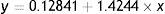Alkaloids contained in Chelidonium majus L. (C. majus) were investigated based on emulsion liquid membrane extraction followed by GC/MS. The oil-in-water emulsion was prepared using polybutadiene-styrene rubber kerosene solution as emulsifier and it is more stable than that of the oil-in-water emulsion prepared with Span 80. The key parameters affecting alkaloids membrane mass transfer rate were investigated and optimized using protopine as reference substance. The optimal emulsion liquid membrane extraction was performed through dispersing the oil-in-water emulsion in aqueous sample extracts solution at room temperature (20°C) for 4min with acetic acid as carrier, which gave much higher extraction efficiency insignificantly than conventional liquid–liquid extraction method. Complete separation of nine alkaloids was achieved within only 4min. A comparison was made of alkaloids from the same C. majus sample in two models: one was the raw and other had undergone an acid bath as the processed sample. The result showed that the alkaloid content in the raw sample is more than that of the processed sample. It means that the content of alkaloid containing in C. majus will be decrease when the raw C. majus has been processed.
Un método simple basado en emulsión por extracción de membrana líquida seguido por GC/MS fue desarrollado para la cuantificación de alcaloides en Chelidonium majus L. (C. majus). La emulsión de aceite en agua se preparó utilizando caucho de polibutadieno-estireno. La solución de queroseno como emulsionante fue más estable que la emulsión de aceite en agua preparada con Span 80. Se investigaron los parámetros clave que afectan a la velocidad de transferencia de los alcaloides a través de la membrana, y se optimizó el uso de protopina como sustancia de referencia. Se realizó la óptima emulsión por extracción de membrana líquida a través de la dispersión de la emulsión de aceite en agua en extractos de solución de muestras acuosas a temperatura ambiente (20°C) durante 4min con ácido acético como portador, lo que otorgó una mayor eficacia a la extracción que el método de extracción líquido-líquido convencional. La separación completa de 9 alcaloides se logró en tan solo 4min. Se realizó una comparación de los 2 métodos de extracción de los alcaloides de la misma muestra de C. majus: uno fue en crudo y otro se sometió a un baño de ácido como la muestra procesada. El resultado mostró que el contenido de alcaloides en la muestra cruda es mayor que el que contiene la muestra procesada. Esto significa que el contenido de alcaloide en C. majus será menor cuando la muestra en crudo haya sido procesada.
Chronic inflammations are known to be responsible for the pathogenesis of many chronic diseases including asthma, rheumatoid arthritis, septic shock, multiple sclerosis, Parkinson's, Alzheimer's diseases and other allergic diseases such as colon cancer.1–3 Antibiotics have little effect on the chronic inflammations. One of the alternatives is to seek novel anti-inflammatory agents in herbal drug plants. During a screening on to find candidates, Chelidonium majus L. (C. majus) has exhibited considerable inhibitory activity in its methanol extract. With being anti-inflammatory, anti-microbial, antiviral, antispasmodic activities, antifungal and fungistatic effects, the C. majus extracts have been reported to control several diseases including dermatoses, liver and gastrointestinal disorders, gastric ulcer, fight fever and treating traumatic injuries and various types of carbuncle, stopping bleeding, relieving pain, alleviating swelling4–8 and parasitic infections in human and animals.9–11
C. majus or Greater celandine belongs to the Papaveraceae family, also named as Shanhuanglian, niujinhua, babujin, duanchangcao and xionghuangcao in Chinese, is a herbaceous perennial plant throughout the world including Asia, Europe, Northwest Africa and North America.12 The main chemical components of C. majus are alkaloids, flavonoids, and phenolic acids,13 in which isoquinoline alkaloids are considered as mainly pharmacological effective component14,15 and in general, have been as indices for estimation of quality of C. majus. Although the C. majus is used as traditional folk medicine for the treatment of diseases with long history, it is also one of potentially hepatotoxic herbs when being used at relatively high concentration, leading an herb induced liver injury.16,17,7
The chemical screening and characterization of extracts is conducted by liquid chromatography (LC), gas chromatography (GC) or capillary electrophoresis (CE) techniques coupled with mass spectrometer (MS) or other different detectors, providing a great deal of preliminary information about the content and nature of constituents.12,18–21 Gas chromatography coupled to mass spectrometry (GC/MS) is one of the most powerful tools for natural products analysis.22–24 With the equipped capillary column, GC/MS has advantages of high separation efficiency, low detection limit and identification easy.25–27
