High species diversity renders benthic diatoms that are useful in assessing environmental impact, as well as an adequate reference for measuring biodiversity in protected areas. Preliminary observations suggested that the Guerrero Negro Lagoon (LGN), located in the Baja California Peninsula, Mexico, is an area with a high diversity of benthic diatoms comprising numerous species of certain genera, orders, class, etcetera, which were not equally diverse or common in other areas, and could thus yield new records for the region. Thus, samples of subtidal sediments from LGN were collected in order to analyze the species composition of epipelic diatoms by means of optical microscopy. The taxonomic study yielded a list with 232 taxa, which comprised 42 centric diatoms (>18%) and 190 pennates from 74 genera; 14 new records for the Mexican Pacific are included. This supported the hypothesis that epipelic diatoms from the LGN subtidal constitute assemblages with a high species richness and numerous taxa characteristic of subtropical regions.
La alta diversidad de diatomeas bentónicas les confiere un uso en la evaluación de impacto ambiental; a la vez, son una referencia adecuada para estimar biodiversidad en áreas protegidas. Observaciones preliminares en la laguna Guerrero Negro (LGN), localizada en la península de Baja California, México, sugirieron que alberga taxocenosis de diatomeas bentónicas con alta diversidad de especies de varios géneros, órdenes, clases, etcétera, que no son igualmente diversas o comunes en otras áreas, por lo que se esperaría encontrar nuevos registros para la región. Con base en esto, se recolectaron muestras de sedimentos del submareal en la LGN con el objetivo de describir la composición de especies de diatomeas epipélicas mediante microscopia óptica. El análisis taxonómico resultó en una lista de 232 taxones que comprende 42 céntricas (>18%) y 190 pennadas contenidas en 74 géneros. Se encontraron 14 nuevos registros para el Pacífico mexicano. Las observaciones respaldan la hipótesis de que las diatomeas epipélicas en el submareal de LGN conforman taxocenosis con elevadas riquezas de especies, conformadas por numerosos taxones característicos de regiones subtropicales.
Benthic diatoms (Bacillariophyta) have been considered the most diverse and productive algal group in marine ecosystems (López-Fuerte, Siqueiros-Beltrones, & Yabur, 2015). Their ecological significance has distinguished them as an adequate reference for estimating biodiversity in marine protected areas (López-Fuerte, Siqueiros-Beltrones, & Navarro, 2010) and as a useful reference for assessing environmental impact (Siqueiros-Beltrones, 2002). Thus, both diversity and ecological aspects of diatoms should be considered essential for having an adequate ecological perspective of any aquatic ecosystem, particularly when elaborating management plans for protected areas.
The Guerrero Negro Lagoon (LGN) is part of the lagoon complex known as the Guerrero Negro-Ojo de Liebre located in the northern and southernmost parts of Baja California Sur (BCS) and Baja California (BC), respectively. It is found within the western mid-part of the Baja California Peninsula and in 1988 it was established as a protected area – Reserva de la Biosfera El Vizcaíno–, which due to its geographic location is considered a biological diversification center (Arellano-Martínez, De La Cruz-Agüero, & Cota-Gómez, 1996). However, notwithstanding the LGN is of utmost importance on environmental issues, and many studies are still required to adequately describe LGN in ecological terms.
Studies on benthic diatom floristics and ecology have been hitherto lacking for the LGN among many other taxonomic and ecological studies, inasmuch that these are scarce for the whole NW Mexican area in general. Most studies on benthic diatoms are related to their role in the diet of abalone (Haliotis spp.), a group of economically important species, and other herbivorous mollusks found in the intertidal ecosystems (Siqueiros-Beltrones & Valenzuela-Romero, 2004). Other studies refer to the structure of benthic diatom assemblages growing on macroalgae and plant substrates (Argumedo-Hernández & Siqueiros-Beltrones, 2008; Siqueiros-Beltrones, 2002); while another comprehensive study describes the epipelic diatom assemblages from mangrove sediments (López-Fuerte et al., 2010).
Preliminary observations of LGN sediments suggested that they could harbor highly diverse assemblages of benthic diatoms with many species of certain taxa (genus, order, class, etcetera) which are not as diverse or common as in other localities of the region, considering both coasts of the Baja California Peninsula. According to this, the LGN could be considered a species diversity hotspot for benthic diatoms, from where certain common and abundant taxa may be exported to other localities, thus being useful to detect connectivity relations with other ecosystems in the NW Mexican region, as well as distributional patterns on the basis of floristics and assemblage structure variations.
Thus, the objective of this study was to describe a significant part of the benthic diatom flora from the LGN based on species richness and composition, focusing on epipelic forms living in the subtidal sediments, including samples from cold and warm seasons. This study also represents the first estimate of benthic diatom species diversity and provides an insight of their biogeographical affinities. We tested the hypothesis that epipelic diatom assemblages from the subtidal sediments in LGN would have a high species richness, containing numerous taxa of distinct biogeographical affinities, as is characteristic in subtropical transitional zones showing high species diversity.
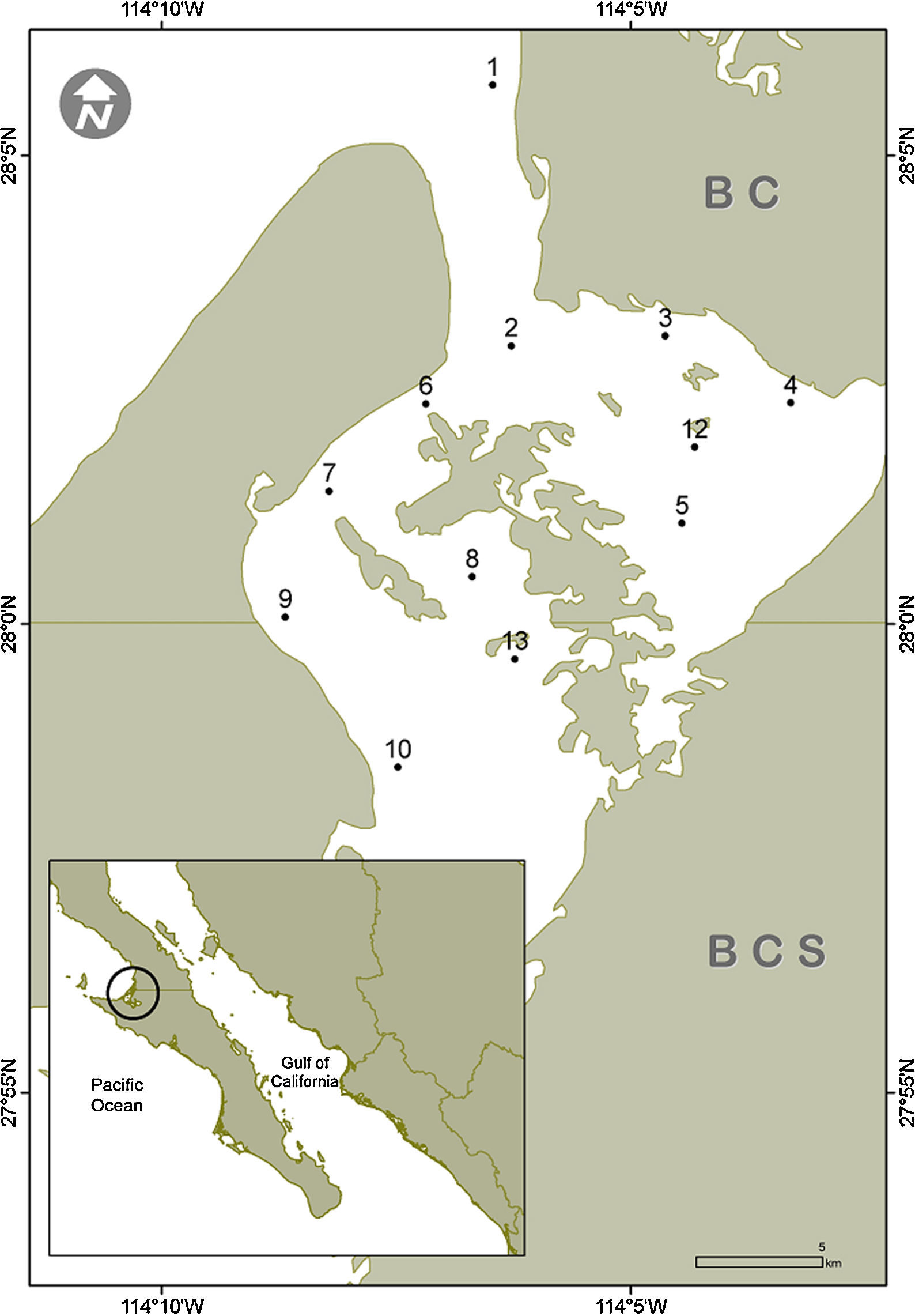
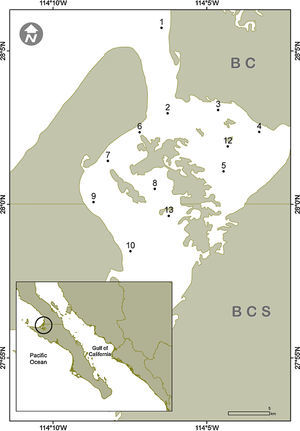
Material and methodsThe Guerrero Negro Lagoon (LGN) is located at 27°35′–27°52′ N, 113°58′–114°10′ W within the northernmost part of BCS and southernmost BC, Mexico (Fig. 1). This lagoon is part of a complex along with the Ojo de Liebre Lagoon, within the boundaries of El Vizcaíno Biosphere Reserve (Arellano-Martínez et al., 1996); the lagoons have separate mouths that drain into to Sebastián Vizcaíno Bay. The climate is arid with a low annual rainfall (mainly during winter) of <100mm (Salinas-Zavala, Llinas, & Rodríguez-Estrella, 1991). The LGN has a rectangular shape that extends approximately 2,100ha, with a maximum length of 13km and width of 8km that is connected to Vizcaíno Bay by a narrow channel (Contreras, 1985). It has a shallow bottom that varies in depth, mostly between 2 and 12m (Lluch-Cota, Castellanos-Vera, Llinas-Gutiérrez, & Ortega-Rubio, 1993) with a maximum of 26m. Eelgrass (Zostera marina) is widely distributed from 6m deep up to the high tide mark (Eberhard, 1966). The lagoon sediments are composed mainly of gray sand mixed with organic alluvial deposits. Both normal and hypersaline salinity gradients have been recorded in the LGN (Lankford, 1977), although it has been considered an isohaline lagoon with salinity values between 35.5–37.5 in winter and 34.7–35.6 in summer (Phleger & Ewing, 1962).
