A new species of stygobitic isopod from Cerro Colorado Cave, Municipality of Apazapan, near Xalapa, Veracruz, is described. The new species is placed in Mexistenasellus due to the lack of eyes and pigmentation, a head distinct from pereonite 1, and 2 well developed pleonites. Other characters, such as the number of setae in the dactyli of the pereiopods, show slight variations from the original description of the genus. The new species can be distinguished from the previously known 7 species in the genus by the shape of the head, the proportions of the pleotelson and length of uropods. Color in life is pink as is characteristic of the species of Mexistenasellus. A key to the species of Mexistenasellus is provided.
Se describe una especie nueva de isópodo estigobítico de la cueva de Cerro Colorado, municipio de Apazapan, cerca de Xalapa, Veracruz. La especie nueva se ubica en Mexistenasellus debido a que no presenta ojos ni pigmentación, una cabeza distinta del pereonito 1 y 2 pleonitos bien desarrollados. Otros caracteres, como el número de setas en los dactilos de los pereiópodos, muestran una leve variación con respecto a la descripción original del género. La especie nueva se puede distinguir de las otras 7 conocidas del género por la forma de la cabeza, las proporciones del pleotelson y el largo de los urópodos. El color en vivo es rosa, como es característico de las especies de Mexistenasellus. Se presenta una clave para las especies de Mexistenasellus.
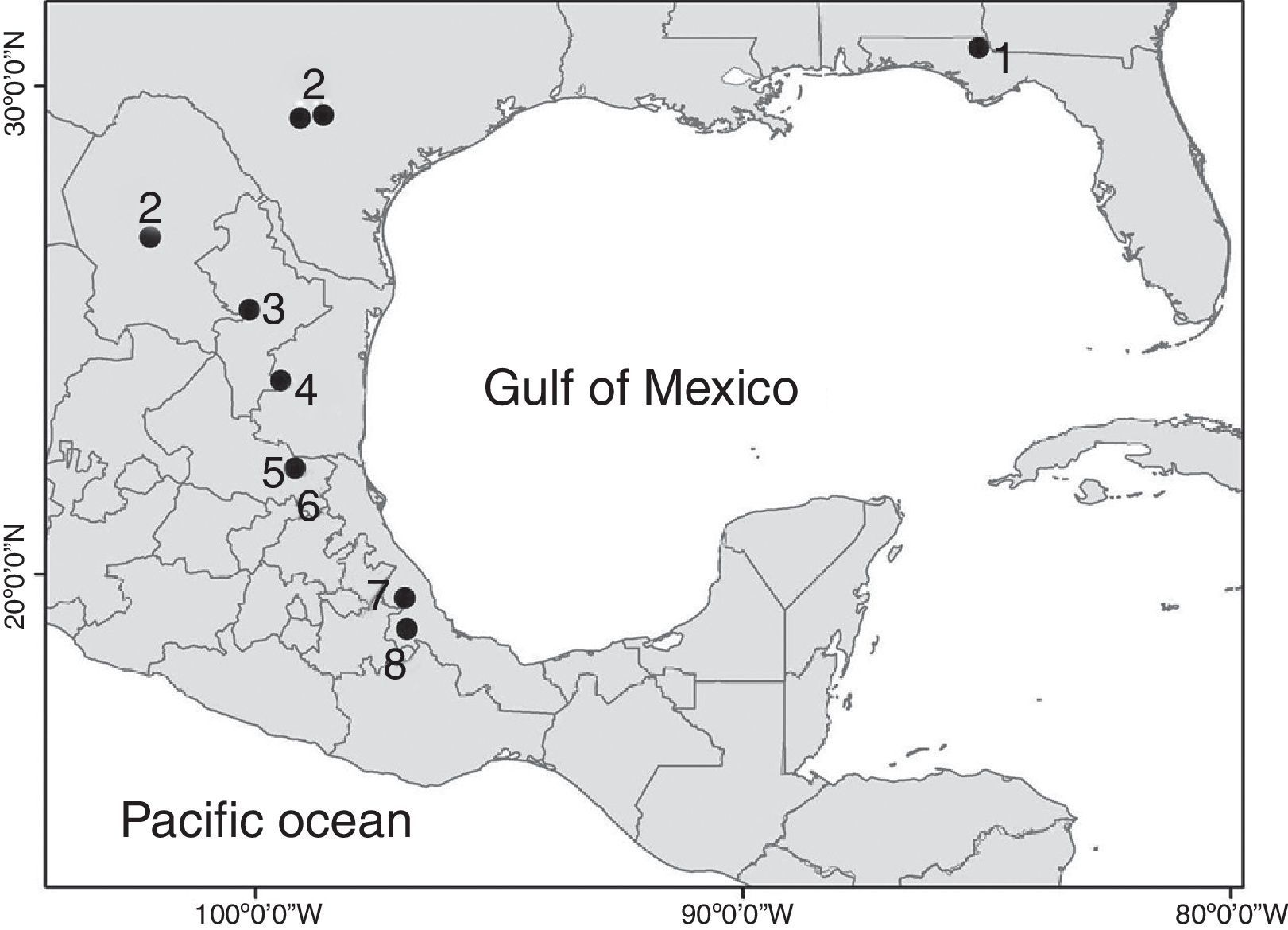
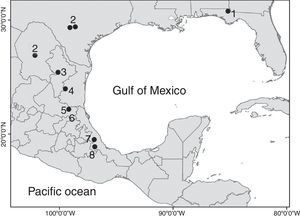
The genus MexistenasellusCole & Minckley, 1972 includes 7 species distributed from Jackson County, Florida, to the west to Bexar and Medina Counties, in southern Texas, USA, through the Mexican States of Coahuila, Tamaulipas, Nuevo León, San Luis Potosí to southern Veracruz (Bowman, 1992; Lewis & Sawicki, 2016; Rocha-Ramírez, Álvarez, Alcocer, Chávez-López, & Escobar-Briones, 2009). All the species in the genus are adapted to cave life. Five of them were collected in caves (M. parzefalliMagniez, 1972; M. wilkensiMagniez, 1972; M. coleiBowman, 1982; M. nulemexBowman, 1982; M. floridensisLewis & Sawicki, 2016), 1 from a well (M. magnieziArgano, 1973) and 1 from the discharge area of a thermal spring (M. coahuilaCole & Minckley, 1972). The new species described herein was also collected in a cave.
