Recent interest in the coadministration of approved pharmaceutical agents has resulted in a wealth of emerging data on the safety and efficacy of dual pharmacological treatment for lower urinary tract symptoms (LUTS). Much evidence supports the coadministration of α-blockers with 5-alpha-reductase inhibitors (5-ARIs) in patients at risk for clinical progression. The use of phosphodiesterase-5 inhibitors (PDE5Is) in combination with 5-ARIs has also demonstrated a good safety and efficacy profile, providing early symptomatic relief and reduction of sexual side effects associated with 5-ARI use, although longer-term studies are needed. Studies investigating the combination of PDE5Is with α-blockers have shown additive effects on each of the individual agents with respect to the International Prostate Symptom Score (IPSS) and the International Index of Erectile Function (IIEF), which holds promise for patients who have shown a poor response to monotherapy. The coadministration of α-blockers and antimuscarinic agents provides an alternative for treatment of storage symptoms in patients who have failed to respond to monotherapy. This review aims to summarize and comment on available evidence regarding the safety and efficacy of combination treatment for LUTS.
El reciente interés en la administración conjunta de agentes farmacéuticos aprobados ha dado lugar a una gran cantidad de nuevos datos sobre la seguridad y eficacia del tratamiento dual farmacológico para los síntomas del tracto urinario inferior. La evidencia científica apoya la co-administración de bloqueadores alfa con inhibidores de la 5-alfa-reductasa (5-ARI) en pacientes con riesgo de progresión clínica.
El uso de inhibidores de la fosfodiesterasa-5 en combinación con 5-ARI también ha demostrado un adecuado perfil de seguridad y eficacia, proporcionando mejoría sintomática y reducción de los efectos sexuales secundarios asociados al uso de 5-ARI, aunque se necesitan estudios a largo plazo. Los estudios de combinación de inhibidores de la fosfodiesterasa-5 con bloqueadores alfa han mostrado efectos aditivos con respecto al Índice Internacional de Síntomas Prostáticos (IPSS) y al Índice Internacional de Función Eréctil (IIEF) sobre cualquiera de los agentes en forma individual, lo que mantiene la eficacia clínica para los pacientes que han mostrado una pobre respuesta a la monoterapia. Además la coadministración de bloqueadores alfa y agentes antimuscarínicos ofrece una alternativa para el tratamiento de los síntomas de almacenamiento en pacientes que no han respondido a la monoterapia. Esta revisión tiene como objetivo resumir y comentar la evidencia disponible sobre la seguridad y eficacia del tratamiento combinado para síntomas del tracto urinario inferior.
The causal link between the prostate and lower urinary tract symptoms (LUTS) has come into question during the past decade, and the correlation between prostate size and symptoms, measured by the International Prostate Symptom Score (IPSS), has been shown to be weak.1 Because of these recent findings and because patients seek help for LUTS rather than for a particular underlying contributing factor such as benign prostatic hyperplasia (BPH) or benign prostatic obstruction (BPO), more recent updates to international clinical management guidelines are now written from the perspective of men who suffer from LUTS, which incorporates a variety of bladder storage, voiding, and/or post-micturition symptoms.2 Storage symptoms typically include daytime urinary frequency, nocturia, urgency, or urinary incontinence; voiding symptoms include slow stream, splitting or spraying, intermittency, hesitancy, straining, and terminal dribble; and post-micturition symptoms refer to the sensation of incomplete emptying or post-micturition dribble.
The evolving understanding about factors that may contribute to LUTS has led to increased interest in the combination of existing treatment approaches, so that multiple modes of action can be used to best manage symptoms. The mainstay of current treatment approaches for LUTS includes the four main drug classes used as monotherapies, and the combination of 5α-reductase inhibitors (5-ARIs) with α-adrenoceptor antagonists (α-blockers).2 The α-blockers are traditionally the first-line treatment for managing signs or symptoms of BPH/LUTS, and this class of agent includes alfuzosin, doxazosin, silodosin, tamsulosin, and terazosin. The 5-ARIs are used to manage benign prostatic enlargement (BPE), and the two key agents are dutasteride and finasteride. Antimuscarinic agents are primarily used to treat overactive bladder (OAB) and other storage symptoms, and the most common in this class include oxybutynin, propiverine, solifenacin, and tolteroidine. The phosphodiesterase-5 inhibitors (PDE5Is) are used for treating the signs or symptoms or BPH with or without erectile dysfunction. The only agent licensed for this indication is tadalafil; the other PDE5Is, vardenafil and sildenafil, are licensed as on-demand erectile dysfunction agents.
The Multinational Survey of the Aging Male-7 reported that among men ≥50 years of age, 43% with mild LUTS, 65.8% with moderate LUTS, and 82.5% with severe LUTS, also suffer from erectile problems.3 This multinational survey concluded that sexual activity is common in a majority of men ≥50 years of age and is an important component of overall quality of life, highlighting the need to consider sexual issues in the management of patients with LUTS.3 Because of the positive impact of PDE5Is on erectile dysfunction, there has been considerable interest in therapeutic regimens that include this class of agent. The purpose of this article is to review evidence on the efficacy and safety of dual pharmacological treatment for LUTS, with the aim of making evidence-based decisions about the suitability of patients with particular symptoms or risk profiles for different types of combination therapy.