Surficial sediment samples were collected by scuba diving in 12 sites along the subtidal bottom of the Guerrero Negro Lagoon in November 2013, January, June and July 2014 (Fig. 1). The sampling depth varied between 3 and 15m. In each site, approximately 150g of sediments where scooped using a 250mL plastic jar. In the laboratory, a 50g subsample was separated, placed in a 100mL beaker, and drinking water was added up to 100mL. The beaker was then placed in an ultrasound bucket for 1min while shaking lightly. Afterwards, the heavier sediments were decanted and disposed of while the remaining sediment suspension was relocated in a 100mL test tube and left to settle for 2h. Again, the overlaying water was removed thus leaving in the bottom a concentrate of diatom frustules. From this concentrate an aliquot was used to make fresh preparation for observation of living cells under the microscope, while the rest was submitted to oxidation of the organic matter. This was done with a mixture of sample, commercial alcohol and nitric acid at a ratio of 1:3:5, varying the amount of the reagents according with the apparent amount of organic matter in each sample (Siqueiros-Beltrones, 2002). Later, the oxidized material was rinsed repeatedly with drinking water until it reached a pH≥6. For each sample 2 permanent slides were mounted using the synthetic resin Pleurax (IR=1.7).
The slides were examined under an optical microscope with phase contrast and planapochromatic optics. Species identification was based on regional literature: Hernández-Almeida and Siqueiros-Beltrones (2008, 2012), López-Fuerte et al. (2010), Moreno-Ruíz, Licea, and Santoyo (1996), Siqueiros-Beltrones (2002, 2006), Siqueiros-Beltrones and Hernández-Almeida (2006), Siqueiros-Beltrones, Argumedo-Hernández, Murillo-Jiménez, and Marmolejo-Rodríguez (2014), as well as on classic literature: Hendey (1964), Hustedt (1959, 1966), Peragallo and Peragallo (1908), Round, Crawford, and Mann (1990), Schmidt et al. (1959), Stidolph, Sterrenburg, Smith, and Kraberg (2012), Witkowski, Lange-Bertalot, and Metzeltin (2000). We mainly followed the classification system of Round et al. (1990). However, the taxonomic status of all taxa were updated according to the Algaease website (http://algaebase.org/search/species/, Guiry & Guiry, 2015). To complement the floristic list, an iconographic catalog was constructed with micrographs taken with a CMOS Konus digital ocular lens microscope at 1000×.
ResultsThe diatom assemblages from the intertidal sediments of LGN comprised 232 taxa distributed within 74 genera, 42 centrics (>18%), and 190 pennates. Out of the total number of taxa, 24 could not be identified to species level (Table 1). An iconographic catalog with most of the observed taxa was constructed to complement the taxonomic analysis (Figs. 2–9).
List of species and infra-specific taxa of benthic diatoms found in subtidal sediments of Laguna Guerrero Negro, BC-BCS, Mexico. NR=new records for the Mexican NW region.
| Class Coscinodiscophyceae Round et R. M. Crawford |
| Order: Anaulales Round et R. M. Crawford |
| Family: Anaulaceae (F. Schütt) Lemmermann |
| Eunotogramma Weisse |
| Eunotogramma laevis Grunow (Fig. 4K, P, Q) |
| Eunotogramma marinum (W. Smith) H. Peragallo et M. Peragallo (Fig. 4R) |
| Order: Coscinodiscales Round et R. M. Crawford |
| Family: Heliopeltaceae W. Smith |
| Actinocyclus Ehrenberg |
| Actinocyclus curvatulus Janisch (Fig. 3A and B) |
| Actinocyclus octonarius var. tenellus (Brébisson) Hendey (Fig. 3C) |
| Actinocyclus ralfsii f. minutae H. Peragallo et M. Peragallo NR |
| Actinoptychus Ehrenberg |
| Actinoptychus adriaticus Grunow (Fig. 2F) |
| Actinoptychus aster J. J. Brun (Fig. 2A) |
| Actinoptychus oppenoorthi T. Reinhold (Fig. 2C) |
| Actinoptychus senarius (Ehrenberg) Ehrenberg (Fig. 2B) |
| Actinoptychus splendens (Shadbolt) Ralfs |
| Plagiogrammopsis Hasle, Stosch et Syvertsen |
| Plagiogrammopsis vanheurckii (Grunow) Hasle, von Stosch et Syvertsen |
| Order: Melosirales R. M. Crawford |
| Family: Hyalodiscaceae R. M. Crawford |
| Hyalodiscus Ehrenberg |
| Hyalodiscus punctatus A. Schmidt |
| Order: Paraliales R. M. Crawford |
| Family: Paraliaceae R. M. Crawford |
| Paralia Heib. |
| Paralia sulcata var. crenulata Grunow |
| Family: Aulacodiscaceae (F. Schütt) Lemmermann |
| Aulacodiscus Ehrenberg |
| Aulacodiscus ehrenbergii C. Janisch (Fig. 2N) |
| Aulacodiscus cf. minimus Hustedt |
| Aulacodiscus sp. NR (Fig. 9A and P) |
| Family: Coscinodiscaceae Kütz. |
| Coscinodiscus Ehrenberg |
| Coscinodiscus concinnus W. Smith |
| Coscinodiscus radiatus Ehrenberg (Fig. 2I) |
| Order: Cymatosirales Round et R. M. Crawford |
| Family: Cymatosiraceae Hasle, von Stosch et Syvertsen |
| Brockmanniella |
| Brockmanniella brockmannii (Hustedt) Hasle, Stosch et Syvertsen |
| Campylosira Grunow |
| Campylosira cymbelliformis (A. Schmidt) Grunow ex van Heurck |
| Order: Triceratiales Round et R. M. Crawford |