The distribution range of Mexistenasellus has its northern limit in Jackson County, Florida, and then follows a north–south axis along the Sierra Madre Oriental from around San Antonio, Texas, USA, south to Córdoba, Veracruz, Mexico (Fig. 1). According to Magniez (1974) and Bowman (1982) such pattern may be the result of the invasion of karst areas along this axis by an ancestral stock that was widely distributed in the shallow embayments that existed at the end of the Cretaceous. However, the distribution of stenasellids in North America, Europe, Eastern Africa and Asia points to a very old origin (Magniez, 1981), suggesting that the group invaded freshwater underground habitats prior to the breakup of Pangaea (Morvan et al., 2013). This new interpretation poses interesting biogeographic questions and a different route of colonization to what Bowman (1982) proposed. In this paper we describe a new species of Mexistenasellus from a locality in Veracruz that is within the range of the genus.
Materials and methodsThe isopods were collected by hand, placed in plastic vials and later preserved in 70% EtOH. Photographs of the complete organisms were taken in a Z16 APO-A Leica microscope. Drawings were made using an Olympus ZH-10 dissecting microscope fitted with a camera lucida and an Olympus BX50 compound microscope.
All the type specimens of the new species are deposited in the Colección Nacional de Crustáceos, Instituto de Biología, Universidad Nacional Autónoma de México, Ciudad de México, Mexico (CNCR). Total length is abbreviated as TL.
DescriptionOrder Isopoda Latreille, 1817
Suborder Asellota Latreille, 1802
Family Stenasellidae Dudich, 1924
Genus Mexistenasellus Cole & Minckley, 1972