α-Blocker/5-ARI combination therapyThis is the most widely investigated combination therapy for LUTS, with many clinical trials building the evidence for efficacy of treatment combinations in this class. The key initial studies were the Prospective European Doxazosin and Combination Therapy (PREDICT) study,4 which investigated finasteride with terazosin, the Alfuzosin Finasteride (ALFIN) study,5 which investigated finasteride with alfuzosin, and the Veterans Affairs Cooperative (VA-COOP) study,6 which investigated finasteride with doxazosin, for 6 or 12 months. All three studies reported significantly improved symptom scores (American Urological Association symptom score [AUA-SS] or IPSS) in the combination therapy groups compared with the baseline and 5-ARI monotherapy groups, with reductions from the baseline between 6.1 and 8.5 points.4–6
Three studies lasted longer than 1 year, the first of which was the Medical Therapy of Prostatic Symptoms (MTOPS) study. It investigated the long-term effects of placebo, doxazosin 1 to 8mg once daily [QD] monotherapy (doxazosin dose was 1mg QD for the first week, increasing to 4mg or 8mg QD, depending on tolerability), finasteride 5mg QD monotherapy, and finasteride/doxazosin combination therapy on BPH clinical progression in 3047 symptomatic patients, with a mean follow-up of 4.5 years.7 Risk for overall clinical progression, defined as an increase above the baseline of at least 4 points in the AUA-SS, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection, was significantly reduced with the doxazosin (39% risk reduction; p<0.001) and finasteride (34% risk reduction; p=0.002) monotherapies compared with placebo, and the risk reduction was significantly greater with the combination therapy (p<0.001) than with either of the agents alone (p<0.001 for both) when compared with placebo.7 Similarly, the risk for acute urinary retention and need for invasive therapy were significantly reduced with the combination therapy (p<0.001) and finasteride monotherapy (p<0.001), but not with doxazosin monotherapy.7 The most common adverse events with doxazosin monotherapy compared with placebo were dizziness, postural hypotension, and asthenia; with finasteride monotherapy, they were erectile dysfunction, decreased libido, and abnormal ejaculation. The adverse events with doxazosin/finasteride combination therapy were similar to those with each drug alone, with the exception of abnormal ejaculation, peripheral edema, and dyspnea – all of which occurred more frequently in patients receiving the combination therapy. The rate per 100 person-years of follow-up of erectile dysfunction was 4.53 with finasteride monotherapy and 5.11 with the combination therapy; both rates were significantly higher than the rate with placebo.7
Another large-scale, long-term follow-up study, the Combination of Avodart and Tamsulosin (CombAT) study, investigated the efficacy and safety of dutasteride 0.5mg QD monotherapy, tamsulosin 0.4mg QD monotherapy, and the dutasteride/tamsulosin combination therapy in reducing the relative risk for acute urinary retention (AUR), BPH-related surgery, and BPH clinical progression over 4 years in 4844 men at increased risk for progression.8,9 The 4-year follow-up results showed that the combination therapy was significantly superior to tamsulosin monotherapy at reducing the relative risk for AUR and BPH-related surgery (p<0.001). It was not superior to dutasteride monotherapy. The combination therapy was, however, also significantly superior to both monotherapies at reducing the relative risk for BPH clinical progression (p<0.001 for both).9 An IPSS reduction of 6.3 points was reported for the combination therapy versus 3.8 points for tamsulosin (p<0.001) and 5.3 points for dutasteride (p<0.001). Superiority of the combination therapy versus tamsulosin from month 9 and versus dutasteride from month 3 was observed and was maintained for the study duration (p<0.001 for all comparisons).9 There were significantly more drug-related adverse events in the combination group. However, the withdrawal rates due to drug-related adverse events were similar across the three treatment groups (6% in the combination group and 4% in the dutasteride and tamsulosin groups). Erectile dysfunction occurred in 5% of the subjects in the tamsulosin group, 7% in the dutasteride group, and 9% in the combination therapy group. Although there was no difference in overall cardiovascular events across treatment groups, the incidence of the composite term “cardiac failure” was higher in the combination (0.9%) and tamsulosin monotherapy groups (0.6%) compared with the dutasteride monotherapy group (0.2%).9
Most recently, the CONDUCT study investigated the first-line treatment of men with BPH with a fixed-dose combination of dutasteride 0.5mg and tamsulosin 0.4mg QD compared with watchful waiting and initiation of tamsulosin 0.4mg QD if symptoms did not improve.10 All patients were given lifestyle advice about symptom management. The change in the IPSS after 24 months of follow-up was significantly greater in the combination group than in the watchful waiting/tamsulosin group (−5.4 vs. −3.6 points; p<0.001), and the combination therapy significantly reduced the relative risk for BPH progression by 43.1% compared with watchful waiting/tamsulosin (p<0.001).10 The most commonly reported adverse events across the combination therapy and tamsulosin monotherapy groups were erectile dysfunction, which occurred in 8% and 5%, and retrograde ejaculation, which occurred in 0% and 4%, respectively. Patients in the combination therapy group also reported loss of libido and ejaculation disorder (2% for each, but neither was reported in the watchful waiting/tamsulosin group).10