| Family: Triceratiaceae (F. Schütt) Lemmermann |
| Auliscus Ehrenberg |
| Auliscus caelatus var. strigillata A. W. F. Schmidt (Fig. 2G) |
| Auliscus punctatus Bailey (Fig. 2H) |
| Cerataulus Ehrenberg |
| Cerataulus californicus A. Schmidt (Fig. 3I, J) |
| Triceratium Ehrenberg |
| Triceratium favus Ehrenberg |
| Family: Plagiogrammaceae De Toni |
| Glyphodesmis Grev. |
| Glyphodesmis sp. |
| Dimeregramma Ralfs |
| Dimeregramma cf. maculatum (Cleve) Frenguelli |
| Dimeregramma minor var. minor (Gregory) Ralfs (Fig. 4A, E, F, J) |
| Dimeregramma sp. (Fig. 4N) |
| Plagiogramma Grevillei |
| Plagiogramma interruptum (Gregory) Ralfs |
| Plagiogramma pulchellum Greville |
| Plagiogramma sp. |
| Plagiogramma wallichianum Greville (Fig. 4L) |
| Family: Biddulphiaceae Kützing |
| Biddulphia Gray |
| Biddulphia tuomeyi (J. W. Bailey) Roper (Fig. 3E) |
| Biddulphia rhombus (Ehrenberg) W. Smith (Fig. 3F) |
| Neohuttonia Kuntze |
| Neohuttonia reichardtii (Grunow) Hustedt (Fig. 4O) |
| Terpsinoë Ehrenberg |
| Terpsinoë americana (Bailey) Grunow |
| Trigonium Cleve |
| Trigonium alternans (Bailey) A. Mann |
| Order: Thalassiosirales Glezer et Makarova |
| Family: Stephanodiscaceae Glezer et Makarova |
| Cyclotella (Kützing) Brébison |
| Cyclotella litoralis Lange et Syvertsen (Fig. 2M) |
| Cyclotella striata (Kützing) Grunow |
| Family: Thalassiosiraceae M. Lebour |
| Ehrenbergiulva Witkowski, Lange-Bertalot et Metzeltin |
| Ehrenbergiulva granulosa (Grunow) Witkowski, Lange-Bertalot et Metzeltin (Fig. 2D and E) |
| Ehrenbergiulva haucki (Grunow) Witkowski, Lange-Bertalot et Metzeltin |
| Thalassiosira Cleve |
| Shionodiscus A. J. Alverson, S. H. Kang et E. C. Theriot |
| Shionodiscus oestrupii (Ostenfeld) A. J. Alverson, S. H. Kang et E. C. Theriot (Fig. 2J) |
| Class Fragilariophyceae Round |
| Family: Psammodiscaceae Round et D. G. Mann |
| Family: Rhaphoneidaceae Forti |
| Delphineis G. W. Andrews |
| Delphineis surirella (Ehrenberg) G. W. Andrews (Fig. 4C, G) |
| Delphineis fasciola var. australis (P. Petit) P. M. Tsarenko (Fig. 4B, D) |
| Diplomenora K. L. Blazé |
| Diplomenora cocconeiformis (A. Schmidt) K. L. Blazé (Fig. 4S) |
| Neodelphineis Takano |
| Neodelphineis sp. |
| Rhaphoneis Ehrenberg |
| Rhaphoneis nitida (W. Gregory) Grunow |
| Rhaphoneis sp. 1 |
| Rhaphoneis surirella var. ceylanica (Cleve) Foged |
| Psammodiscus Round et D. G. Mann |
| Psammodiscus calceatus T. Watanabe, T. Nagumo et J. Tanaka NR (Fig. 2L) |
| Psammodiscus nitidus (W. Gregory) Round et D. G. Mann (Fig. 2K) |
| Grammatophora Ehrenberg |
| Grammatophora hamulifera Kützing |
| Order: Thalassionematales Round |
| Family: Thalassionemataceae Round |
| Thalassionema Grunow |
| Thalassionema nitzschioides (Grunow) Mereschkowsky |
| Order: Climacospheniales Round |
| Family: Climacospheniaceae Round |
| Climacosphenia Ehrenberg |
| Climacosphenia moniligera Ehrenberg |
| Family: Fragilariaceae Grev. |
| Opephora Petit |
| Opephora marina (W. Gregory) Petit |
| Opephora marina (W. Gregory) Petit var.? (Fig. 4M) |
| Opephora pacifica (Grunow) Petit (Fig. 4H) |
| Opephora schwartzii (Grunow) Petit ex Pelletan (Fig. 4I) |
| Opephora sp. 1 NR (Fig. 9G, H) |
| Podocystis J. W. Bailey |
| Podocystis adriatica (Kützing) Ralfs |
| Staurosirella D. M. Williams et Round |
| Staurosirella pinnata (Ehrenberg) D. M. Williams et Round |
| Trachysphenia P. Petit |
| Trachysphenia australis P. Petit |
| Trachysphenia australis var. rostellata Hustedt |
| Class Bacillariophyceae Haeckel |
| Order: Achnanthales Silva |
| Family: Achnanthaceae Kützing |
| Achnanthes Bory |
| Achnanthes danica (Flögel) Grunow (Fig. 5A) |
| Achnanthes fimbriata (Grunow) Ross (Fig. 5B) |
| Achnanthes tenera Hustedt |
| Family: Achnanthidiaceae D. G. Mann |
| Achnanthidium Kützing |
| Achnanthidium sp. 1 |
| Planothidium Round et Buktiyarova |
| Planothidium delicatulum (Kützing) Round et Bukhtiyarova |
| Planothidium hauckianum (Grunow) Round et Bukhtiyarova |
| Planothidium lilljeborgei (Grunow) Witkowski Planothidium polaris (Østrup) Witkowski, Lange Bertalot et Metzeltin (Fig. 5R) |
| Family: Cocconeidaceae Kützing |
| Amphicocconeis |
| Amphicocconeis disculoides (Hustedt) Stefano et Marino |
| Anorthoneis Grunow |
| Anorthoneis eurystoma Cleve (Fig. 5D) Anorthoneis excentrica (Donkin) Grunow (Fig. 5D) |
| Anorthoneis hyalina Hustedt (Fig. 5E) |
| Cocconeis Ehrenberg |
| Cocconeis californica var. kerguelensis Heiden (Fig. 9Q) |
| Cocconeis cf. nugalas M. H. Hohn et J. Hellerman |
| Cocconeis cf. pelta A. Schmidt NR (Fig. 9L, M) |
| Cocconeis discrepans A. W. F. Schmidt |
| Cocconeis distans W. Gregory (Fig. 5S) |
| Cocconeis guttata Hustedt et Aleem (Fig. 5O, P) |
| Cocconeis latecostata Hustedt (Fig. 5L) |
| Cocconeis neodiminuta Krammer |
| Cocconeis peltoides Hustedt |
| Cocconeis pinnata W. Gregory ex Greville |
| Cocconeis placentula var. euglypta (Ehrenberg) P.T. Cleve |
| Cocconeis pseudomarginata Gregory |
| Cocconeis sp.1 |