Mexistenasellus atotonoztok sp. nov.
Type material. Holotype: male, 6.2mm TL; Mexico, Cerro Colorado Cave, Municipality of Apazapan, 30km SE from the capital city of Xalapa, Veracruz (19°21.373′N, 96°41.967′W, 531m asl); June 26th, 2014; coll. A. Guillén-Servent; CNCR 29943. Female paratype, 5.8mm TL; same locality, date and collector; CNCR 29944. Other paratypes: 8 males, 5.1–6.3mm TL, and 9 females, 5.7–6.6mm TL; same locality, date and collector; CNCR 29945.
Diagnosis. Head trapezoidal, anterior margin concave. Pereonites 5–7 with posterolateral angles acute. Pleopod II protopod rounded, longer than wide, with 4–5 setae on distal margin; exopod one-third length of protopod, with horizontal suture between articles; endopod longer than protopod and exopod combined, tapering distally, with groove on distal half where projection folds around cannula.
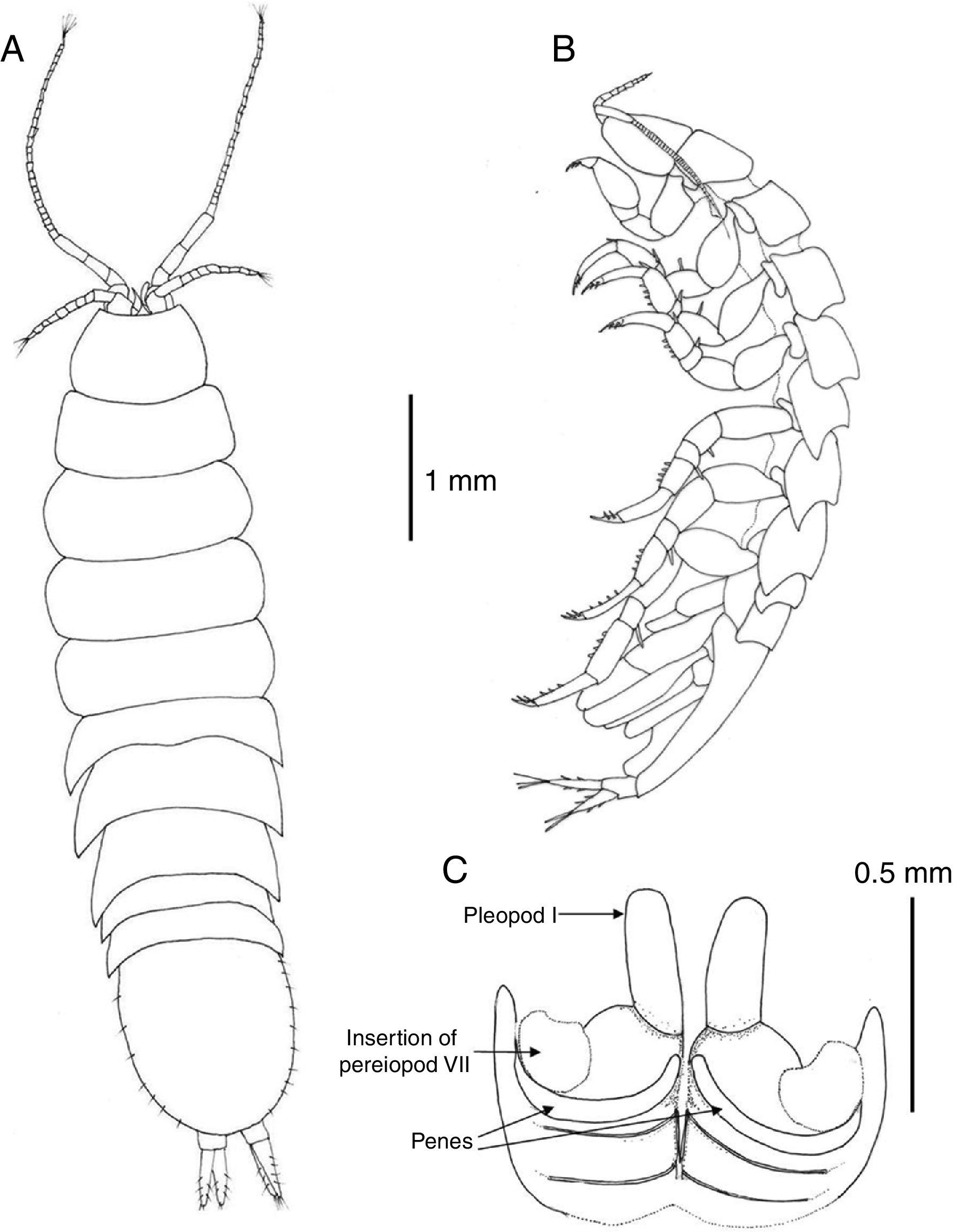
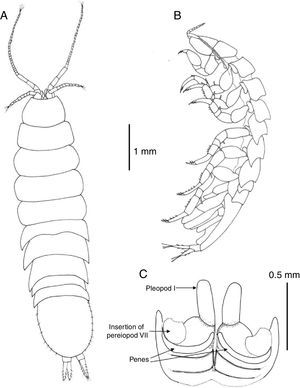
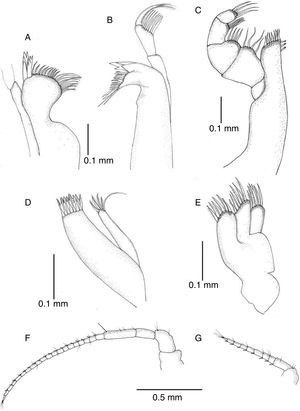
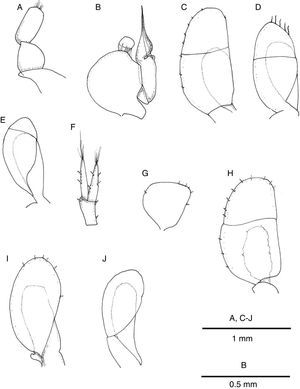
Description. Body 3.7 as long as wide, surface smooth with scattered short setae (Fig. 2A); length of male holotype 6.2mm. Cephalon 1.5 as wide as maximum length; anterior margin slightly concave, without rostrum; lateral margins anteriorly rounded, converging, posterior half subparallel; posterior margin convex (Fig. 3A). Pereonite length increasing from first to third which is longest, pereonites 4–7 shorter, subequal in length; pereonites 3–4 widest. Pereonites 1–4 with lateral margins rounded, pereonites 5–7 with posterolateral margins projected posteriorly, acute (Fig. 2B). Coxae of pereonites visible in lateral view. Pleotelson 1.12 as long as broad, oval-shaped, with scattered marginal setae (Fig. 3A).