Generally, the isolation of bioactive compounds from bio-matrix requires a preliminary fractionation, and thus, a cleanup pretreatment of herbal drug plants is needed prior to GC/MS analysis. In recent years, the emulsion liquid membrane extraction (ELME) has been proven to be a powerful sample extraction technique,28,29 which can give a channel from the feed phase to the trip phase for weak alkaline organic compound, and thus, alkaloids in herbs can be enriched from its extracts. Separation of alkaloids from traditional Chinese medicines (TCMs) using ELME, as was done in the current study, provides a discriminating benefit with respect to attractant analysis. It is known that at least some of the alkaloids will be transferred to strip phase. The use of ELME is believed to allow for preferential concentration of attractive compounds while minimizing collection of organic impurities.30,31 However, ELME suffers from the emulsion instability, which limits its applications at a broader scale. To overcome the emulsion instability, the alternative was added some polymeric compounds to the organic phase in preparation of emulsion, which was considered as a good way for improvement of emulsion stability.31
Our previous works have been the focus of attention for developing new analytical methodology for the use of GC/MS combining a sample pretreatment to determine the alkaloids in plants.32–39 In the present work, a simple and rapid method based on ELME followed by GC/MS was developed for the simultaneous identification and determination of isoquinoline alkaloids in C. majus. The emulsion for pretreatment of C. majus extracts was prepared by adding rubber additive as emulsifier instead of Span 80 and its superiority over conventional emulsion for the extraction of isoquinoline alkaloids from C. majus was investigated and demonstrated.
Experiments comparing ethanol extracts from the crude and processed C. majus have been briefly discussed in the literature. This method of pretreatment was reported to provide more material than extraction of alkaloids from TCMs. The purpose of this manuscript is to report the identities of alkaloids that are contained in crude and processed C. majus and then analyzed using the method described by us in a previous paper.32 The results reported herein provide the foundation for the next manuscript in this series, in which a comparison is made of alkaloids content from the crude and processed same TCMs sample for which alkaloids has a markedly different content.
ExperimentalMaterials and chemicalsC. majus L. (dried) was purchased from herbal medicine market (Anguo, Hebei, China) and identified with their voucher numbers. The protopine reference standard was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Matrine was purchased from Chinese control of pharmaceutical and biological products (Beijing, China). Acetone of HPLC grade was supplied by Beijing RUIZHX Technology Company (Beijing, China). The solvents and other commercial-grade reagents were obtained from local distributors. Kerosene (boiling point range of 200–240°C) and butadiene styrene rubber (BR9000) were obtained from manufacturers (Yanshan, Beijing). The water used in the experiments was doubly distilled water prepared in our laboratory.
Samples treatmentThe sample of C. majus was divided two group, one was undergone a processing drug and other was not. The processing drug was performed with being soaked raw C. majus in HCl solution (2mol/L) for 1h and dried in the air. Both samples were extracted with ethanol aqueous solution (60%) in Soxhlet extractor until the sample extracted solution colorless. After ethanol was recovered, two samples extracts solutions (50mL) were obtained, marked E1 for raw C. majus sample and E2 for processed C. majus sample.
Emulsion extraction of alkaloidsThe preparation procedural details of the emulsion employed to extract alkaloids were described in the reference.32 A volume of 7mL 0.1M H2SO4 was added to 13mL the membrane phase (kerosene and rubber emulsifier) and 0.4mL carrier (36% of acetic acid) in a 25mL beaker and the mixture was vortexed (WX500CY, Weiyu, Shanghai, China) at 10,000rpm for several min, before obtaining about 20mL fresh water in oil (W/O) emulsion. The feed phase was prepared through diluting the sample solution E1 or E2 with water 2-fold and adjusting pH 12 with NaOH solution (9.8%), respectively. The fresh emulsion (10mL) was dispersed in the feed phase (40mL) forming a water-oil-water (W/O/W) emulsion for the extraction of alkaloids under magnetic stir (150rpm).
After emulsion extraction, the emulsion was separated and demulsified. The resulting demulsification product strip phase solution was basified using 9.8% aqueous NaOH and then were extracted with methylene dichloride (3× 3mL). The organic solvent in extracts was evaporated under N2 blow and the dried extracts were dissolved in 10mL of acetone of HPLC grade in glass vials. The final products for GC/MS analysis were limpid.