| Cocconeiopsis Witkowski, Lange-Bertalot et Metzeltin |
| Cocconeiopsis cf. kantsinensis (Giffen) Witkowski |
| Cocconeiopsis patrickae (Hustedt) A. Witkowski, Lange-Bertalot et Metzeltin (Fig. 5Q) |
| Cocconeiopsis regularis (Hustedt) Witkowski |
| Order: Bacillariales Hendey |
| Family: Bacillariaceae Ehrenberg |
| Fragilariopsis Hustedt |
| Fragilariopsis doliolus (Wallich) Medlin et P. A. Sims |
| Hantzschia Grunow |
| Hantzschia virgata (Roper) Grunow |
| Nitzschia Hassall |
| Nitzschia dissipata (Kützing) Rabenhorst |
| Nitzschia distans Gregory |
| Nitzschia fluminensis Grunow (Fig. 8G) |
| Nitzschia grossestriata Hustedt (Fig. 8I) |
| Nitzschia sigma (Kützing) W. Smith (Fig. 8H) |
| Tryblionella |
| Tryblionella cf. coarctata (Grunow) D. G. Mann |
| Family: Anomoeoneidaceae D. G. Mann |
| Staurophora Mereschkowsky |
| Staurophora cf. salina (W. Smith) Mereschkowsky |
| Staurophora sp. |
| Order: Lyrellales D. G. Mann |
| Family: Lyrellaceae D. G. Mann |
| Lyrella Karayeva |
| Lyrella abrupta (Gregory) D. G. Mann |
| Lyrella approximatoides (Hustedt) D. G. Mann |
| Lyrella atlantica (A. Schmidt) D. G. Mann |
| Lyrella clavata var. caribaea (Cleve) Siqueiros Beltrones |
| Lyrella clavata var. elongata (H. Peragallo) Siqueiros Beltrones |
| Lyrella clavata var. indica (Greville) Moreno |
| Lyrella excavata (Greville) D. G. Mann |
| Lyrella exsul (A. Schmidt) D. G. Mann |
| Lyrella fogedii Witkowski, Lange-Bertalot et Metzeltin |
| Lyrella fundata (Hustedt) Siqueiros Beltrones |
| Lyrella granulata (Grunow) E. Nevrova, A. Witkowski, M. Kulikovskiy et Lange-Bertalot |
| Lyrella hennedyi var. crassa (Peragallo) Siqueiros Beltrones |
| Lyrella hennedyi var. furcata (Peragallo et Peragallo) Siqueiros Beltrones |
| Lyrella impercepta (Hustedt) J. L. Moreno |
| Lyrella implana (Hustedt) J. L. Moreno |
| Lyrella irrorata (Greville) D. G. Mann |
| Lyrella lyra (Ehrenberg) Karayeva |
| Lyrella lyra var. constricta (Peragallo) Siqueiros Beltrones |
| Lyrella lyra var. subtypica (Hustedt) Siqueiros Beltrones |
| Lyrella spectabilis (Gregory) D. G. Mann |
| Lyrella sp. 2 cf. spectabilis (Gregory) D. G. Mann |
| Petroneis Stickle et D. G. Mann |
| Petroneis granulata (J. W. Bailey) D. G. Mann (Fig. 8E) |
| Order: Mastogloiales D. G. Mann |
| Family: Mastogloiaceae Mereschkowsky |
| Mastogloia G. H. K. Thwaites ex W. Smith |
| Mastogloia binotata (Grunow) Cleve |
| Mastogloia crucicula (Grunow) Cleve v. crucicula |
| Mastogloia pusilla Grunow (Fig. 5J) |
| Mastogloia gieskesii Cholnoky NR (Fig. 5I) |
| Mastogloia sp. |
| Order: Naviculales Bessey |
| Family: Amphipleuraceae Grunow |
| Frustulia Rabenhorst |
| Frustulia sp. 1 |
| Halamphora (Cleve) Levkov |
| Halamphora subangularis (Hustedt) Levkov |
| Halamphora terroris (Ehrenberg) P. Wang |
| Halamphora turgida (Gregory) Levkov (Fig. 6O) |
| Halamphora wisei (M. M. Salah) I. Álvarez-Blanco et S. Blanco |
| Parlibellus Cox |
| Parlibellus sp. 1 |
| Parlibellus sp. 2 |
| Family: Diadesmidaceae D. G. Mann |
| Caloneis Cleve |
| Caloneis cf. consimilis (A. Schmidt) Cleve NR (Fig. 5A) |
| Caloneis liber (W. Smith) Cleve |
| Caloneis liber var. linearis Cleve (Fig. 5T) |
| Caloneis westii (W. Smith) Hendey (Fig. 5X) |
| Family: Cosmioneidaceae D. G. Mann |
| Cosmioneis D. G. Mann et Stickle |
| Cosmioneis sp. 1 NR (Fig. 9S, T) |
| Cosmioneis sp. 2 NR (Fig. 8F) Family: Scoliotropidaceae Mereschkowsky Biremis D. G. Mann et E. J. COX Biremis cf. ridicula (M. H. Giffen) D. G. Mann (Fig. 5F) |
| Fogedia Witkowski, Lange-Bertalot, Metzeltin et Bafana |
| Fogedia finmarchica (Cleve and Grunow) A. Witkowski, Metzeltin et Lange-Bertalot (Fig. 7P) |
| Fogedia geisslerae A. Witkowski, Metzeltin et Lange-Bertalot (Fig. 7R) |
| Diploneis Ehrenberg |
| Diploneis crabro (Ehrenberg) Ehrenberg (Fig. 7A–C) |
| Diploneis litoralis (Donkin) Cleve (Fig. 7F) |
| Diploneis litoralis var. clathrata (Østrup) Cleve (Fig. 7K) |
| Diploneis notabilis (Greville) Cleve (Fig. 7U, V) |
| Diploneis obliqua (J.-J. Brun) Hustedt (Fig. 7M, N) |
| Diploneis papula (A. W. F. Schmidt) Cleve (Fig. 7H, I, X) Diploneis papula var. constricta Hiustedt (Fig. 7J) |
| Diploneis smithii (Brébisson) Cleve (Fig. 7G) |
| Diploneis splendida (W. Gregory) Cleve (Fig. 7D) |
| Diploneis suborbicularis (W. Gregory) Cleve (Fig. 7L) |
| Family: Naviculaceae Kütz. |
| Navicula Bory |
| Navicula bipustulata A. Mann |
| Navicula borneoensis Hustedt NR (Fig. 8R) |
| Navicula cancellata Donkin (Fig. 8P) |
| Navicula carinifera Grunow (Fig. 8L) |
| Navicula cf. arenaria var. rostellata Lange-Bertalot |
| Navicula cf. bipustulata A. Mann (Fig. 8U) |
| Navicula cf. parva (Ehrenberg) Ralfs |
| Navicula cf. diserta Hustedt |
| Navicula digitoradiata (W. Gregory) Ralfs (Fig. 8J) |
| Navicula directa (W. Smith) Ralfs (Fig. 8W) |
| Navicula distans (W. Smith) Ralfs (Fig. 8V) |
| Navicula diversistriata Hustedt (Fig. 5C) |
| Navicula longa (W. Gregory) Ralfs (Fig. 8X) |
| Navicula parva (Ehrenberg) Ralfs |
| Navicula pennata A. Schmidt |