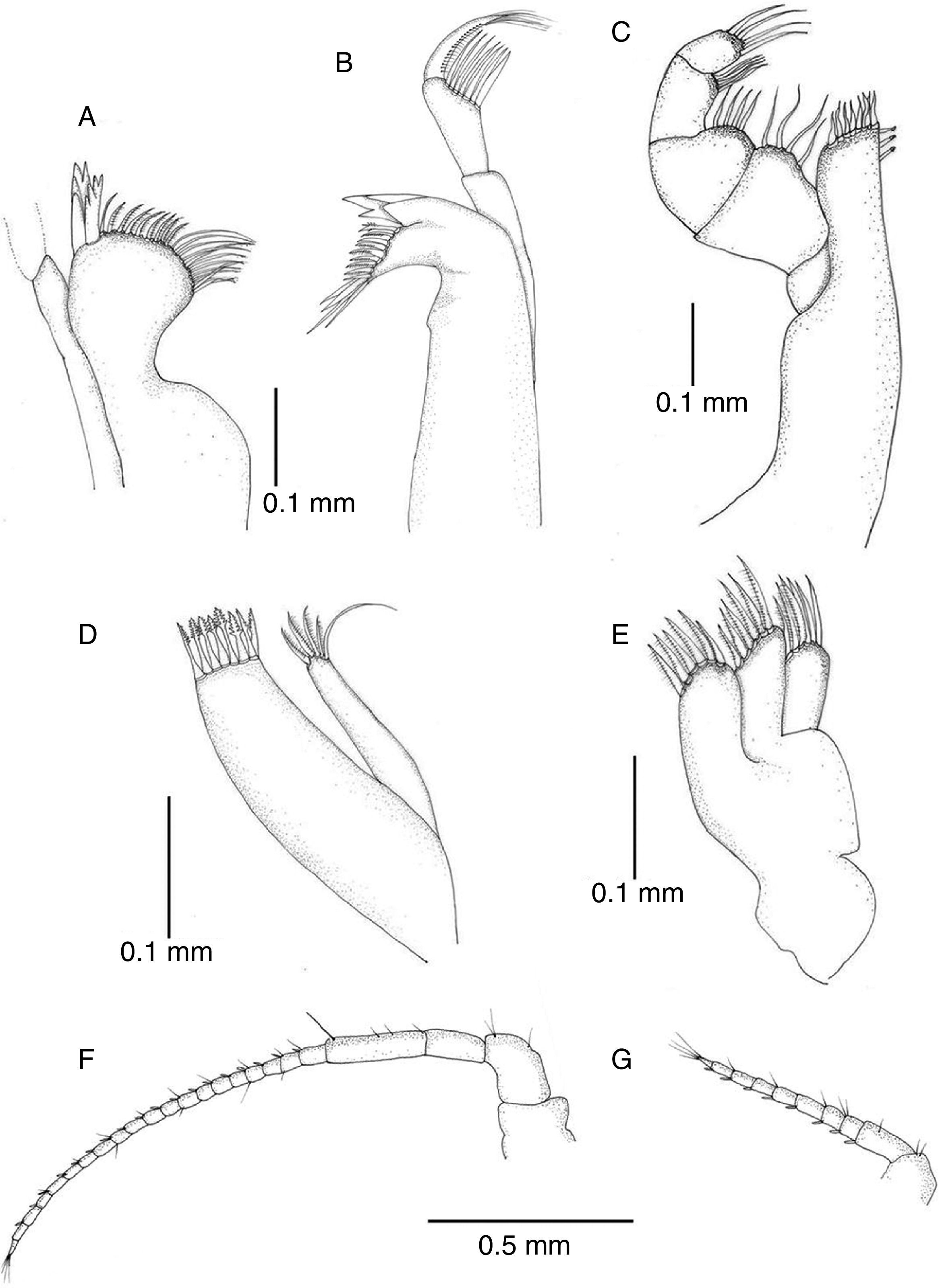
Antennula reaching posterior margin of cephalon, flagellum with 7 articles (Figs. 3B and 4G). Antenna reaching posterior margin of pereonite 2; peduncle with fourth article longest, flagellum composed of 20 articles (Fig. 4F). Flagellum articles of both, antennula and antenna, each one with 1 aesthetasc and 1 or several setae. Left mandible with incisor process 4-cuspate, adjacent setal row with 2 serrate setae followed by 7 setae, intermediate 5 with setules, proximal tip with 6 long simple setae; articles of palp subequal in length, second one becoming wider distally, with 7 long setae, third one with row of short setae along internal margin and terminal tuft of setae (Fig. 4B). Distal portion of right mandible rounded, incisor process and lacinia 4-cuspate; palp similar to that of right mandible (Fig. 4A). Maxillula with outer ramus subrectangular with 8 spines along distal margin; inner ramus shorter, slender, with 5 spines on distal margin (Fig. 4D). Maxilla with 3 lobes, inner lobe with 7 distal setae, middle and outer ones with 6 setae (Fig. 4E). Maxilliped, endite with 7 spines along distal margin, 2–3 subapical retinacula; palp typical (Fig. 4C).