The long-term follow-up results from the MTOPS, CombAT, and CONDUCT studies are considered the most relevant due to the long-term effect of the 5-ARI drug class. However, the patient populations were slightly different among the studies: the MTOPS study included men with LUTS, regardless of prostate volume or symptom score, whereas the CombAT study included those ≥50 years of age with prostate volumes ≥30mL, IPSS ≥12 and prostate-specific antigen (PSA) 1.5–10ng/mL, and the CONDUCT study enrolled subjects with prostate volumes ≥30mL, IPSS 8–19, and total serum PSA≥1.5ng/mL.7,9,10 The results from these long-term studies favored the combination therapy over either of the monotherapies, in terms of the AUA-SS and IPSS, as well as maximal flow rate (Qmax) and delay in disease progression. It is not known whether the observed difference in clinical outcomes persists for longer than a 4-year period. In addition, although 5-ARI monotherapy has a modest effect on sexual function (penile erection, ejaculation, and sexual desire) in men with LUTS, the MTOPS and CombAT studies found that when adding select α-blockers, the sexual side effects on ejaculation dysfunction were additive.11 Similarly, the CONDUCT study reported increased sexual side effects (erectile dysfunction, retrograde ejaculation, loss of libido) with the combination therapy compared with the watchful waiting/tamsulosin monotherapy treatment.10
Clinical management implicationsThe primary use of this treatment combination seems to be in patients with moderate to severe symptoms (IPSS>7 or AUA-SS>8) who show risk factors for clinical progression, although much of the long-term data have come from studies that included only subjects with large prostate volumes (≥30mL). The most recent American Urological Association (AUA) guideline (2010) advises that the α-blocker/5-ARI combination is effective for the treatment of LUTS with demonstrable prostatic enlargement based on volume measurement or PSA level as a proxy for volume and/or enlargement on digital rectal examination.12 Similarly, the 2013 European Association of Urology (EAU) guidelines also recommend this treatment combination in subjects with enlarged prostates2 and the 2010 National Institute for Health and Clinical Excellence (NICE) guidelines suggest offering this treatment combination to men with large prostates or PSA>1.4ng/mL and who are considered at risk for progression due to, for example, older age.13
The results of three studies that examined withdrawal of the α-blocker after initial combination therapy indicate that when this treatment combination is used, the α-blocker may be discontinued once the maximum effect of the 5-ARI has been achieved in patients with mild to moderate symptoms, usually after 6–12 months of combination treatment.14–16 In the Symptom Management After Reducing Therapy (SMART-1) study, 84% of men with baseline IPSS<20 changed to 5-ARI monotherapy after 24 weeks without noticeable deterioration in symptoms. However, 42.5% of men with severe baseline symptoms (IPSS≥20) reported worsening of symptoms after withdrawal of the α-blocker.14 Rates of sexual side effects were similar between the treatment groups. In the group that received combination therapy for 36 weeks, 7% experienced ejaculation disorders, 4% decreased libido, and 5% erectile dysfunction; in the group that switched from combination therapy to dutasteride monotherapy, 7% experienced ejaculation disorders, 6% decreased libido, and 2% erectile dysfunction.14 A more recent study of 103 BPH patients who had been on α-blocker/dutasteride combination therapy for 12 months and were then randomized to either continuation of combination therapy or withdrawal of the α-blocker, showed that 89% of patients with IPSS<20 who changed to dutasteride monotherapy, switched without noticeable deterioration in their symptoms.16 Of the men with severe baseline symptoms who switched to dutasteride monotherapy, 34% reported a worsening of their symptoms compared with 20% in the group that continued to receive combination therapy.16 The optimal duration of combination therapy before α-blocker discontinuation has not yet been determined.12
PDE5I/α-blocker combination therapyThree main PDE5Is have been used for some time for the treatment of erectile dysfunction on an on-demand dosing schedule: tadalafil,17 sildenafil,18 and vardenafil.19 Sildenafil and vardenafil are similar agents in terms of their pharmacokinetic profiles, with a short time to maximum plasma concentration (1hour) and elimination half-life (4–5hours).20 In comparison, tadalafil has a similar time to maximum plasma concentration (2hours) although markedly lower systemic clearance relative to other PDE5Is, with a half-life of 17.5hours, making it suitable for QD use.21 The positive results of the tadalafil monotherapy QD registration trial may have led to the current interest in the use of PDE5Is as part of combination therapy.
The primary international clinical trial of tadalafil 5mg QD monotherapy against an active control compared tadalafil with placebo and tamsulosin 0.4mg QD in 511 men with LUTS/BPH, IPSS≥13, and Qmax≥4 to ≤15mL/s over a 12-week treatment period.22 Oelke et al. reported statistically significant improvements in total IPSS from the baseline to week 12 for both tadalafil (LS mean±SE: −2.1±0.6; p=0.001) and tamsulosin (−1.5±0.6; p=0.023) compared with placebo. The IPSS change from the baseline was −6.3±0.5 in the tadalafil group and −5.7±0.5 in the tamsulosin group. No direct comparison between tadalafil and tamsulosin was reported. Qmax increased significantly versus placebo with both tadalafil (2.4mL/s; p=0.009) and tamsulosin (2.2mL/s; p=0.014).22 In sexually active men with erectile dysfunction, erectile function also showed significant improvement with tadalafil (change in the International Index of Erectile Function [IIEF] score 4.0; p<0.001), but not with tamsulosin, compared with placebo. The authors concluded that tadalafil and tamsulosin once-daily resulted in significant and numerically similar improvements versus placebo in LUTS/BPH and Qmax.22 In another study, Egerdie et al. investigated two doses of tadalafil (2.5 and 5mg) in a placebo-controlled trial in sexually active men with erectile dysfunction and BPH/LUTS, and found significant improvements in IPSS with the 5mg dose (LS mean −4.6; p<0.001) and in IEFF Erectile Function Domain (IIEF-EF) scores versus placebo with both tadalafil doses (both p<0.001).23 Tadalafil 5mg has been licensed since 2011 for once-daily use in the treatment of BPH with or without erectile dysfunction.