| Navicula rolandii W. Wunsam, A. Witkowski et Lange-Bertalot (Fig. 8K) |
| Navicula sp. 1 (Fig. 8S) |
| Navicula torifera Hustedt NR (Fig. 8Q) |
| Trachyneis Cleve |
| Trachyneis aspera (Ehrenberg) Cleve (Fig. 8B) |
| Trachyneis velata A. Schmidt (Fig. 8C) |
| Family: Pinnulariaceae D. G. Mann |
| Craspedopleura M. Poulin |
| Craspedopleura cf. kryophila (Cleve) M. Poulin NR (Fig. 9Q) Craspedopleura sp. NR (Fig. 9R) |
| Oestrupia Heiden |
| Oestrupia powelli (Lewis) Heiden |
| Pinnularia Ehrenb. |
| Pinnularia rectangulata (W. Gregory) Rabenhorst (Fig. 5G, H) |
| Pinnularia cf. cruciformis (Donkin) Cleve |
| Pinnularia cf. trevelyana (Donkin) Rabenhorst |
| Family: Pleurosigmataceae Mereschowsky |
| Gyrosigma Hassall |
| Gyrosigma simile (Grunow) Boyer |
| Pleurosigma W. Smith |
| Pleurosigma angulatum var. genuinum (Queckett) W. Smith |
| Pleurosigma inflatum Shadbolt Pleurosigma naviculaceum Brébisson (Fig. 8D) |
| Family: Scolioneidaceae D. G. Mann |
| Scolioneis D. G. Mann |
| Scolioneis brunkseiensis (Hendey) D. G. Mann |
| Family: Scoliotropidaceae Mereschkowsky |
| Progonoia H.-J. Schrader |
| Progonoia musca (Gregory) Schrader |
| Family: Sellaphoraceae Mereschkowsky |
| Fallacia Stickle et D. G. Mann |
| Fallacia cf. tenera (Hustedt) D. G. Mann (Fig. 7Z) |
| Fallacia forcipata (Greville) Stickle et D. G. Mann (Fig. 7S) |
| Fallacia hummii (Hustedt) D. G. Mann (Fig. 5M) |
| Fallacia inscriptura (Hendey) Witkowski, Lange-Bertalot et Metzeltin (Fig. 7T) |
| Fallacia litoricola (Hustedt) D. G. Mann |
| Fallacia nummularia (Greville) D. G. Mann (Fig. 7O) |
| Fallacia nyella (Hustedt) D.G. Mann |
| Fallacia oculiformis (Hustedt) D. G. Mann (Fig. 7Y) |
| Fallacia sp. 1 (Fig. 5K) |
| Fallacia pseudoforcipata (Hustedt) D. G. Mann |
| Fallacia schoemaniana (Foged) Witkowski (Fig. 7W) |
| Fallacia subforcipata (Hustedt) D. G. Mann (Fig. 7Q, ZA) |
| Fallacia versicolor (Grunow) D. G. Mann |
| Fallacia vittata (Cleve) D. G. Mann (Fig. 5N) |
| Stauroneis Ehrenberg |
| Stauroneis tackei (Hustedt) Krammer et Lange-Bertalot |
| Order: Rhopalodiales D. G. Mann |
| Family: Rhopalodiaceae (Karsten) Topachevs’kyj et Oksiyuk |
| Rhopalodia pacifica Krammer |
| Order: Surirellales D. G. Mann |
| Family: Surirellaceae Kützing |
| Psammodictyon D. G. Mann |
| Psammodictyon panduriforme var. abruptum (Peragallo) D. G. Mann |
| Psammodictyon panduriforme var. latum (Wittrock) D. G. Mann (Fig. 8N) |
| Psammodictyon roridum (M. H. Giffen) D. G. Mann (Fig. 8M, O) |
| Psammodictyon sp. 1 |
| Surirella Turpin |
| Surirella fastuosa Ehrenberg (Fig. 8A) |
| Surirella fastuosa var. recedens (A. Schmidt) Cleve |
| Surirella sp. NR (Fig. 9N, O) |
| Order: Thalassiophysales D. G. Mann |
| Family: Catenulaceae Mereschkowsky |
| Amphora Ehrenberg |
| Amphora amoena Hustedt (Fig. 6M) |
| Amphora arenaria Donkin (Fig. 6I) |
| Amphora arenicola Grunow (Fig. 6F) |
| Amphora beaufortiana Hustedt |
| Amphora biggiba Grunow (Fig. 6S) Amphora binodis v. bigibba Grunow (Fig. 6V) |
| Amphora contracta Grunow |
| Amphora crassa W. Gregory (Fig. 6X) |
| Amphora crassa W. Gregory var. (¿?) (Fig. 6K) |
| Amphora delicatissima Krasske (Fig. 6T) |
| Amphora elegantula Hustedt (Fig. 6P) |
| Amphora exilitata Giffen (Fig. 6N) |
| Amphora maletracta var. constricta (H. Heiden) Simonsen (Fig. 6R) |
| Amphora marina W. Smith (Fig. 6L, W) |
| Amphora ostrearia Brébisson ex Kützing (Fig. 6Q) |
| Amphora pediculus (Kützing) Grunow ex A. Schmidt |
| Amphora proteus W. Gregory (Fig. 6B, E, U) |
| Amphora proteus var. contigua Cleve (Fig. 6C) |
| Amphora proteus var. kariana Grunow (Fig. 6A, D) |
| Amphora sp. 1 (Fig. 6G) |
| Amphora sp. 2 |
| Amphora spectabilis Gregory (Fig. 6J) |
| Catenula Mereschkowsky |
| Catenula adhaerens (Mereschkowsky) Mereschkowsky |
| Class: Bacillariophyta incertae sedis |
| Order: Bacillariophyta incertae sedis |
| Family: Bacillariophyta incertae sedis |
| Neodetonia |
| Neodetonia superba (C. Janisch) S. Blanco NR |
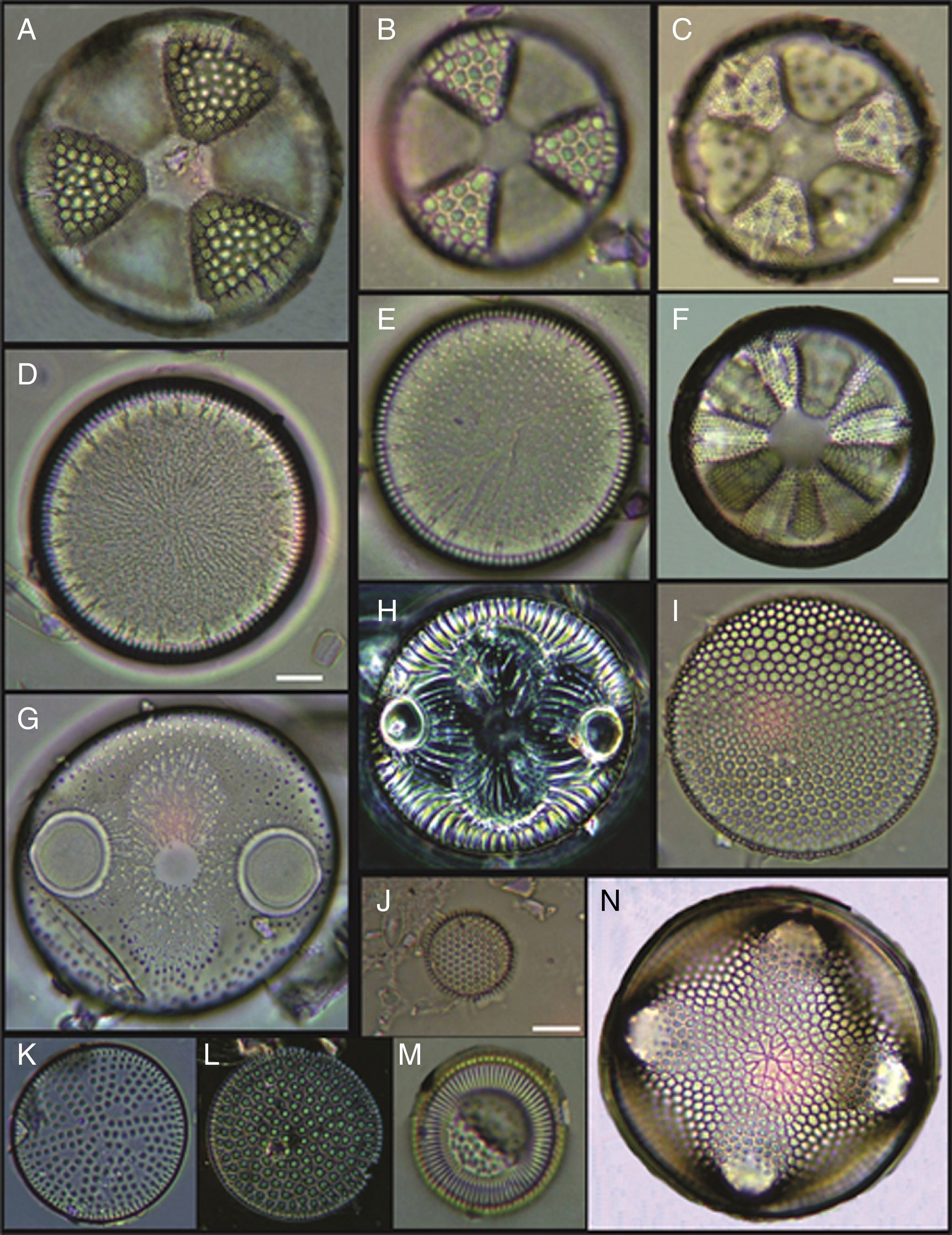
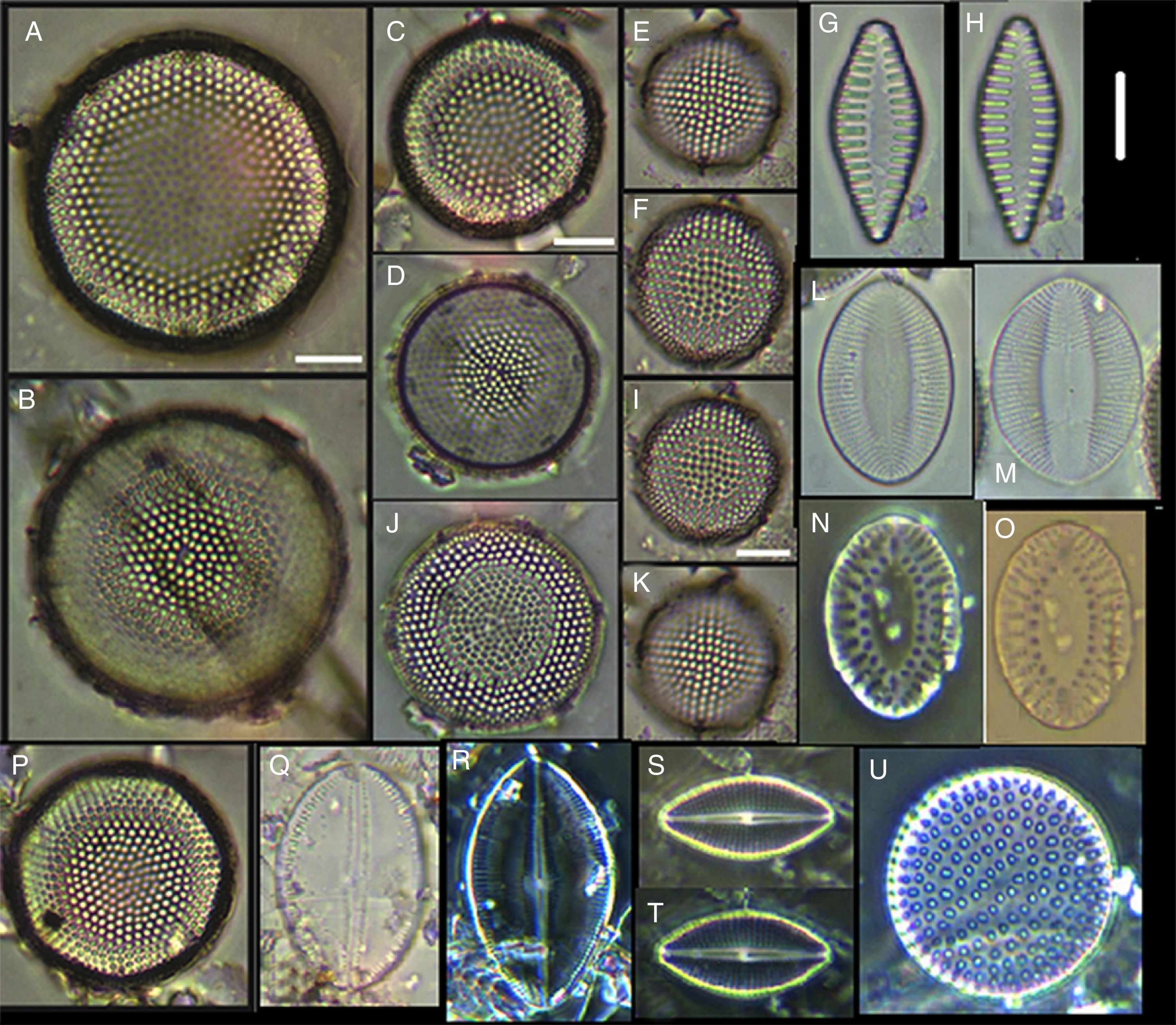
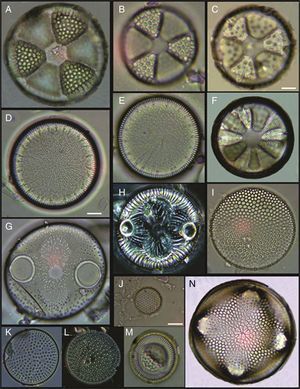
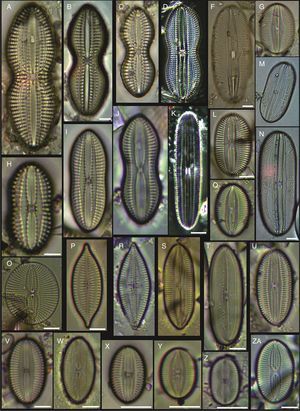
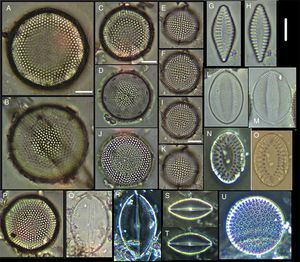
(A) Actinoptychus aster, (B) Actinoptychus senarius, (C) Actinoptychus oppenoorthi, (D, E) Ehrenbergiulva granulosa, (F) Actinoptychus adriaticus, (G) Auliscus caelatus var. strigillata, (H) Auliscus punctatus, (I) Coscinodiscus radiatus, (J) Shionodiscus oestrupii, (K) Psammodiscus nitidus, (L) Psammodiscus calceatus, (M) Cyclotella litoralis, (N) Aulacodiscus ehrenbergii. Bars=10μm.
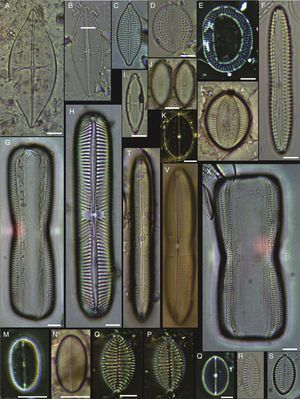
(A, E, F, J) Dimeregramma minor var. minor, (B, D) Delphineis fasciola var. australis, (C, G) Delphineis surirella, (H) Opephora pacifica, (I) Opephora schwartzii, (K, P, Q) Eunotogramma laevis, (L) Plagiogramma wallichianum, (M) Opephora marina, (N) Dimeregramma sp., (O) Neohuttonia reichardtii, (R) Eunotogramma marinum, (S) Diplomenora cocconeiformis. Bars=10μm.