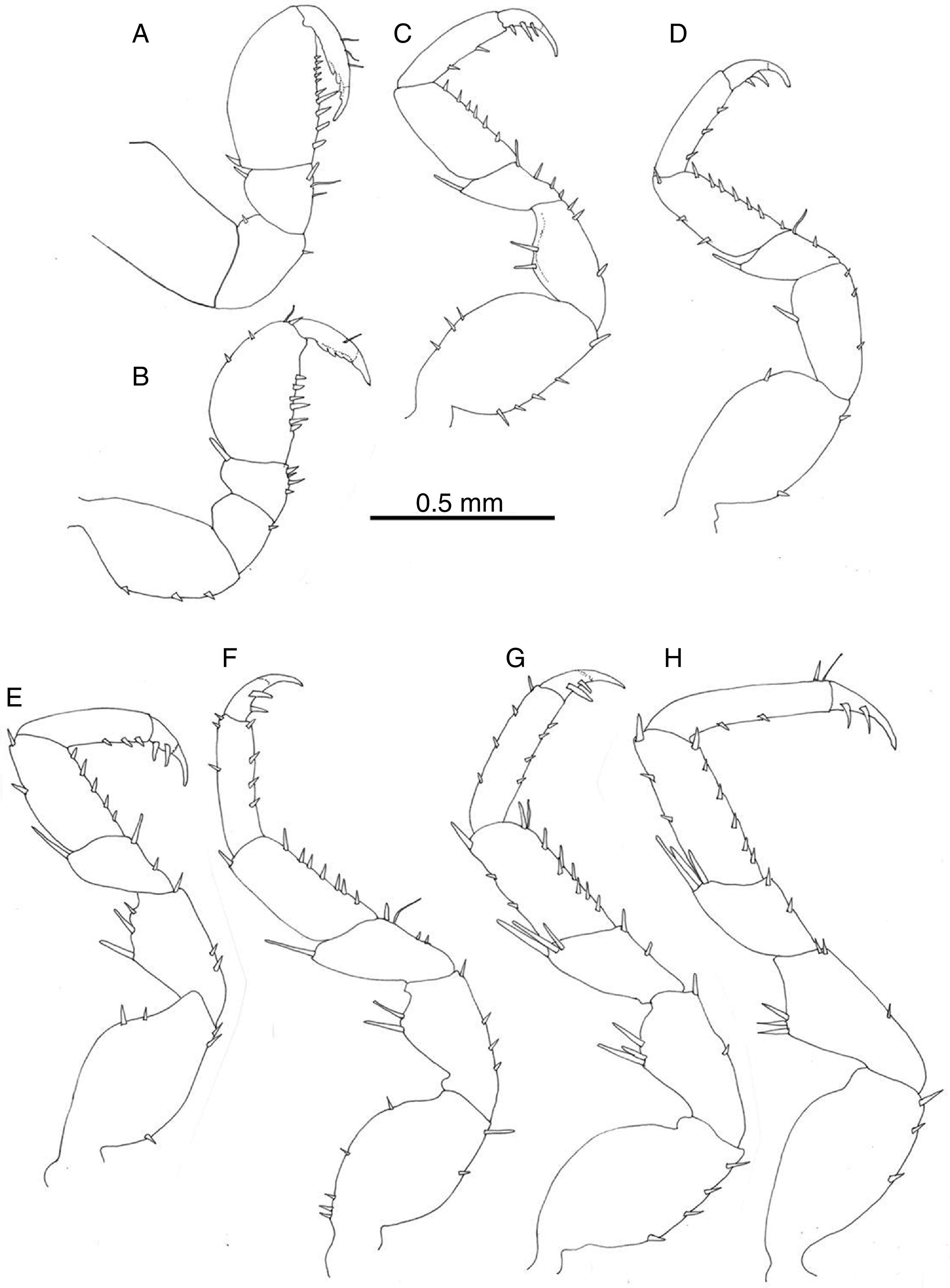
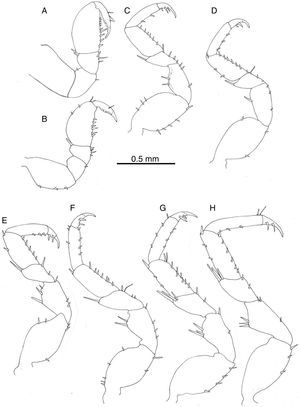
Pereiopod I of male prehensile; merus with scattered setae, propodus length 1.5 width, lateral margin rounded, palmar margin straight with row of irregularly sized setae, dactyl with terminal claw and 3 subterminal blunt spines (Fig. 5A); pereiopod I of female similar to that of male (Fig. 5B). Pereiopods II–VII ambulatory, similar in shape and size, becoming stouter and slightly longer posteriorly (Fig. 5C–H). Pereiopods II–VII ischia with 1–5 setae on lateral margin; meri and carpi with large setae on distolateral angle; propodi with short setae along palmar margin; dactyli with variable number of short setae on lateral and opposing margins, tip with claw plus 2 setae, occasionally 3.
Pereonite 7 of male with curved, elongate, simple penes, reaching midline of sternal plate, articulated next to base of pereiopod VII coxae (Fig. 3C).