Crossover safety studies of PDE5Is as add-on therapyA number of initial crossover studies were performed to investigate the safety of the PDE5I/α-blocker treatment combination, although they primarily focused on the intermittent addition of a PDE5I to a regular α-blocker regimen for improvement of erectile dysfunction. These studies were designed with the principal aim of detecting any potential hemodynamic effects of the treatment combination. A crossover study, with 18 patients in each arm, was performed to investigate the potential hemodynamic interactions of two doses of tadalafil (10 and 20mg QD) in combination with either doxazosin 8mg QD or tamsulosin 0.4mg QD.24 The study reported that tadalafil augmented the hypotensive effects of doxazosin, but had little hemodynamic interaction with tamsulosin. Another crossover study assessed the pharmacodynamic effects of two doses of coadministered vardenafil (10mg or 20mg QD) in patients with BPH undergoing stable tamsulosin therapy (0.4 or 0.8mg QD). No evidence of clinically significant hypotension was observed, and no patient exhibited symptomatic hypotension (systolic blood pressure <85mmHg with dizziness).25 A third crossover study investigating alfuzosin 10mg QD with a single dose of tadalafil 20mg QD detected no clinically relevant interactions.26 The combination therapy elicited a maximal decrease in standing systolic blood pressure (mean difference 4.35mmHg) that was not significantly different from placebo.
The hemodynamic effects of tamsulosin 0.4mg QD and sildenafil 100mg QD combination therapy were investigated in 16 patients with BPE.27 Both the sildenafil monotherapy (−11±2mmHg [mean±standard error of the mean]) and sildenafil/tamsulosin combination therapy (−14±2mmHg) groups experienced decreased supine systolic arterial blood pressure compared with placebo (−2±4mmHg; p<0.05). Heart rate, diastolic arterial pressure, stroke index, cardiac index, and arterial pulse wave velocity were not significantly affected by any of the treatments compared with placebo.27 Another crossover study investigated the effects of the doxazosin gastrointestinal therapeutic system with vardenafil 10mg QD on the hemodynamic status of 37 patients with BPH and erectile dysfunction.28 The combination therapy was associated with a maximum decrease in standing systolic blood pressure of 6.18mmHg (p=0.039 versus placebo). One patient had an asymptomatic standing systolic blood pressure <85mmHg, but otherwise, no symptomatic hypotension or clinically significant adverse cardiovascular events were reported during the study.28
More recently, a larger study was performed to investigate the safety of the daily coadministration of α-blockers with tadalafil in 318 men with LUTS secondary to BPH. Eligible men ≥45 years receiving stable α-blocker therapy (uroselective: alfuzosin, silodosin, and tamsulosin; non-uroselective: doxazosin and terazosin) were randomized to tadalafil 5mg QD or placebo for 12 weeks.29 There was no significant difference in the proportion of men who reported treatment-emergent dizziness (tadalafil/α-blocker 7.0%; placebo/α-blocker 5.7%; p=0.403). A trend was seen for increased hemodynamic signs and symptoms in men taking non-uroselective α-blockers, particularly doxazosin, although this association was not detected with the other α-blocker combinations.29 The pilot studies testing α-blocker therapy with both intermittent and continuous PDE5I treatment report that the treatment combination is safe, with minor hemodynamic changes not considered clinically relevant.24–28
Prospective combination therapy studiesA recent meta-analysis of five trials that tested PDE5Is in combination with α-blockers in 437 patients (264 in the combination group and 173 in the PDE5I group) found significantly lower IPSS scores in the combination group than in the control group (pooled estimate −4.21; 95% CI −7.09 to 1.32; p=0.004).30 The same meta-analysis examined the effect of combination therapy versus PDE5I monotherapy on Qmax across four studies involving 204 patients, reporting a statistically significant improvement in Qmax in the combination group compared with the control group (pooled estimate 1.43; 95% CI 0.38–2.47; p=0.007).30