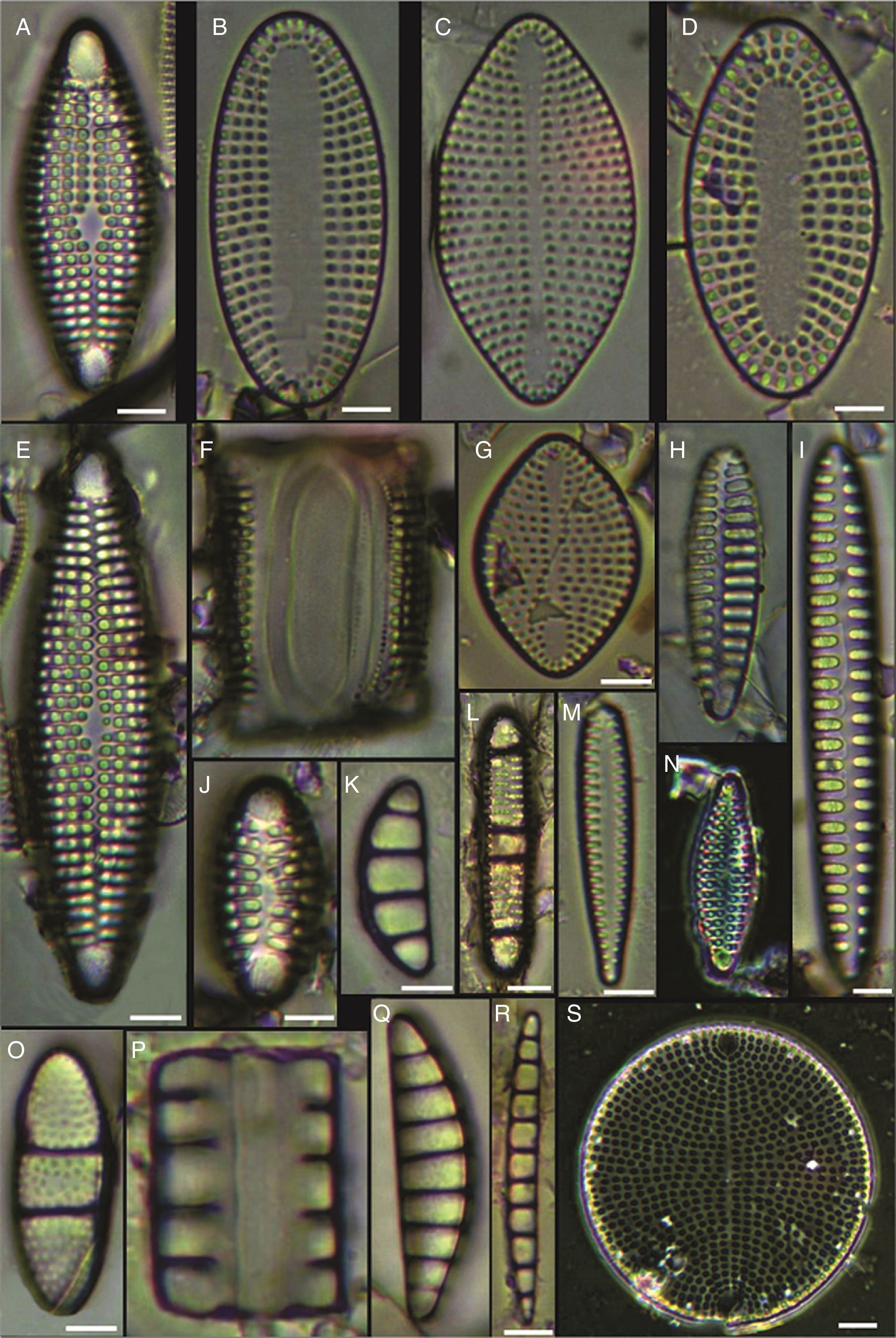
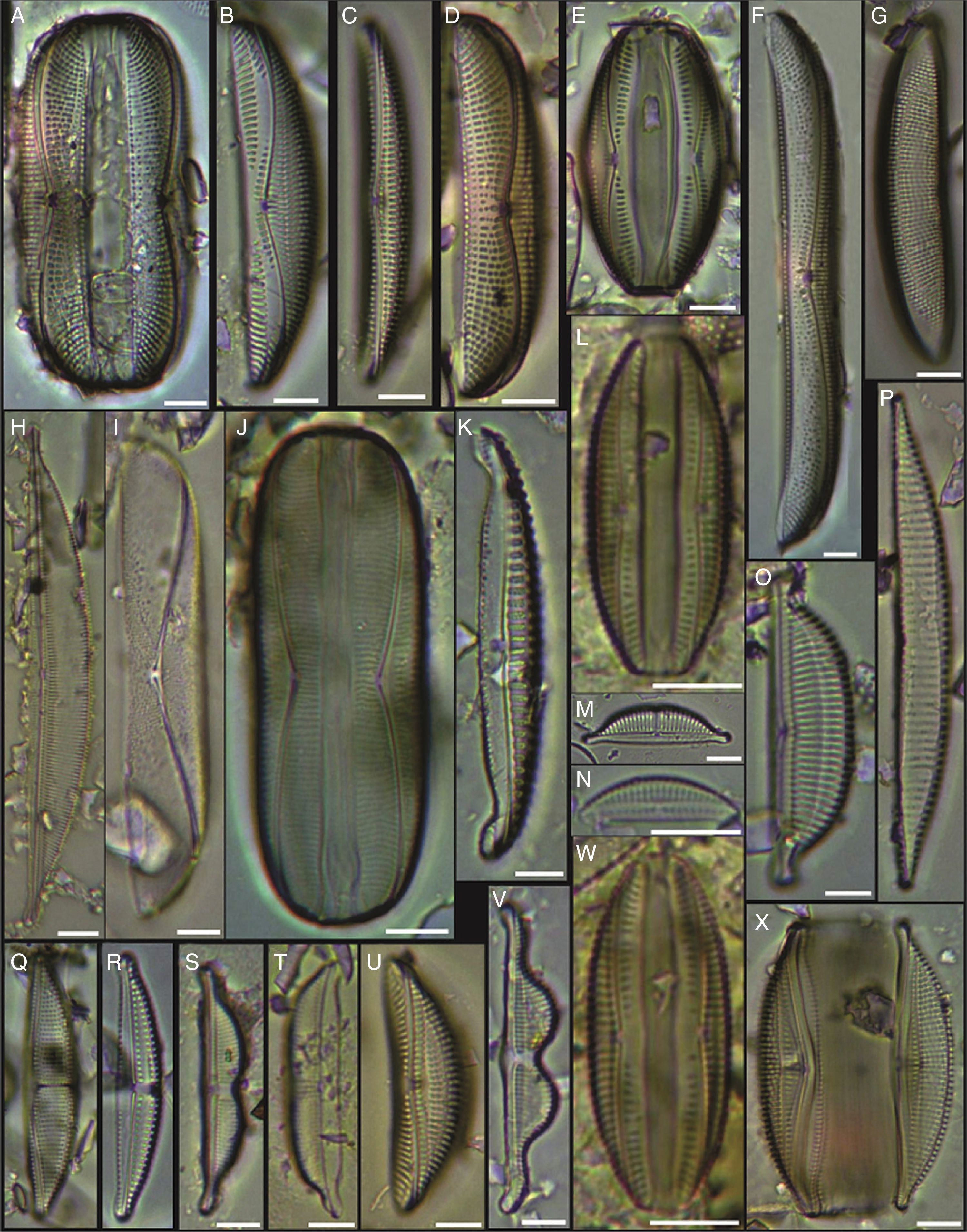
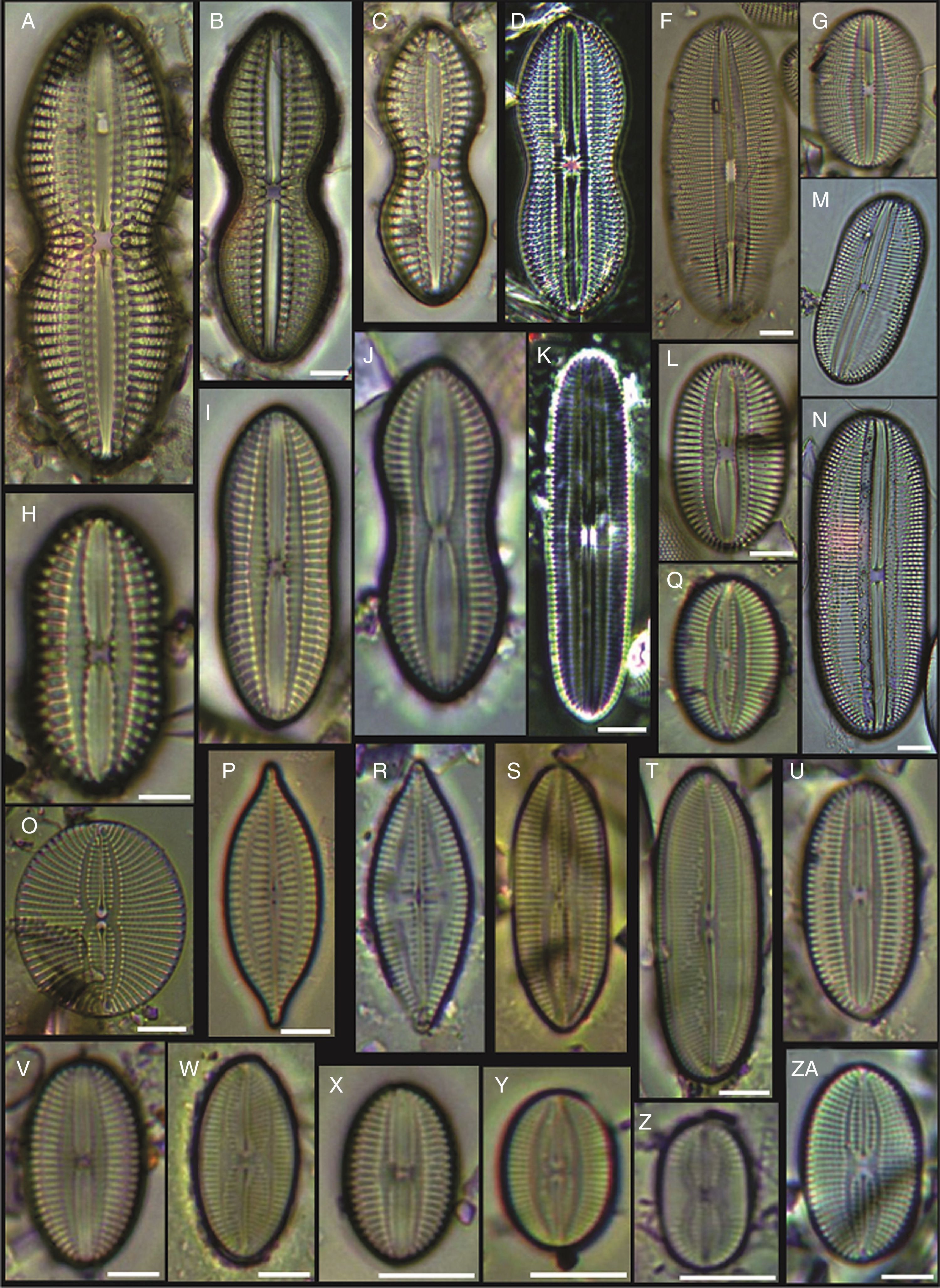
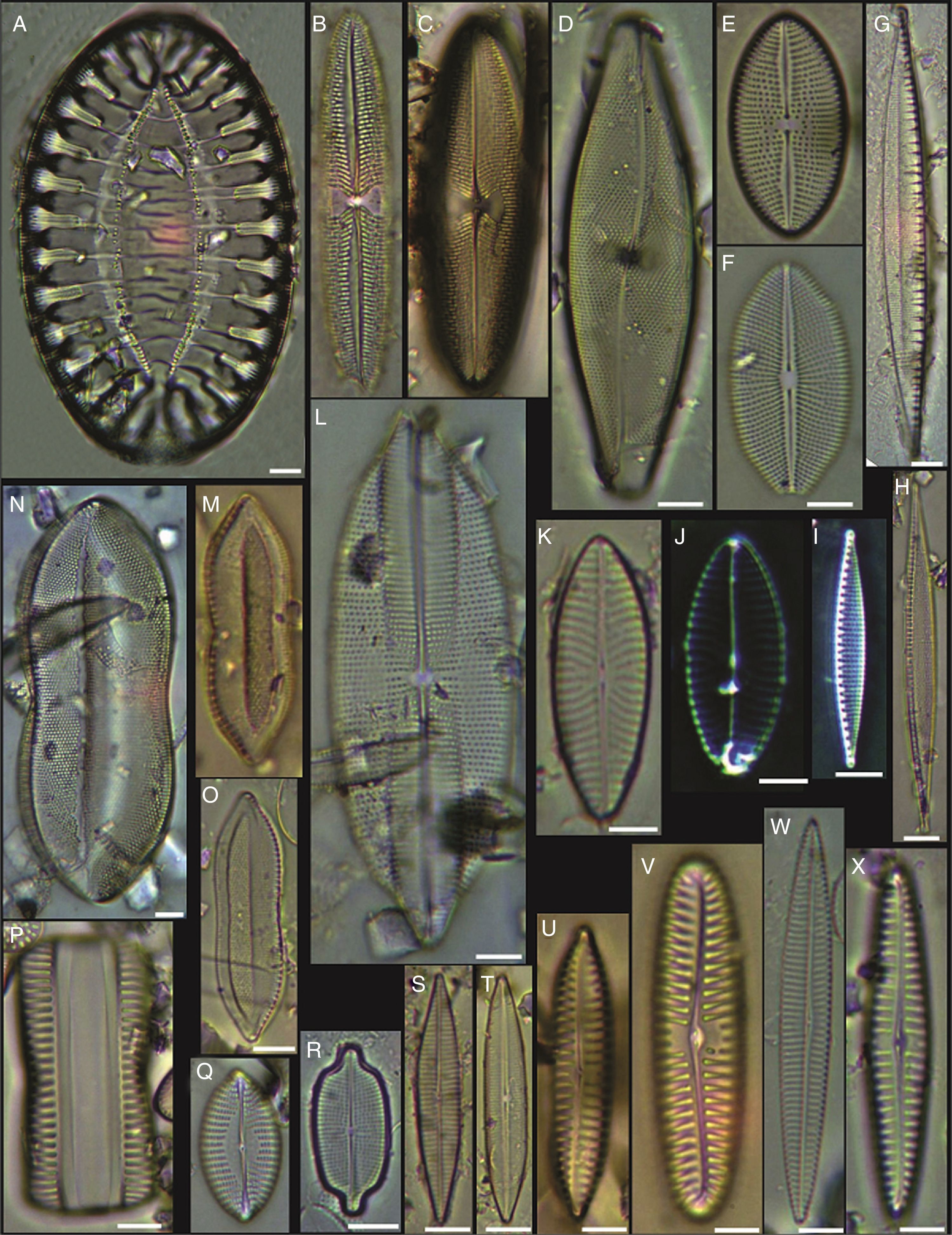
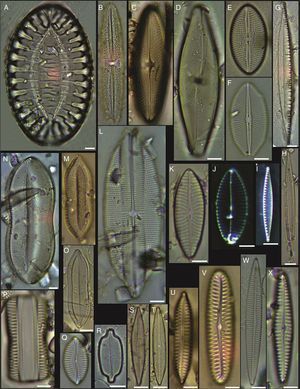
(A) Achnanthes danica, (B) Achnanthes fimbriata, (C) Navicula diversistriata, (D) Anorthoneis eccentrica, (E) Anorthoneis hyalina, (F) Biremis cf. ridicula, (G, H) Pinnularia rectangulata, (I) Mastogloia gieskesii, (J) Mastogloia pusilla, (K) Fallacia sp., (L) Cocconeis latecostata, (M) Fallacia hummii, (N) Fallacia vittata, (O, P) Cocconeis californica var. kerguelensis, (Q) Cocconeiopsis patrickae, (R) Planothidium polaris, (S) Cocconeis distans, (T) Caloneis liber var. linearis, (X) Caloneis westii, (V) Caloneis cf. consimilis. Bars=10μm.
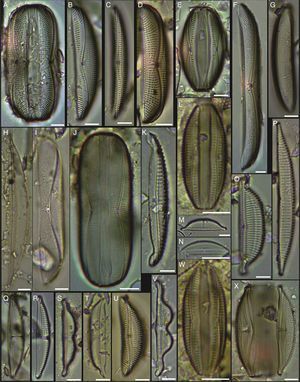
(A, D) Amphora proteus var. kariana, (B, E, U) Amphora proteus, (C) Amphora proteus var. contigua, (F) Amphora arenicola, (G) Amphora sp. 1, (H) Halamphora terroris, (I) Amphora arenaria, (J) Amphora spectabilis, (K) Amphora crassa var.? (L, W) Amphora marina, (M) Amphora amoena, (N) Amphora exilitata, (O) Halamphora turgida, (P) Amphora elegantula, (Q) Amphora ostrearia, (R) Amphora maletracta var. constricta, (S) Amphora bigibba, (T) Amphora delicatissima, (V) Amphora binodis v. bigibba, (X) Amphora crassa. Bars=10μm.