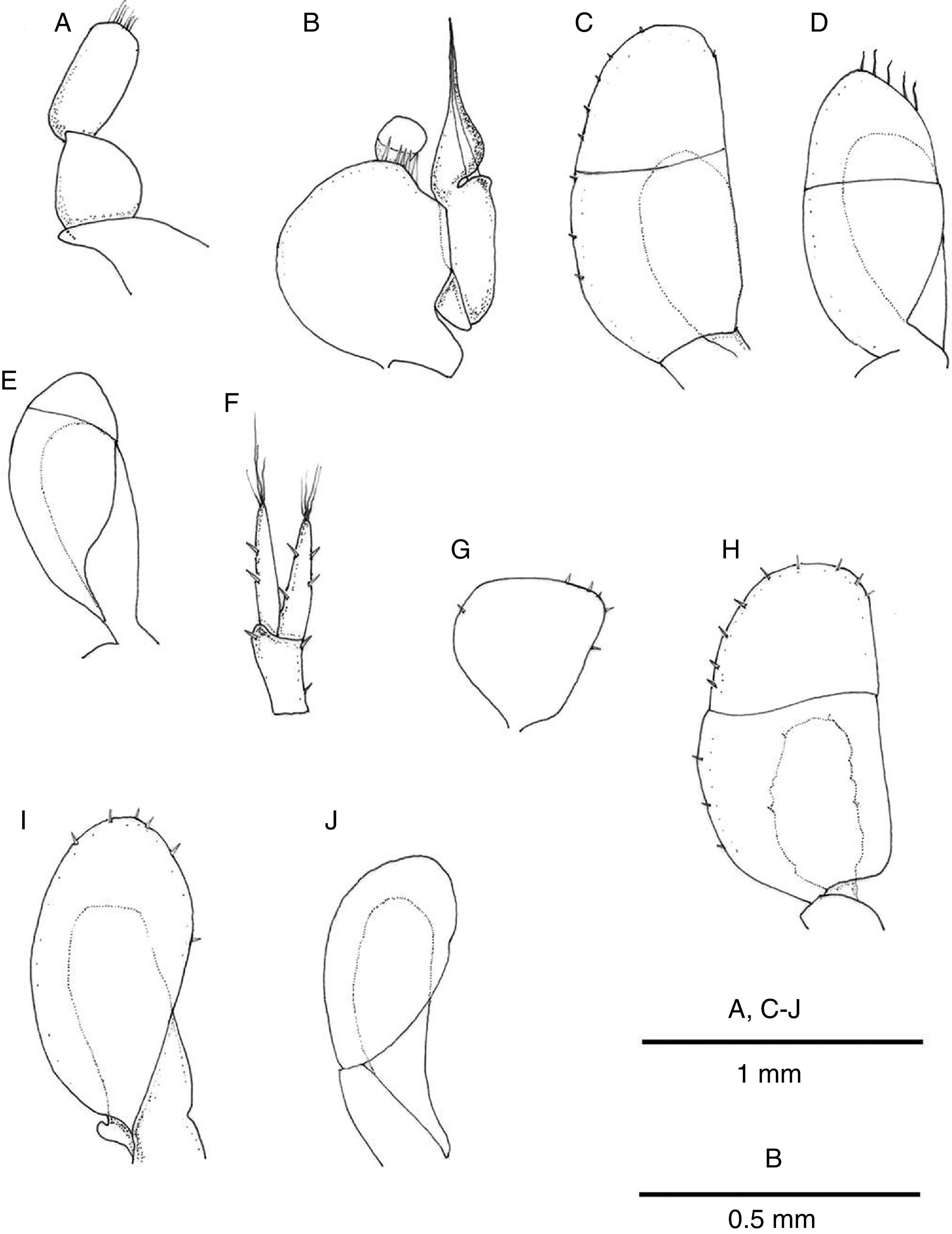
Pleopod I of male oval, smooth, with short row of marginal setae (Fig. 6A). Pleopod II of male protopod length 1.15 width, with 5 setae on distal margin; exopod semicircular with horizontal suture between articles; endopod longer than protopod and exopod combined, tapering distally, with groove on distal half where projection folds around cannula (Fig. 6B). Pleopod III exopod with regularly spaced short setae along margin, suture on distal half, endopod 0.54 length of exopod (Fig. 6C); pleopod IV exopod with 5 long distal setae, suture on distal half, endopod 0.67 length of exopod (Fig. 6D); pleopod V exopod margin devoid of setae, suture on distal third running oblique to longitudinal axis of blade, endopod 0.8 length of exopod (Fig. 6E).
Pleopod II of female pyriform, with few scattered marginal short setae (Fig. 6G). Pleopod 3 with exopod oval, transverse suture slightly sinuous on distal half, with few scattered marginal short setae; endopod about half as long as exopod (Fig. 6H). Pleopod IV exopod oval with few marginal short setae, endopod subrectangular, 0.7 as long as exopod (Fig. 6I). Pleopod V exopod oval, endopod 0.8 as long as exopod (Fig. 6J).
Uropods with protopod and rami with few scattered short spines; endopod slightly shorter than exopod, rami with distal tuft of setae (Fig. 6F). In dorsal view uropods 0.37 length of pleotelson (Fig. 3A).
Taxonomic summaryEtymology. The specific epithet “atotonoztok” is derived from the Nahuatl language words “atotonik” (hot water) and “oztotl” (cave), to construct a genus plus species name meaning Mexistenasellus from the hot water cave. The Nahuatl language was spoken by the native people that inhabited the region around the cave in prehispanic times.
Distribution. The new species is only known from the type locality in central Veracruz, Mexico. So far, only the gallery accessed through Cerro Colorado pit has been explored. However, other unexplored caves with thermal waters and high content of H2S (hydrogen sulfide), potentially connected to this, exist within 1km around the same limestone hill where the pit opens. Other sulfidic hot springs exist in the region, opening the possibility of a larger distribution of M. atotonoztok in the area.
