomy as the indicated technique to guarantee patient's airway.
This technique must be conducted by qualified personnel in patients afflicted with maxillofacial trauma and head and neck tumors; in these patients they recommend conducting the technique after lesion resection in order to predict neck's edema and trachea's position.
In our case, after taking in consideration the lesion’ s size and location, it was decided to plan a presurgical tracheostomy, after considering the difficulty existing to guarantee a suitable airway by conventional intubation.
Treatment of PA varies according to location and extension of the lesion. Lingram et al1 described surgical excision as the preferred treatment. Previous studies have shown that enucleation is not recommended, since there is a high recurrence rate, reported to be between 2 and 45%.6 Microscopically this can be due to the focal absence of the capsule surrounding the lesion and/or formation of satellite nodules caused by epithelium invagination towards the capsule.1,6
In our case, it was decided to perform a type II B Brown maxillectomy, due to the large size of the lesion and the lesion’ s invasion towards adjacent anatomical structures without involving the ipsilateral orbit floor and contralateral jaw.13
When dealing with large lesions, many authors14,15 report use of extra-oral approaches to excise these tumors.
Traditional extra-oral techniques, such as the Weber-Ferguson approach provide suitable surgical exposition, nevertheless, they possess the disadvantage of causing external scarring.14,15 When treating benign conditions, it is suggested to perform intra-oral approaches so as to avoid esthetic facial complications.
In our case, it was decided to perform an intraoral approach for a Lefort I osteotomy, since this procedure allows wide bilateral and symmetrical vision of the nostrils and maxillary sinuses in order to expose the lesion through maxillary osteotomies.
Other types of approaches have been described for resection of tumors in the oral-pharyngeal region, such as Mediofacial Degloving proposed by Casson et al16,17 in 1974. Authors like Buchwald et al18 consider that in medial maxillectomy and some cases of radical maxillectomy, this approach must be used as the first option.
With respect to immediate reconstruction alternatives, Kreft et al19 describe use of obturators and prosthetic reconstructions to restore the patient's function after performing the maxillectomy; in our case we concurred with this treatment.
Authors such as Genden20 and Germain et al21 support performing free flaps which allow reconstruction of large defects besides solving the problems of shutter plate prosthesis, nasal discharge, cleansing and constant prosthetic refinement. It must be taken into consideration that econstruction of free flaps implies certain procedural complexities as a consequence of prolonged surgical times; this is associated to the need to be able to count with a multidisciplinary team as well as post-operative intensive therapy, which entails use of high-cost surgeries. Conversely, manufacturing of a shutter plate prosthesis significantly reduces surgery time and offers the possibility to perform suitable and immediate dental rehabilitation.
To conclude, progressively can be said that PA is a slow and progressively growing lesion, for this reason, when treatment is not undertaken at its initial phase, it can reach large dimensions. Recommended treatment is surgical resection of the lesion, since this type of lesions exhibit high recurrence rate. Likewise, approach will always be decided by the surgeon and will depend on size and location, nevertheless, it is suggested to perform conservative approaches, such as the intraoral approach, which allows us to achieve tumor resection without compromising the patient's esthetics and immediately restoring function.
Este artículo puede ser consultado en versión completa en http://www.medigraphic.com/facultadodontologiaunam