Liquid–liquid extraction (LLE)Samples solution E1 or E2 (approximately 20mL) was mixed with 20mL of hydrochloric acid solution (2mol/L). After refluxed under heating for 1h, the mixture was defatted using methylene dichloride (3× 15mL). Next, the aqueous layer was collected and basified to pH 12 using 9.8% aqueous NaOH solution and then was extracted using methylene dichloride (3× 20mL). The organic solvent in extracts was evaporated under N2 blow and the dried extracts were dissolved in 10mL of acetone of HPLC grade in glass vials. The final products for GC/MS analysis were limpid.
GC/MS apparatus and measurement conditionsChromatographic separation was performed using an Agilent 6890N GC system equipped with a fused silica capillary column (DB-5MS UI, length: 30m, i.d.: 0.32mm, film thicknesses: 0.5μm). The carrier gas was Helium at a constant flow-rate of 1min−1. The column temperature program was 50°C, held for 1min, then increased at 40°Cmin−1 to 200°C, and was further increased at rate 4°Cmin−1 to 280°C, finally, held for 10min. The injections (1μL each) were of the split mode (10:1) with the injector and interface line temperature set at 260 and 280°C, respectively. The GC was coupled to a 5975C MS. The measurement was operated in positive electron impact (EI) ionization mode at 70eV; EM voltage was 1423V. The temperature of ion source and quadrupole was 230°C and 150°C, respectively. The mass spectra were obtained in a full scan mode (33–550amu) at 0.25s/scan scan rate.
Calibration was performed using the standard stock solution (STD) and one working solution made from the STD with its dilution. The STD of protopine was dissolved 10mg of protopine in the mixture of acetone (HPLC) and methylene dichloride (8:2, v:v). Reference standard solution (200mg/L) of matrine was prepared in water containing 40mg of matrine in a 200mL of volumetric flask.
A series of working standard solutions were prepared by dilution of the STD of protopine with acetone (HPLC). All the solutions were stored at −20°C until use. Prior to injection, the solutions were each added 50μL of the matrine reference standard.
The calibration curve was made by plotting the ratio of peaks area of the analyte protopine to matrine in function of the injected mass of the analyte. The correlation coefficient (R2) of the calibration curve was between 0.99713.
where y was the concentration of solution and x was the ratio of peaks area of the analyte protopine to matrine.Results and discussionEffect of emulsification timeStability of a prepared W/O emulsion is one of the most important factors impacting the extraction efficiency. Breakage of emulsion nullifies the separation efficiency by transferring back of the separated solute to the feed phase. Our recent work has emphasized a stable W/O emulsion can be prepared using a rubber emulsifier instead of Span 80.40 The most important factors governing the emulsion stability were studied. These factors involve emulsification speed and time, strip phase pH, emulsifier concentration and volume ratio of the strip aqueous phase to organic phase etc. Extending emulsification time, the W/O emulsion prepared with rubber emulsifier is obvious consistence, which affect the permeation process.