Tadalafil treatment combinationsTamsulosin is the most studied α-blocker in combination with tadalafil. In a crossover pilot study, Bechara et al. compared tamsulosin 0.4mg QD with and without tadalafil 20mg QD in 27 patients with a history of BPH/LUTS; patients received each treatment regimen for 45 days.31 Improvements from the baseline in the IPSS score and IPSS-quality of life (QOL) were significant in both treatment groups (p<0.001 for both), although the IPSS mean change from the baseline was significantly larger in the combination group compared with the tamsulosin group (−9.2 vs. −6.7; p<0.05). Improvements in Qmax and decreases in post-void residual (PVR) volume from the baseline were similar in both treatment groups. The IEFF-EF showed significant improvement only in the combination group (p<0.001) and the global assessment quality showed that all patients preferred the combination scheme. During the study, two adverse event-related discontinuations occurred, one with headache (combination group) and the other with moderate cutaneous rash (tamsulosin group). No serious adverse events (SAE) were reported.31
Regadas et al. performed urodynamic studies to compare tadalafil 5mg/tamsulosin 0.4mg QD with placebo/tamsulosin 0.4mg QD in 40 patients with LUTS secondary to BPH.32 At the end of the 30-day study period, the primary endpoint, detrusor pressure at maximum flow, was significantly reduced in the tadalafil/tamsulosin group (13±17.0) compared with the placebo/tamsulosin group (−1.2±14.4; p=0.03). Similarly, total IPSS, and the storage and voiding subscores improved significantly in the tadalafil/tamsulosin group compared with the placebo/tamsulosin group (p≤0.05 for all).32
In a larger study of 133 patients with LUTS due to BPH, Singh et al. compared tadalafil 10mg/tamsulosin 0.4mg QD combination therapy with tadalafil 10mg QD monotherapy and tamsulosin 0.4mg QD monotherapy.33 At the end of the 3-month study period, significant improvement in the IPSS score was reported across all treatment groups (tamsulosin: −50.9%, p<0.05; tadalafil: −33.5%, p<0.05; tadalafil/tamsulosin combination: −53.9%, p<0.05). Similarly, significant improvements in the IIEF-5 score, Qmax, PVR volume, and QOL were reported in the three treatment groups. In the tamsulosin, tadalafil and tamsulosin/tadalafil combination groups, the IIEF-5 score increased [+39.3% (p<0.05), +46.0% (p<0.05), and +60.2% (p<0.05), respectively]; Qmax increased [+34.0% (p<0.05), +29.8% (p<0.05), and +37.0% (p<0.05), respectively]; PVR volume was reduced [−60.9% (p<0.05), −49.5% (p<0.05), and −63.0% (p<0.05), respectively]; and QOL scores also improved significantly [−73.4% (p<0.05), −70.3% (p<0.05), and −79.7% (p<0.05), respectively].33 Five adverse event-related treatment discontinuations (3.7%) occurred during the study, but there were no severe adverse events or SAEs. Adverse events leading to discontinuation were worsening of LUTS (tadalafil monotherapy), severe myalgia and backache (one each in the tadalafil monotherapy and tadalafil/tamsulosin groups), flushing, headache, myalgia, and hypotension (tadalafil monotherapy), and an increase in intraocular pressure in a patient previously diagnosed with glaucoma (tadalafil monotherapy).33