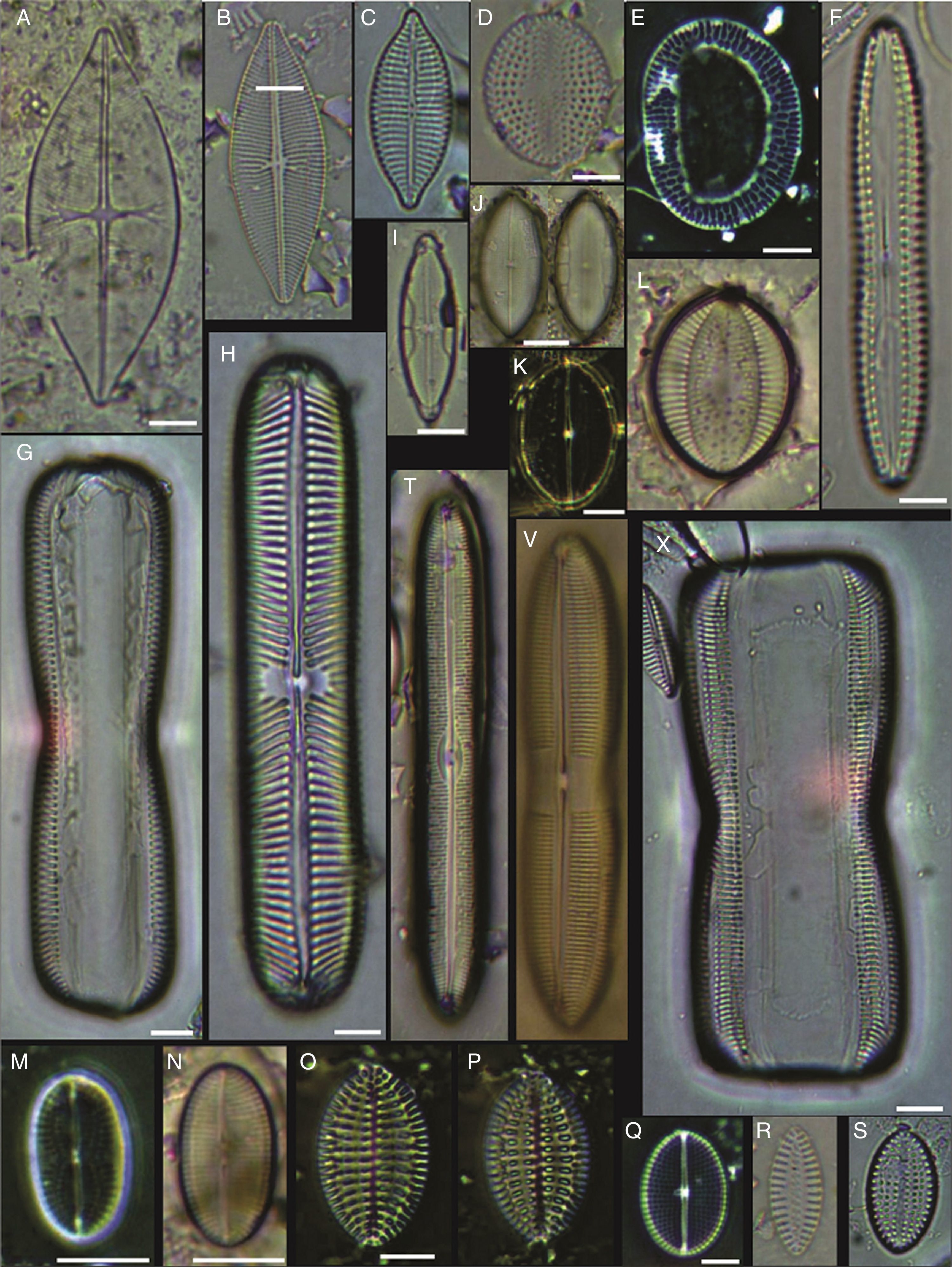
(A–C) Diploneis crabro, (D) Diploneis splendida, (F) Diploneis litoralis, (G) Diploneis smithii, (H, I, X) Diploneis papula, (J) Diploneis papula var. constricta, (K) Diploneis litoralis var. clathrata, (L) Diploneis suborbicularis, (M, N) Diploneis obliqua, (O) Fallacia nummularia, (P) Fogedia finmarchica, (Q, ZA) Fallacia subforcipata, (R) Fogedia cf. geissleriana, (S) Fallacia forcipata, (T) Fallacia inscriptura, (U, V) Diploneis notabilis, (W) Fallacia shoemaniana, (Y) Fallacia oculiformis, (Z) Fallacia cf. tenera. Bars=10μm.
(A) Surirella fastuosa, (B) Trachyneis aspera, (C) Trachyneis velata, (D) Pleurosigma naviculaceum, (E) Petroneis granulata, (F) Cosmioneis sp. 2, (G) Nitzschia fluminensis, (H) Nitzschia sigma, (I) Nitzschia grossestriata, (J) Navicula digitoradiata, (K) Navicula rolandii, (L) Navicula carinifera, (M, O) Psammodictyon roridum, (N) Psammodyction panduriformis var. latum, (P) Navicula cancellata, (Q) Navicula torifera, (R) Navicula borneoensis, (S) Navicula sp. 1, (T) Parlibellus sp. 1, (U) Navicula cf. bipustulata, (V) Navicula distans, (W) Navicula directa, (X) Navicula longa. Bars=10μm.
The species list includes 14 new records (NR) for the Mexican Pacific (Table 1). The genus Lyrella I. Karayeva stands out with the highest number of species and infra-specific taxa (21) and 6 NR, which were reviewed separately. Likewise, a high number of species of Amphora Ehrenberg (25, including 4 Halamphora (Cleve) Levkov) was recorded, and although no NR of this genus occurred, there were 2 unidentified taxa. Also, Navicula Bory included 16 species and 2 NR; 14 species of Fallacia Stickle et Mann, a genus that comprises mainly epipelic forms. The genus Cocconeis Ehrenberg was represented by 14 species (1 NR); these are mainly epilithic and epiphytic forms. The above contrasts with the few (6) species of Nitzschia Hassall (1 NR) and 5 Mastogloia Thwaites (1 NR). There is also included 1 new record of Craspedopleura M. Poulin and 2 of Cosmioneis Mann et Stickle.
DiscussionThe above results back up the proposed hypothesis that: a) the epipelic diatoms from the intertidal of the Guerrero Negro Lagoon constitute assemblages with a high species diversity, and b) the occurrence of 193 diatom taxa previously recorded hitherto in the region, i.e., 83%, reflects the wide biogeographical spectrum recorded in the neighboring subtropical transition zone to the south and the east coast of the Baja California Peninsula. Many of these taxa have been recorded previously as tychoplankton, either in the Gulf of California (Moreno-Ruíz et al., 1996) e.gr., species of Auliscus Ehrenberg and Psammodiscus Round et Mann or, as most of the taxa in our list, in benthic substrata from mangrove systems on both coasts of Baja California Sur (Hernández-Almeida & Siqueiros-Beltrones, 2012; López-Fuerte & Siqueiros-Beltrones, 2006; López-Fuerte et al., 2010; Siqueiros-Beltrones & Morzaria-Luna, 1999; Siqueiros-Beltrones & Sánchez-Castrejón, 1999; Siqueiros-Beltrones et al., 2014; Siqueiros-Beltrones, 2006).
In the most recent study on diatoms from sediments in the region (Siqueiros-Beltrones et al., 2014) a typical assemblage of diatoms was described with a species richness of 182 taxa, where only 13 were centrics (7%). However, few species of Lyrella (4) were observed. In contrast, in mangrove sediments from Magdalena Bay, out of 327 diatom taxa recorded, 15.5% (50) were centrics, and 15 of Lyrella (López-Fuerte, 2004). A suggestive similarity stands out, inasmuch in our species list, besides the 43 centrics, 21 species and infra-specific-taxa of Lyrella were identified, including 8 new records for the Mexican coasts and 11 for the NW of México (Siqueiros-Beltrones et al., in press).
The relatively high number of centric forms in the LGN, combined with the many representatives of Lyrella, could be reflecting particular conditions that distinguish it from the other environments in the west coast of BCS. The LGN is located farther north than the accepted latitudinal distribution for mangrove forests along the west coast of the Baja California Peninsula (González-Zamorano, Nava-Sánchez, León-de la Luz, & Díaz-Castro, 2011). However, it has been considered within a subtropical region due to the influence of a tropical water mass (Hernández-Rivas, Jiménez-Rosenberg, Funes-Rodríguez, & Saldierna-Martínez, 2000). However, the occurrence and effect of the California current and local upwelling events, typical of this area should be acknowledged. The low temperature of the water that these currents provide related to a certain type of characteristic biota – recorded by Eberhard (1966)–, is also found in San Quintín Bay and in the Punta Banda estuary farther north in Baja California. This defines a transitional region that has been observed in the phytoplankton assemblages of the Magdalena Bay lagoon complex (Gárate-Lizárraga & Siqueiros Beltrones, 1998). Furthermore, López-Fuerte et al. (2015) recently recorded a particular benthic diatom flora in the coast of Guadalupe Island located farther north, off the coast of Baja California, for which no particular biogeographical affinity could be determined. There, many tropical forms were observed, v.gr. Mastogloia spp., including recent records from the Mexican Caribbean (López-Fuerte, Siqueiros-Beltrones, & Hernández-Almeida, 2013).
According to the above, the recorded species of the epipelic diatom assemblage in the LGN reflect the transitional biogeographical nature of the region. Likewise, the high species richness of benthic forms in the lagoon is evidenced, considering that only 1 type of substratum was analyzed. Moreover, the 24 still unidentified species also show that much exploration is required for this region on benthic diatoms
In view of the potential regarding this floristic reference for further ecological and biogeographical studies which are necessary for managing protected areas, the scenario calls for estimating ecological parameters of the benthic diatom assemblages comprising other substrates and seasons.
This study was partially financed by Project SIP-20150537 of the IPN where DASB is Comisión de Operación y Fomento de Actividades Académicas (COFAA) and Estímulos al Desempeño de los Investigadores (EDI) fellow. FOLF currently holds a postdoctoral research grant from the Conacyt. The reviews by 2 anonymous referees helped to improve this manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.