Aiming to determine an appropriate protocol for short-term storage of the sperm of San Pedro Mártir trout, Oncorhynchus mykiss nelsoni, wild fish previously matured in captivity were anesthetized and the semen, obtained by abdominal pressure, was suspended in Erdhal and Graham extender at 1:3, 1:6 and 1:9 dilution ratios and kept at 4° C for 9 days with an undiluted sample as control. The percentages of sperm motility and membrane integrity were assessed daily. The results indicated that sperm suspended at a dilution of 1:9 can be stored at 4° C with ≥ 80% motility and ≥ 65% membrane integrity for 7 and 6 days respectively. In addition, the results show that membrane integrity decreased more rapidly than motility, which suggests that it may be used as an early indicator of sperm damage during short-term cold storage. The results obtained are important to support future conservation programs of this trout.
Con el objetivo de determinar un protocolo apropiado para el almacenamiento a corto plazo de los espermatozoides de trucha de San Pedro Mártir, Oncorhynchus mykiss nelsoni, se anestesiaron peces silvestres previamente madurados en cautiverio y el esperma, obtenido por presión abdominal, fue suspendido en una solución extender de Erdhal y Graham a tasas de dilución de 1:3, 1:6 y 1:9 y mantenido a 4° C durante 9 días con una muestra control sin diluir. Los porcentajes de movilidad e integridad de membrana fueron evaluados diariamente. Los resultados indicaron que los espermatozoides suspendidos en una dilución de 1:9 se pueden almacenar a 4° C con movilidad ≥ 80% e integridad de membrana ≥ 65% por 7 y 6 días respectivamente. Además, los resultados muestran que la movilidad disminuye más lentamente que la integridad de la membrana, lo que sugiere que ésta puede utilizarse como un indicador temprano del daño al esperma durante el almacenamiento a corto plazo en bajas temperaturas. Los resultados obtenidos son importantes para apoyar los programas de conservación de esta trucha.
The anthropogenic pressures on marine and terrestrial ecosystems, accompanied by depletion of natural stocks have aggravated the degradation of some native and threatened fish species. The strategies for the conservation of their genetic resources should include the application of reproductive strategies, since it would allow continuous fry production throughout the year. However, continuous breeding may be impossible due to lack of spermiating males or asynchronous maturation of male and female broodstock (Bozkurt and Seçer, 2005; Mañanós et al., 2009). Thus, appropriate short-term storage techniques to maintain viable sperm for hours or days, with no alteration in fertilizing ability, may be of great importance to enhance fish production (Scott and Baynes, 1980; Hatipoðlu and Akçay, 2010).
Short-term storage has been considered a useful strategy for preservation of threatened or endangered fish species (Basavaraja and Hegde, 2005; Maria et al., 2006a; Hatipoðlu and Akçay, 2010). Therefore, it may be a worthy strategy for the conservation of the endemic San Pedro Mártir trout (SPMT), Oncorhynchus mykiss nelsoni, which inhabits the streams of the western slopes of Sierra San Pedro Mártir (Ruiz-Campos and Pister, 1995) and is considered the southernmost coastal rainbow trout in North America (Behnke, 2002). It is resistant to high temperatures during summer and has a non-migratory nature, which are bioecological attributes different from those of other rainbow trout (Ruiz-Campos, 1993).
Due to its restricted distribution and low abundance, SPMT is subject to special protection (Jelks et al., 2008; Semarnat, 2010). Therefore, conservation programs are required but efforts to maintain it in captivity for conservation and restocking have been difficult, mainly due to the difficulty of synchronizing male maturity and female spawning under controlled conditions (Ruiz-Campos, 1993; Aguilar-Juárez, 2010).
Short-term storage of SPMT sperm until eggs become available for reproduction in captivity may enhance programs for species conservation. This would also allow transportation of sperm to hatcheries with fertile female broodstock, or when difficulties arise in obtaining synchronization of male-female maturity. In addition, due to the high genetic diversity of this species (Camarena-Rosales et al., 2008), short-term sperm conservation could solve the problems of inbreeding of other native species currently held in captivity (De los Santos-Camarillo, 2008).
In this paper we describe a protocol for short-term storage of SPMT sperm at low temperatures (4° C), and assess the effect of this procedure on sperm motility and membrane integrity.
Materials and methodsCapture and maintenance of fishIn March 2007, 11 wild SPMT males were captured and maintained at the Banco Periférico de Germoplasma del Noroeste of the Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE, BPGN). The organisms were kept under artificial photoperiod during 7.5 months in a 250-L fiberglass tank connected to a recirculating system until maturation (Aguilar-Juárez et al., 2011).