Sildenafil treatment combinationsThe efficacy of combination therapy with sildenafil 25mg QD and alfuzosin 10mg QD was investigated by Kaplan et al. in a pilot study of 62 men with LUTS suggestive of BPH and erectile dysfunction.34 Improvements in the IPSS were significant across the three treatment groups, although they were greatest in the men receiving the combination therapy (−24.1%) compared with alfuzosin (−15.6%) or sildenafil alone (−16.9%; p<0.03). Frequency, nocturia, PVR volume, and Qmax were significantly improved with both alfuzosin monotherapy and the combination treatment, and improvement in the IIEF was marked with sildenafil (49.7%), although greater with the combination treatment (58.6%). The rate of adverse event-related treatment discontinuation was 11% overall (seven patients: two in the alfuzosin group [dizziness], two in the sildenafil group [flushing and dyspepsia], and three in the combination group [dizziness; gastric upset]). No SAEs or evidence of hypotension or syncope occurred during the 12-week treatment period.34
An 8-week study comparing sildenafil citrate 25mg four times/week monotherapy with tamsulosin 0.4mg QD or the combination of these agents in 60 men with LUTS suggestive of BPH and erectile dysfunction was reported in 2010.35 The IPSS, Qmax, PVR volume, Sexual Health Inventory of Male (SHIM) scores, and the third and fourth questions on the IIEF (relating to achieving and sustaining penetration, respectively) were significantly improved in all treatment groups compared with the baseline (p<0.001 for all). Improvement in IPSS was greater in the combination group (40.1%) and tamsulosin group (36.2%) compared with the sildenafil group (28.2%; p<0.001). Similarly, PVR volume reductions were also significantly greater in the combination and tamsulosin monotherapy groups compared with sildenafil monotherapy (p<0.001 for both). Improvement in Qmax was significantly higher in the combination therapy group (42.0%) compared with either of the monotherapy groups (p<0.001). Tuncel et al. concluded that the combination treatment of sildenafil/tamsulosin was not superior to tamsulosin monotherapy in improving voiding symptoms.35
Vardenafil treatment combinationsGacci et al. compared the safety and efficacy of tamsulosin 0.4mg/placebo QD with tamsulosin 0.4mg/vardenafil 10mg QD combination therapy in 60 men with persistent storage LUTS after a 2-week run-in period with tamsulosin.36 Significant improvements compared with the baseline in the Qmax, average flow rate (Qave), irritative-IPSS subscores, and the IIEF were reported with the combination therapy over tamsulosin monotherapy (p≤0.039 for all) at the 12-week follow-up. No differences in the incidence of common, treatment-related adverse events were found between the groups, and no patient reported any SAEs.36
Clinical management implicationsThe combination of α-blocker and PDE5I has been shown in recent studies to be more effective than PDE5I monotherapy for managing erectile dysfunction in men with LUTS/BPH. Considering that 43.0% to 82.5% of patients suffering LUTS also experience erectile dysfunction,3 this treatment combination holds much promise, particularly in patients who have shown a poor response to monotherapy with either of these classes of agent. The PDE5I/α-blocker combination therapy has been shown to improve Qmax, PVR volume, and detrusor pressure at maximum flow compared with placebo, and also results in additive effects with respect to the IEFF and IPSS compared with PDE5I or α-blocker monotherapy. The mechanism for the additive improvement in symptoms produced with combination therapy is not yet fully elucidated. It has been hypothesized that the agents work via two distinct mechanisms of action, but on common target organs.33
Since both α-blockers and PDE5Is have vasodilatory effects, there has been particular interest in determining whether an additive decrease in blood pressure exists with combination treatment. As described, the primary objective of the trial reported by Goldfischer et al. was to investigate the potential hemodynamic effects of daily coadministration of tadalafil 5mg with various α-blockers over a 12-week treatment period.29 The changes in hemodynamic signs and symptoms were similar in men receiving tadalafil or placebo in combination with an α-blocker, and no cases of syncope or SAEs attributable to hypotension were reported. Although the study was not