Habitat. The cave where the isopods were found consists of a 300m long gallery, oriented in an E–W direction. It traverses Upper Cretaceous limestone and Quaternary travertine (Servicio Geológico Mexicano, 2010). The internal dimensions of the passage are very variable, with some relatively high (∼15m) and wide (∼12m) chambers and very narrow sections. It is accessed by a vertical shaft about 18m deep, that opens at the ceiling through a relatively narrow circular entrance (2m diameter) about 100m from the eastern end of the walkable passage. Several warm water springs flow from the floor inside the cave. The air inside the cave was very warm (probably over 30°C) and humid, and had a distinctive hydrogen sulfide odor, making the atmosphere suffocating. The hydrothermal sulfidic current might be related to the volcanic activity of the nearby Citlaltépetl-Cofre de Perote volcanic range, but the fermenting biotic activity over the bat guano must contribute to the high temperature (>25°C) and air composition. Toward the western end of the cave the water flows superficially draining underground between rocks at the end of the passage. After a restriction the cave continues and probably contains large chambers further in, since many bats were flying in and out through the narrow opening. Toward the eastern end of the cave a stream flows through large mounds of bat guano. The stream looks silvery, with the water crystal clear, with a bluish tinge, and the bottom white, as if the organic matter and other compounds were being degraded by substances contained in the water (Fig. 8). Near that end of the cave is a man-made reservoir resembling a water trough in which several ponds form. Sparse groups of isopods were observed in the ponds, and great numbers were present in the shallow areas near the edge of the largest pond, where density reached about 3 isopods per cm2. The water in this pond had a temperature of 33.8°C, 0.98mg/l of dissolved oxygen, conductivity of 1162μS/cm, salinity of 0.5ppm (measured “in situ” with an YSI Model 85 Handheld Dissolved Oxygen, Conductivity, Salinity and Temperature System, YSI Inc.), and pH of 8.0 (measured “in situ” with colorimetric paper, pH 2.0–9.0 colorpHast, EMD Chemicals, and with a bench pH-meter Mi150, Milwaukee Instruments, Inc., in samples brought to the laboratory). No other aquatic macroinvertebrate or fish were observed in a superficial quick survey. There were many cockroaches (Periplaneta sp.) and some beetles on the guano piles, and many small dipterans flying in the cave corridor.
RemarksThe new species described herein shows some variation relative to the original diagnosis of Mexistenasellus given by Cole and Minckley (1972). In particular, in the new species the inner plate of the maxillula can have 5 setae instead of 4, and the dactyli of pereiopods II–VII have a claw plus 2 setae and sometimes 3, instead of pereiopod I with 1 setae and pereiopods II–VII with 2 setae. Other species also show variations noticeably in the length of the uropods, which could be much longer than the pleotelson as opposed to shorter than pleotelson.
Although unpigmented, the species of Mexistenasellus and other stenasellids, have been reported to be bright red (M. coahuila) to faint red (M. magniezi) due to the color of the respiratory pigment contained in their haemolymph. However, stenasellids living in cool waters are whitish in color (Magniez, 1999), which may indicate that the reddish pigment represent an adaptation to environments with low concentrations of oxygen (Gorr, Cahn, Yamagata, & Bunn, 2004). For example, the bright red M. coahuila lives in thermal springs at 30–34°C (Cole & Minckley, 1972). Mexistenasellus atotonoztok n. sp. lives in still warm waters with abundant organic matter and an overlaying hydrogen sulfide rich atmosphere, and perhaps hypoxic, that must demand special adaptations to low oxygen concentrations. These are probably expressed in the haemolymph pigments that give them a pink color in life (Fig. 7).
The external morphology of the species of Mexistenasellus is very uniform. The main characters used to separate them are: the shape of the cephalon, which can be trapezoidal or subquadrangular, with a straight or sinuous anterior margin, and as wide as pereionite 1 or narrower; the lateral margins of pereionites, which can be rounded to subacute, setose or with scattered setae; the relative length and width of the 2 visible pleonites; the size of the pleotelson relative to total length, and its shape, that varies from subrectangular to oval, with a continuous posterior margin or 1 with 2 slight concavities; and the length of the uropods. Other characters that can separate species are the shape and size of the pleopods, and in males the form of pleopod II.
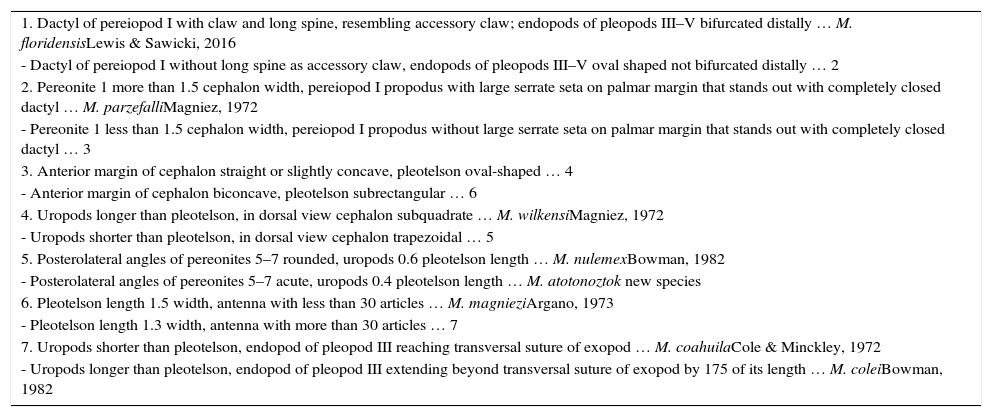
The variation found in the characters described above is, so far, enough to separate all 8 species of Mexistenasellus. In particular, the sympatric M. parzefalli and M. wilkensi can be recognized, the former, by the narrow cephalon relative to the width of pereonite 1, and the latter by a cephalon as wide as pereinoite 1 (Magniez, 1972, 1973). Mexistenasellus nulemex is a small species reaching 5.9mm in total length, has a trapezoidal cephalon, an oval-shaped pleotelson, the dorsal surfaces of the body segments appear slightly separated, and the 2 pleonites are very short and barely visible in dorsal view. This species is similar to M. parzefalli in the shape of pereiopod I, with a propodus that is proximally much wider (1.4–1.7) than carpus, with an internal angle armed with strong spines (Bowman, 1982). In M. colei the cephalon is 1.5 times as wide as long and it has the longest uropods (1.7) relative to pleotelson length. Mexistenasellus magniezi has a cephalon with a sinuous anterior margin and the most lengthened pleotelson, 1.5 as long as wide. Mexistenasellus coahuila has a cephalon with straight lateral margins and a sinuous anterior margin, whereas the uropods are much shorter than the pleotelson. In M. atotonoztok the posterolateral angles of pereionites 5–7 and pleonite 1 are acute. In M. floridensis pleonites 1–2 are very short and the endopods or pleopods II–V are bifurcated distally (Lewis & Sawicki, 2016).