Fish were fed initially with a daily ration of frozen mysids (Ocean Aquatics, Kelowna, British Columbia) equivalent to 3% of their wet weight, which was replaced gradually with commercial food (42% protein and 15% lipid, 3.5 mm, floating, El Pedregal Silver Cup, Toluca, Mexico). When fish were fully acclimated, the total feed ration was equivalent to 5% of their wet weight supplied in 2 equal parts at 08:00 and 18:00 hours.
Sperm collectionEight of the 11 males matured. These were starved for 2 d to avoid feces production during milt collection and anesthetized in a water bath with 100 μL/L of a clove oil/ethanol (1:9 v/v) mixture during 10-15 min (Keene and Noakes, 1998), after which the genital opening of each male was cleaned, dried with paper towels and the semen was obtained by applying gentle abdominal pressure. The first sample was discarded to avoid contamination with urine and feces, and the clean milt samples were collected in 1.8-mL plastic pipettes, placed on crushed ice and transported immediately (~2 min) to the BPGN.
Evaluation of motilityImmediately after collection, triplicate 0.5 μL subsamples of each sample were placed on a clean microscope slide and mixed with 20 μL of activation medium DIA 532 prepared with NaCl 0.009 M, glycine 0.05 M and 7-9 Sigma tris 0.02 M and 87.40mOsmol/kg (Herráez et al., 2009). The percentage of rapid, vigorous, and forward-moving spermatozoa was evaluated at 400X using dark-field microscopy (Nikon H600L, Eclipse 80i). All samples had ≥ 80% motility, and were pooled and used for the experiment.
Evaluation of sperm concentrationAfter determination of volume (µL), the sperm concentration of each sample fixed with a 1% lugol solution was evaluated on triplicate subsamples under a microscope at 400X (Nikon H600L, Eclipse 80i, Nikon Corporation, Tokio, Japan) with a hematocytometer (0.1 mm, Brightline, Hausser Scientific, Horsham, Pennsylvania, USA).
Dilution and short-term storageIncreasing dilutions (1:3, 1:6, or 1:9) of the pooled sperm suspended in Erdhal and Graham’s (EG) antibiotic-free extender solution (CaCl2•2H2O 0.001 M, MgCl2•6H2O 0.001 M, Na2HPO4 0.002 M, KCl 0.034 M, citric acid 0.001 M, glucose 0.055 M, 10mL of a 0.002 M KOH solution, 20mL of a bicine 0.006 M solution, 323mOsmol/kg and pH 7.4) were prepared for short-term storage. All chemicals were analytical grade (Sigma Chemical Co., St. Louis Missouri, USA).
Triplicate sperm samples (100 µL) were mixed with 300, 600 and 900 µL of EG solution (dilution 1:3, 1:6 and 1:9 respectively), placed in 0.6-mL (control sample and 1:3 dilution) or 1.5-mL (1:6 and 1:9 dilutions) conical Eppendorf tubes hermetically sealed to avoid evaporation, and kept in a conventional refrigerator at 4° C for 9 days, during which the percentages of sperm motility and membrane integrity were assessed daily. Three undiluted sperm samples (250 µL in 0.6-mL tubes) were used as control.
Motility was estimated as described previously, using 0.5 µL aliquots of all samples. Membrane integrity was evaluated with the live/dead sperm viability kit (L-7011 Molecular Probes, Inc., Eugene, Oregon, USA), according to the Molecular Probes (2005) protocol. For this, 10 µL aliquots of each sperm dilution were mixed with 0.05 µL of SYBR-14 (20nM final concentration) and incubated in the dark at ~19 C for 10 min. After addition of 0.50 µL propidium iodide (PI, 12 µM final concentration), samples were incubated for an additional 10 minutes. Membrane integrity was evaluated in triplicate. A total of 10 fields (100 cells per field) were observed under a fluorescence microscope at 200 or 400X, using a blue (490 nm) excitation filter. Green and red fluorescence were noted as indicative of viable or damaged cell membranes, respectively.
Statistical analysisMean motilities and membrane integrities of the treatments, after each day of storage, were compared using 2-way ANOVA tests after arcsine square-root transformation. Specific differences were identified with the Student-Newman-Keuls (SNK) tests. All statistical analyses were performed with α=0.05.
ResultsMilt volumes ranged from 90 to 355 µL, and sperm concentrations and motilities varied from 0.8 to 1.2 × 109 cells/mL and from 80 to 95% respectively. Since the milt volumes were insufficient to perform experiments with the individual samples, the sperm of the 8 fishes was pooled giving a total volume of 1,805 µL, a sperm concentration of 1 x 109 cells/mL and average motility ~87%.