powered for subgroup analysis, when stratified by α-blocker type, a trend was observed for increased hemodynamic signs and symptoms in men taking non-uroselective α-blockers, particularly doxazosin. This was not seen with the coadministration of uroselective α-blockers, such as alfuzosin, silodosin, and tamsulosin.29 More generally, across the studies investigating PDE5I/α-blocker coadministration, no new safety signals were detected, with discontinuations due primarily to the adverse events of myalgia, dizziness, sensation of heaviness, and headache. No SAEs were reported in these clinical trials.
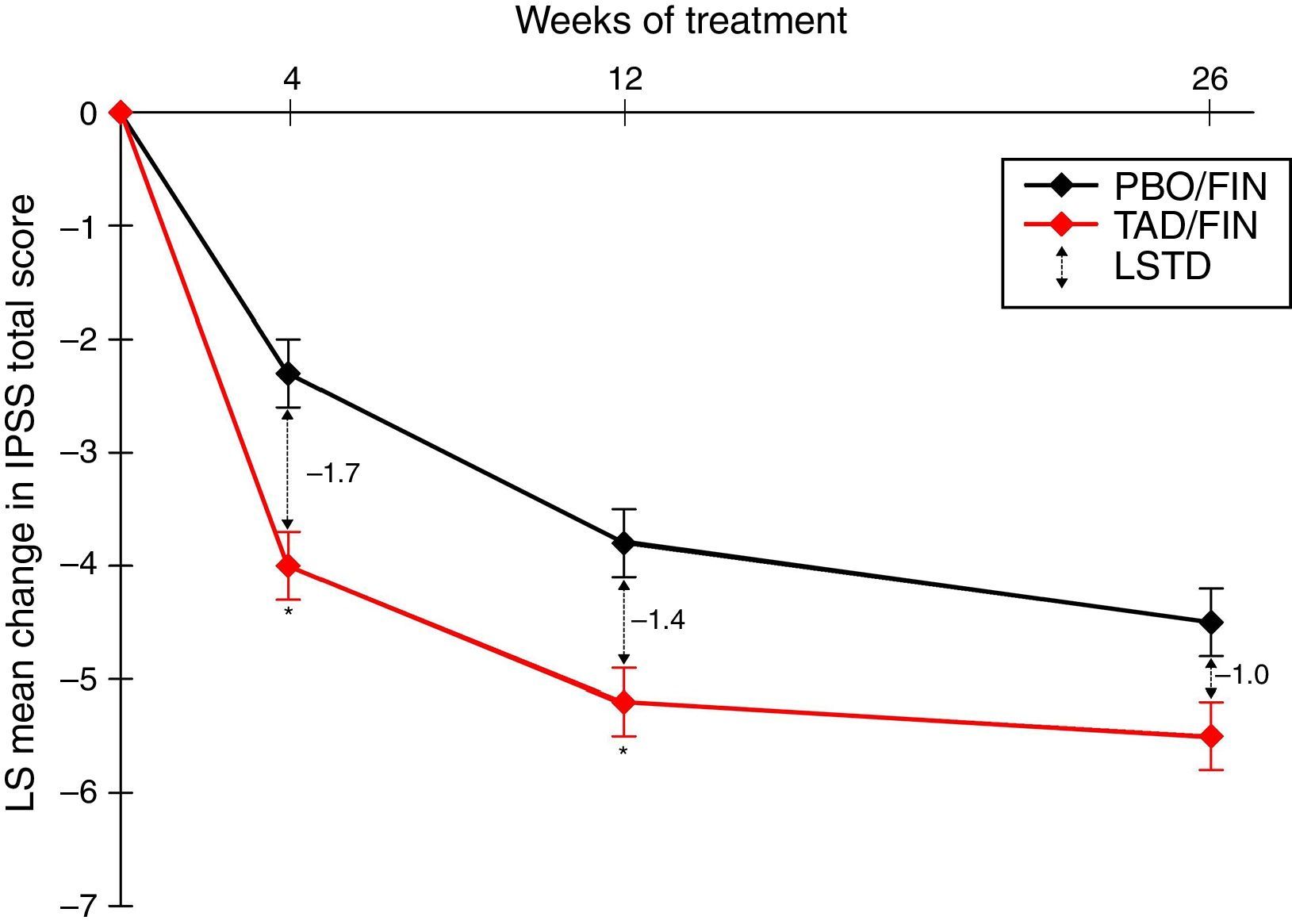
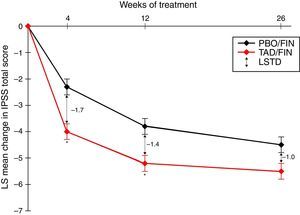
PDE5I/5-ARI combination therapyThe use of a PDE5I together with a 5-ARI has been suggested to offer earlier symptomatic relief in LUTS than with 5-ARIs alone, and it also seems that the PDE5I may reduce the sexual side effects associated with 5-ARI use, including erectile dysfunction, decreased/lost libido, and ejaculation delay/failure. In a recent study investigating the coadministration of tadalafil 5mg QD and finasteride 5mg QD, 350 5-ARI-naïve men with IPSS≥13 and LUTS secondary to BPH were treated with placebo/finasteride and 345 with tadalafil/finasteride for 26 weeks.37 Least squares mean changes from the IPSS baseline after 4, 12, and 26 weeks of combination therapy were −4.0, −5.2, and −5.5, respectively, and represented significantly greater changes from the baseline than the placebo/finasteride group at all visits (p≤0.022) (Fig. 1). The IPSS subscores for storage and voiding and the QOL index were numerically improved with the combination treatment, although there was no statistically significant difference. Least squares mean changes from the baseline in erectile function, measured as the IIEF-EF score, were 3.7 after 4 weeks and 4.7 after 12 and 26 weeks of combination therapy, while the corresponding scores with placebo/finasteride were −1.1, 0.6, and −0.0 (p<0.001 at all visits).37
LS mean change from the baseline in the International Prostate Symptom Score (IPSS) total score over a 26-week treatment period in patients who were randomized to the study treatment and received ≥1 dose of the double-blind study drug (TAD/FIN 345 and PBO/FIN 345). Results adapted from Casabé et al., 2014.37 * p≤0.001. Error bars represent standard error. Abbreviations: IPSS, International Prostate Symptom Score; LS, least squares; LSTD, least squares treatment difference; PBO/FIN, placebo/finasteride 5mg once daily; TAD/FIN, tadalafil 5mg+ finasteride 5mg once daily.
The overall safety profile did not deviate from that previously established for tadalafil or finasteride monotherapy. Most treatment-emergent adverse events were mild to moderate in severity, and the incidence of SAEs and discontinuations related to adverse events was low and showed no significant difference between treatment groups. Fewer patients receiving combination therapy reported reduced libido or erectile dysfunction compared with those in the placebo/finasteride group: five patients in the placebo/finasteride group and one patient in the tadalafil/finasteride group reported erectile dysfunction. Five patients reported decreased or lost libido and two patients reported ejaculation delay/failure in the placebo/finasteride group, while no patients in the tadalafil/finasteride group reported decreased/lost libido or adverse ejaculation issues.37
Given that LUTS improvements are generally observed after 6–12 months of 5-ARI monotherapy, it would be worthwhile to investigate this treatment combination in a longer-term study with treatment duration of at least 12 months.6,38,39 Although the generalizability of these findings is limited to men with IPSS≥13 and large prostate volumes (≥30mL), this category of combination therapy is very appealing in managing BPH/LUTS symptoms in patients with the potential for disease progression who wish to maintain or improve sexual function.