The morphological differences among species cannot be associated to a latitudinal gradient. For example, while M. nulemex (Cueva La Boca, 34km SE of Monterrey, Nuevo León), M. parzefalli and M. wilkensi (Cueva del Huizache, Ciudad Valles, San Luis Potosí) have a pereiopod I with the basal portion of the propodus much wider than the carpus, the other 5 species from Florida, Texas, Coahuila and Veracruz, have a slender propodus as wide as the carpus. Mexistenasellus nulemex and M. floridensis, from Nuevo León and Florida, are similar in having very short pleonites unlike the rest of the species with longer pleonites. Instead of a gradient we find a mosaic of variation in most characters. In the case of M. atotonoztok it is similar in the external morphology to M. parzefalli in the shape of the cephalon and the pleotelson; however, the uropods of the new species are much shorter and the posterolateral angles of pereionites 5–7 are acute. Pleopods III–V of the new species are similar to those of M. coahuila and M. colei; but pleopod II is unique in that it has an endopod that is longer than the protopod and exopod combined. In summary, at this point it is not clear what the relationships among species within the genus are, and the recent discovery of M. floridensis and M. atotonoztok suggests first, that more species in the genus await to be discovered and described; and second, that the real extent of morphological variation within the genus is still undetermined.
Key to the species of Mexistenasellus.
| 1. Dactyl of pereiopod I with claw and long spine, resembling accessory claw; endopods of pleopods III–V bifurcated distally … M. floridensisLewis & Sawicki, 2016 |
| - Dactyl of pereiopod I without long spine as accessory claw, endopods of pleopods III–V oval shaped not bifurcated distally … 2 |
| 2. Pereonite 1 more than 1.5 cephalon width, pereiopod I propodus with large serrate seta on palmar margin that stands out with completely closed dactyl … M. parzefalliMagniez, 1972 |
| - Pereonite 1 less than 1.5 cephalon width, pereiopod I propodus without large serrate seta on palmar margin that stands out with completely closed dactyl … 3 |
| 3. Anterior margin of cephalon straight or slightly concave, pleotelson oval-shaped … 4 |
| - Anterior margin of cephalon biconcave, pleotelson subrectangular … 6 |
| 4. Uropods longer than pleotelson, in dorsal view cephalon subquadrate … M. wilkensiMagniez, 1972 |
| - Uropods shorter than pleotelson, in dorsal view cephalon trapezoidal … 5 |
| 5. Posterolateral angles of pereonites 5–7 rounded, uropods 0.6 pleotelson length … M. nulemexBowman, 1982 |
| - Posterolateral angles of pereonites 5–7 acute, uropods 0.4 pleotelson length … M. atotonoztok new species |
| 6. Pleotelson length 1.5 width, antenna with less than 30 articles … M. magnieziArgano, 1973 |
| - Pleotelson length 1.3 width, antenna with more than 30 articles … 7 |
| 7. Uropods shorter than pleotelson, endopod of pleopod III reaching transversal suture of exopod … M. coahuilaCole & Minckley, 1972 |
| - Uropods longer than pleotelson, endopod of pleopod III extending beyond transversal suture of exopod by 175 of its length … M. coleiBowman, 1982 |
To Susana Guzmán from IB-UNAM for the photographs used in Figure 2 and Raquel Hernández for preparing the final versions of them. We thank the Fideicomiso Público del Fondo Ambiental Veracruzano (Public Trust of the Environmental Fund of Veracruz) for providing the funds necessary for field work with the project “Conservación de las Colonias de Murciélagos de la Cueva de Cerro Colorado, Apazapan, a través del Ecoturismo Educativo”, agreement F.A.V./013/2014, managed by the Secretaría de Medio Ambiente (Environmental Agency, Sedema) of the government of the state of Veracruz. Part of the field work was funded by the Instituto de Ecología A.C. institutional project number 20035-30919 entitled “Conocimiento de la hidro-geobiología y la biota de los sistemas cavernícolas de la cuenca del río Pescados para su conservación y exploración de aplicaciones”. We thank Moisés Ruiz, Jesús Hernández and Juan Antonio Catzim for their assistance during field work, and the anthropologist Román Güemes for his advising for the correctness of the Náhuatl language based species name.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.