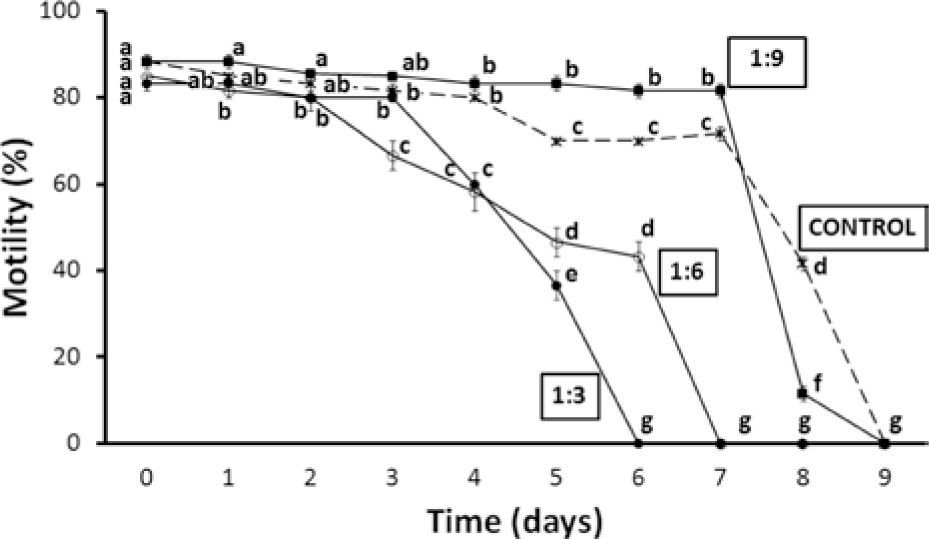
After dilution, the best results were achieved with sperm suspended in the 1:9 dilution. Sperm was maintained for 7 days with mean motilities > 80%, while after day 4 the controls were already at ≥ 70%. Motility of ≥ 80% lasted only for the first 3 days in the case of the other treatments (Fig. 1).
Percentage of motility (mean ± standard error) of pooled sperm samples of O. mykiss nelsoni stored at 4° C for 9 days in different dilutions. Equal or common letters: lack of significant differences, which are indicated by different letters (2-way ANOVA and Student-Newman-Keuls tests, α=0.05). a> b> c> d> e> f> g.
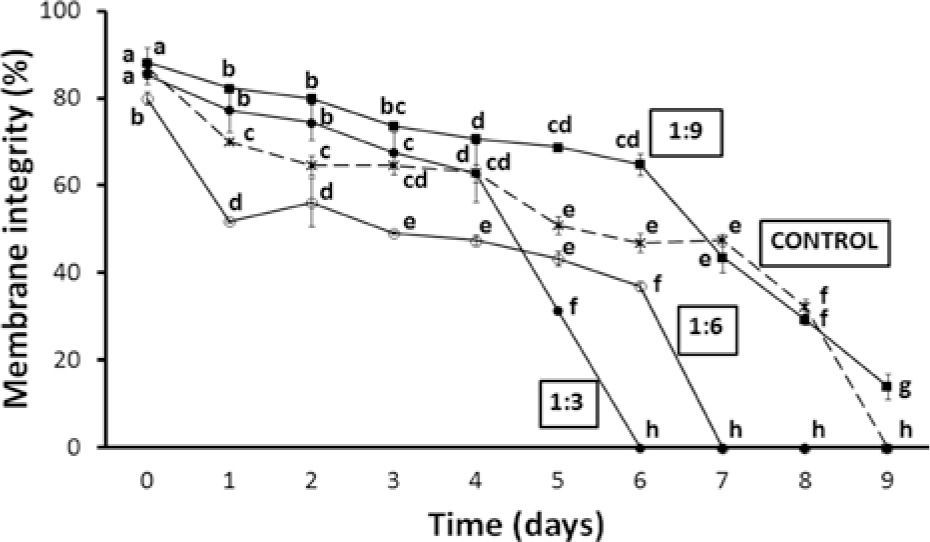
Membrane integrity showed a similar trend. The best result was found in sperm suspended in 1:9 dilution, in which > 65% mean membrane integrity lasted 6 days while that of the control samples was < 50%. Motility and membrane integrity decreased more rapidly in treatments 1:3 and 1:6 (0% membrane integrity after 6 and 7 days, respectively) than in the control and 1:9 dilution (Fig. 2). Statistical analysis showed that significant differences (p< 0.001 in all cases) were related to time of storage, dilution ratio, as well as to their interaction.