α-Blocker/antimuscarinic combination therapyTraditionally, α-blockers have been used to manage LUTS associated with BPH and antimuscarinics used for OAB, but we now have increasing data on the combination of these agents. The majority of studies investigating this combination have looked at antimuscarinics as add-on to baseline α-blocker therapy: studies have investigated tamsulosin with add-on solifenacin,40,41 propiverine,42 and oxybutynin,43 α-blockers with add-on tolterodine,44 or propiverine extended-release.45
The two main studies that prospectively assigned combination treatment with an α-blocker and antimuscarinic have shown improvements over monotherapy, particularly regarding storage symptoms. An 8-week study comparing doxazosin 4mg QD controlled-release gastrointestinal therapeutic system with and without propiverine hydrochloride 20mg QD in 211 men with OAB and BPO reported significant benefits in urinary frequency, average micturition volume, storage, urgency, and patient satisfaction in the combination group (p<0.029 for all).46 Improvement in voiding symptoms was similar in the two groups, but improvement in storage symptoms was significantly higher in the combination therapy group (p=0.029). This was accompanied by higher overall adverse event rates, although similar discontinuation- and adverse event-related discontinuation rates were observed between treatment groups.46 The TIMES study was a 4-arm prospective study that compared 12 weeks of treatment with placebo, tolterodine 4mg QD extended-release, tamsulosin 0.4mg QD, and tolterodine 4mg extended-release/tamsulosin 0.4mg combination therapy in 879 men with OAB and BPH.47 Men with clinically significant bladder outlet obstruction (PVR volume>200mL and Qmax<5mL/s) or PSA>10ng/mL with a risk for prostate cancer were excluded from the study. A total of 80% of men receiving combination therapy reported treatment benefit by week 12 compared with 62% receiving placebo, 71% receiving tamsulosin, and 65% receiving tolterodine. Only the combination therapy resulted in a significant improvement in quality of life and total IPSS compared with placebo (p=0.03 for both). The most commonly occurring adverse event was dry mouth, with two and five patients discontinuing treatment due to dry mouth in the tamsulosin and combination therapy groups, respectively. The incidence of AUR requiring catheterization was low (combination treatment 0.4%, tolterodine 0.4%, and 0% in the other groups).47
Two more recent studies investigated solifenacin/tamsulosin combination therapy.48,49 Kaplan et al. compared two doses of solifenacin (6mg and 9mg QD) with the tamsulosin 0.4mg QD oral controlled absorption system combination versus placebo for 12 weeks in a safety study. This study focused on the outcomes of Qmax and detrusor pressure at Qmax and both were reported to be non-inferior to placebo in each of the interventional groups.48 Urinary retention was reported in one patient, who was receiving combination therapy (lower dose solifenacin). Since prostate size and PSA levels were not measured in this study, the generalizability of these results is limited.48 In the NEPTUNE study, 1334 men with storage and voiding LUTS were randomized to one of four treatment arms: placebo, tamsulosin 0.4mg QD oral controlled absorption system (TOCAS), solifenacin 6mg QD plus TOCAS, or solifenacin 9mg QD plus TOCAS for 12 weeks.49 Reductions in the IPSS and total urgency and frequency score were reported in all treatment arms, with solifenacin 6mg plus TOCAS significantly improving storage symptoms compared with placebo (p<0.001) and TOCAS monotherapy (p<0.01) and significantly improving voiding symptoms compared with placebo (p≤0.05). Urinary retention occurred in eight patients during the study (seven cases were considered drug-related and five cases required catheterization: one case with TOCAS [0.3%] and one with solifenacin 6mg plus TOCAS [0.3%] and three cases with solifenacin 9mg plus TOCAS [0.9%]).49
Although all of the prospective studies investigating the α-blocker/antimuscarinic combination have been short in duration (8–12 weeks), the findings of these studies confirm that the short-term combination therapy response rates are improved compared with monotherapy in men with moderate to severe storage and voiding symptoms. As such, this combination may represent a good treatment alternative for patients who have not responded to monotherapy with an α-blocker or antimuscarinic drug. This treatment approach is supported in clinical guidelines, which recommend offering antimuscarinics to men with OAB, and α-blocker/antimuscarinic combination treatment for those with persisting storage symptoms.50
ConclusionsThe evidence base supporting the coadministration of two agents for the clinical management of LUTS is expanding. Although α-blockers are traditionally the first-line treatment option for patients with voiding symptoms, PDE5Is were recently shown to have similar efficacy in improving IPSS and Qmax. The combination of these classes of agents was found to be more effective than either of the monotherapies for providing symptomatic relief (as assessed by the IPSS and IEFF) and particularly effective in resolving erectile dysfunction. Investigations of hemodynamic changes with this treatment combination showed similar changes with tadalafil/α-blocker therapy compared with placebo/α-blocker therapy, with a trend for increased hemodynamic signs only with non-uroselective α-blockers, most notably doxazosin. No new safety signals were detected with this treatment combination.24–28,31–34,36,51
Antimuscarinic agents remain the first-line treatment for patients with moderate to severe storage symptoms and OAB, although the addition of an α-blocker may further improve storage and voiding symptoms in those men who have not responded to antimuscarinic monotherapy, and is supported by clinical treatment guidelines.50 Longer-term studies assessing the efficacy of this treatment combination would add to the existing evidence showing improvement with 8 to 12-week treatment duration.46–49
5-ARIs are the mainstay of treatment for patients at risk for LUTS clinical progression, and there is a wealth of evidence for the concomitant use of α-blockers in older men and/or those with large prostates.7–9 The optimal treatment duration before α-blocker discontinuation has not yet been determined, although the results of two studies indicate that discontinuation may only be suitable for patients with mild to moderate symptoms, as many of those with severe baseline symptoms deteriorated after α-blocker withdrawal.14,16 The use of PDE5Is in combination with 5-ARIs in a similar patient population at risk for clinical progression has been the subject of recent investigation, which has shown it offers early symptomatic relief and also reduces the sexual side effects associated with 5-ARI use.37 This treatment combination warrants further investigation through large-scale clinical trials with treatment duration of at least 6–12 months.
Financial disclosureThe development of this article was sponsored by Eli Lilly and Company.
Conflict of interestLuis Reyes-Vallejo is an employee of Eli Lilly and Company. Ignacio Barragán Arteaga has been a speaker and advisory board member for Asofarma, Eli Lilly and Company and GSK.
Medical writing support was provided by Lucy Rutherford, and editorial support was provided by Angela Lorio, of inVentiv Health Clinical. Medical writing and editorial support were funded by Eli Lilly and Company.