Percentage of membrane integrity (mean ± standard error) of pooled sperm samples of O. mykiss nelsoni stored at 4° C for 9 days in different dilutions. Equal or common letters: lack of significant differences, which are indicated by different letters (2-way ANOVA and Student-Newman-Keuls tests, α=0.05). a> b> c> d> e> f> g.
Given the need to pool sperm samples because of the small volumes of individual production, our data may be taken as representative of the entire lot of male spawners rather than of the individual sperm response to short term storage, which for this reason has been advocated as an effective strategy to reduce variability in sperm preservation experiments (Ciereszko et al., 2011), although according to Babiak and Dabrowski (2003) individual variability is low in the case of rainbow trout sperm.
The percentage of motility obtained after 7 days of storage with the 1:9 dilution was higher than that obtained for other fish species such as common carp, Cyprinus carpio; abant trout, Salmo trutta abanticus; streaked prochilod, Prochilodus lineatus; grass carp, Ctenopharyngodon idella, and the major carp, Labeo calbasu (Marques and Pereira-Godinho, 2004; Bozkurt and Seçer, 2005; Bozkurt et al., 2009; Hatipoðlu and Akçay, 2010; Hassan et al., 2012).
It has been suggested that, at least for some fish species, sperm viability may improve during short-term storage in an oxygen-rich atmosphere (Christensen and Tiersch, 1996; Benic et al., 2000; Jenkins-Keeran et al., 2001; Marques and Pereira-Godinho, 2004; Bobe and Labbe, 2009; Donaldson et al., 2011; Ciereszko et al., 2011). Although SPMT sperm showed higher motility and viability than other species after storage without oxygen, an enriched gaseous atmosphere could be tested as a possible additional treatment for the further improvement of the results obtained in this study.
Dilution is another factor that plays an important role in improving sperm quality of aquatic species (Valdebenito et al., 2009; Bobe and Labbe, 2009; Ciereszko et al., 2011; Glenn III et al; 2011) and its effect could be conducive to the success of short-term sperm storage and production of viable offspring for repopulation programs. In this study, we obtained better results with dilution ratios increasing progressively from 1:3 to 1:9. This is consistent with the results obtained with 1:10 sperm suspensions of rohi, Labeo rohita; piracanjuba, Brycon orbignyanus, and Labeo calbasu (George and Mitra, 1994; Maria et al., 2006b; Hassan et al., 2012).
Higher dilutions (1:20 or 1:50) have even been suggested for the yellow catfish Mystus nemurus and the european eel, Anguilla anguilla (Muchlisin et al., 2004; Peñaranda et al., 2010). However, dilution may have different effects on sperm quality, depending on the target species (Bobe and Labbe, 2009; Glenn III et al, 2011). For example, a 1:10 dilution did not improve motility, preservation or fertilization of several salmonid species (Erdhal et al., 1984), and a stepwise increase of the dilution ratio from 1:1 to 1:20 did not show significant differences in motility of common carp sperm C. carpio (Glenn III et al., 2011). Additionally, dilution ratios higher than 1:2 were not recommended for silver carp, Hypophthalmichthys molitrix and bighead Aristichthys nobilis (Chen et al., 1992).
The sensitivity to dilution of the sperm of some species may be due to the balance between favorable (urine dilution, sperm density reduction, pH control) and unfavorable factors (dilution of some important seminal plasma components), which control optimum conditions for storage at low temperatures (Bobe and Labbe, 2009).
The low resistance to storage obtained with the lower dilutions (1:3, 1:6) seems to indicate a deterioration of the protective effect of the seminal fluid, possibly due to the dilution of its nutrient contents (ions or proteins), which have been suggested as important elements for sperm quality (Billard, 1983; Morisawa and Morisawa, 1986; Rurangwa et al., 2004; Lahnsteiner, 2010; Cosson, 2010; Ciereszko et al., 2011). On the other hand, dilution would play a positive role in the case of the 1:9 dilution, possibly because the extracellular nutrients and the diluted sperm may have reached isosmotic conditions, or because of the increased amount of Ca2+ and Mg2+ divalent cations added with the extender solution, which are antagonic to the inhibitory effect of sperm motility of K+ cations (Baynes et al., 1981; Billard, 1987; Alavi and Cosson, 2006). Given the species-specific sperm composition and balance between the opposite effects of these cations, this might explain the wide range of dilution ratios suggested for short-term storage of the sperm of different species.
The best indicator of sperm quality is its fertilization ability (Lahnsteiner et al., 1998; Honeyfield and Krise, 2011; Migaud et al., 2013). However, other criteria that do not depend on the availability of eggs, such as motility or membrane integrity, may be used as shortcuts for quality assessment (Ogier de Baunly et al., 1997; Segovia-Quintero et al., 2000; Flajšhans et al., 2004; Rurangwa et al., 2004; Fauvel et al., 2010). Although it has been shown that it is not necessarily related to fertilizing ability (Herráez et al., 1993; Rurangwa et al., 2004; Mongkonpunya et al., 2011), motility has been the most common method used to evaluate sperm quality (Gleen III et al., 2011). In addition, Foster et al. (2011) suggested that motility and membrane integrity assess separate aspects of sperm quality. Thus, the integration of all this information may determine more accurately the performance of sperm at the moment of fertilization.
Activation of sperm motility in freshwater species such as common carp and rainbow trout depends on different mechanisms. In carp, Krasznai et al. (2000) proposed membrane hyperpolarization (K+ efflux) followed by depolarization (Ca2+ influx into the cell), while in salmonid fish 2 different regulatory mechanisms have been suggested, 1 depending on phosphorylation of the 15 kDa protein by tyrosine kinase at the basal part of the flagellum, and the second due to phosphorylation of the 22 kDa DLC by a cAMP-dependent protein kinase along the sperm flagellum (Inaba et al., 1998; Itoh et al., 2003).
However, in anadromous species such as Morone saxatilis, motility can be activated in hypo-, iso- and hypertonic solutions (He and Woods III, 2003), suggesting that in this case mechanisms different from those proposed for carp and trout could be involved in the control of sperm motility (He et al., 2004).
In this study there were no initial differences between percentages of motility and membrane integrity, but the damage of the membrane increased more rapidly than motility. This is in agreement with Membrillo-Ortega et al. (2003), who mentioned that the least sensitive part of the cell to low temperatures is the sperm tail. Thus, although further studies are needed to elucidate these mechanisms, the rapid rate of damage to membrane integrity rather than to sperm motility suggests that the factors involved in membrane integrity may be different from those causing activation of sperm motility in the case of SPMT.
A variety of techniques has been developed for salmonid sperm short-term storage (Scott and Baynes, 1980; Babiak and Dabrowski, 2003; Cabrita et al., 2009a; Tiersch and Green, 2011), and in some cases the results exceed those obtained in this study. As an example, rainbow trout sperm was stored for 14 days at 1.4° C in ziploc bags without extender solution, with a final sperm motility of ~50% (Babiak and Dabrowski, 2003). It has been suggested that the use of sperm dilution depends on the knowledge of the physiology of the species used, especially for those with external fertilization, which require sperm activation (Cabrita et al., 2009b).
Also, dilution with an extender may reduce the sperm concentration and facilitate simultaneous activation (Cabrita et al., 2009b) and, as an added advantage to the improved duration of sperm storage, diluting the samples may maximize the availability of sperm in the case of fish that produce low milt volumes (Ciereszko et al., 2011; Huiping and Tiersch, 2011), which is the case of SPMT.
The only limitation to the use extenders for sperm dilution could be their cost and availability, which on the other hand might be balanced out by improved activation or longer duration of viability. For this, the technique needs to be assessed and appropriately modified for each species. The protocol developed in this study allows maintaining SPMT sperm at low temperatures, undiluted or diluted to 1:9 in Erdhal and Graham’s extender solution, with ≥ 80% motility for 7 days and ≥ 65% membrane integrity for 6 days, a length of time which we deem sufficient to obtain eggs from other sources or for transportation to other fish farms, as well to conduct experiments for long term storage (Huiping and Tiersch, 2011; Tiersch, 2011; Hassan et al., 2012).
Although it may be possible to improve these results either using appropriate diets for an adequate protection of sperm membranes (Aguilar-Juárez, 2010), keeping the broodstock at an appropriate thermal regime (Labbé and Maisse, 1996) or enriching the storage solutions with fatty acids (Lahnsteiner et al., 2009), our results demonstrate the feasibility of short-term storage of the sperm for this species, and could be useful for further studies on cryopreservation, which are essential to support the management and reproduction programs of many species of fish.
This work was supported by Semarnat Project 2002-COI-0393, in partial fulfillment towards the Ph.D. degree of the first author at Universidad Autónoma de Baja California. C. Ochoa, A. Ramírez, A. Torrero, M. Valle, E. Lopez gave technical assistance. D. Voltolina helped with the English manuscript. The permit SGPA/DGVS 08473 for trout collection was granted by Dirección General de Vida Silvestre, México.