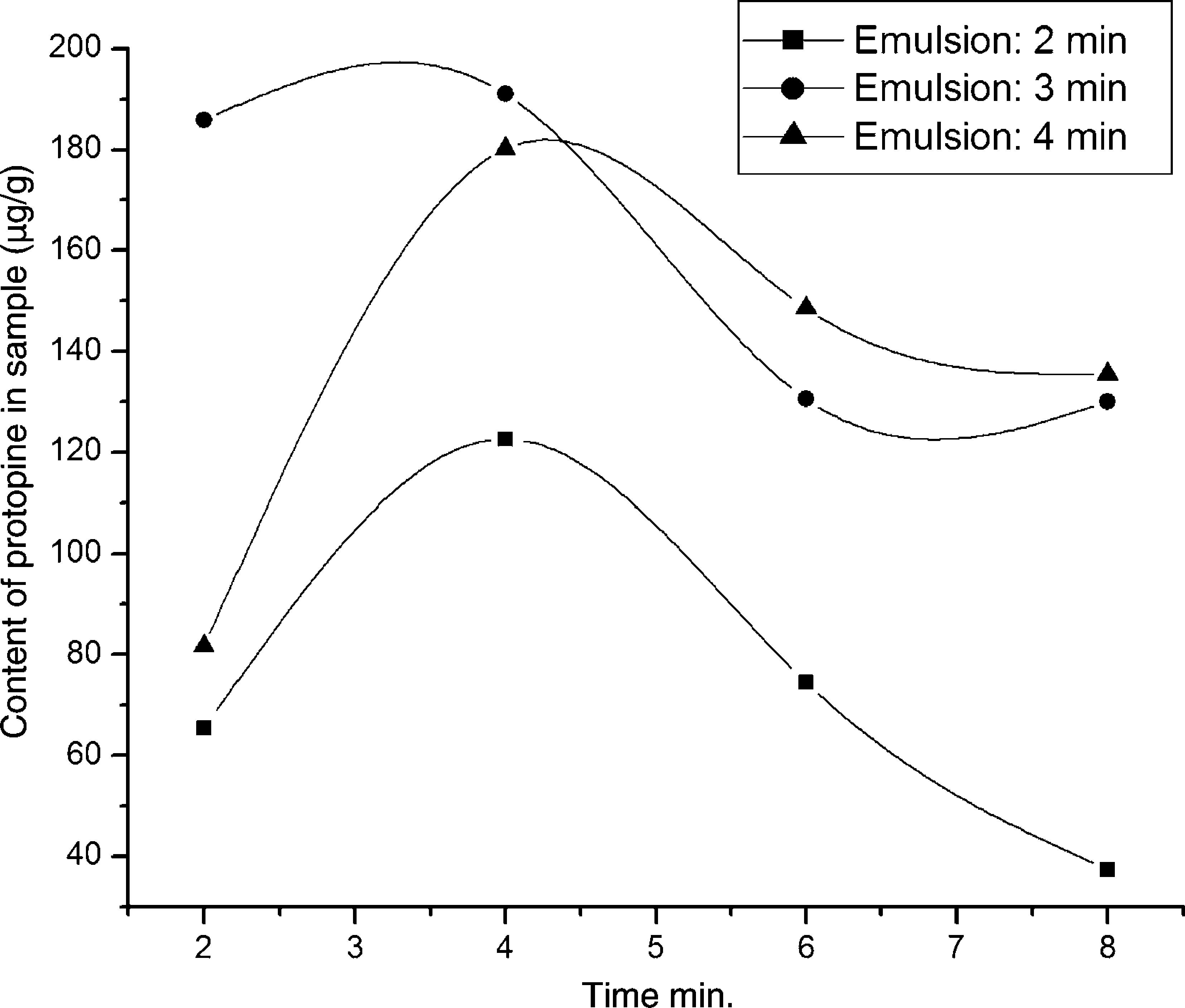
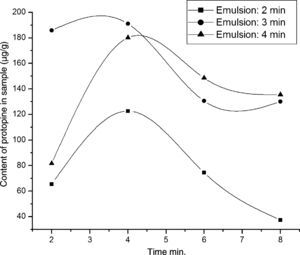
Experiments were conducted in the light of “Emulsion extraction of alkaloids” section described with emulsification time from 2 to 4min. Content of protopine per gram C. majus (CPC) extracted by this method was regarded as the assessment for assessing the effect of emulsification time on mass transfer rate.
Quantification employed external standard method using matrine as reference substance with different concentration of protopine. The standard curve of protopine was achieved by measuring the ratio of two peaks areas as a function of the concentration of protopine solution, which showed a linear relationship with a good correlation coefficient in the protopine concentration range from 2 to 25μg/mL (y=0.12841+104244x, R2=0.9971). Each experiment was performed at least triplicates and the mean values were calculated.
The effect of emulsification time shows that the CPC is increased with emulsification time increased from 2 to 3min and decreased for further increase in emulsifying time to 4min (Fig. 1). For insufficient emulsification time (<3min), the effect of coalescence was great because the droplets have a large size, which leads to their coalescence. In contrast, for a long emulsification time, the breakage plays a role because of high internal shearing conducive to a very high number of small droplets by volume unit. This increases collision frequency between small droplets conducting to emulsion breakage. Therefore, an emulsification time of 3min was taken as the optimum for further studies in the following experiments.
Effects of emulsification time on the extraction protopine. Experimental conditions – emulsions: volume ratios of rubber emulsifier to kerosene to internal strip phase (0.1M sulfuric acid aqueous solution) are 1:12:7; emulsification speed is 10,000rpm; C. majus alcohol extracts aqueous solution (pH 12) is feed phase; 10mL of the emulsion is dispersed 40mL the feed phase for 4min at 150rmp of magnet stirring speed.
From Fig. 1, whatever the emulsification time was used, an abnormal CPC decrease with increase of extraction time beyond 4min was observed. This phenomenon was also observed in our previous similar work extracting dl-anabasine from Alangium platanifolium root.32 The CPC decrement caused by extending extracting time is not clear yet. Fortunately the decrement is not a fatal fault because the most target compounds can be transferred within 4min. In the other ward, a 4min of extracting time is long enough for an emulsion extraction and the abnormal mass transport can be avoid as long as limiting the extracting time in 4min.
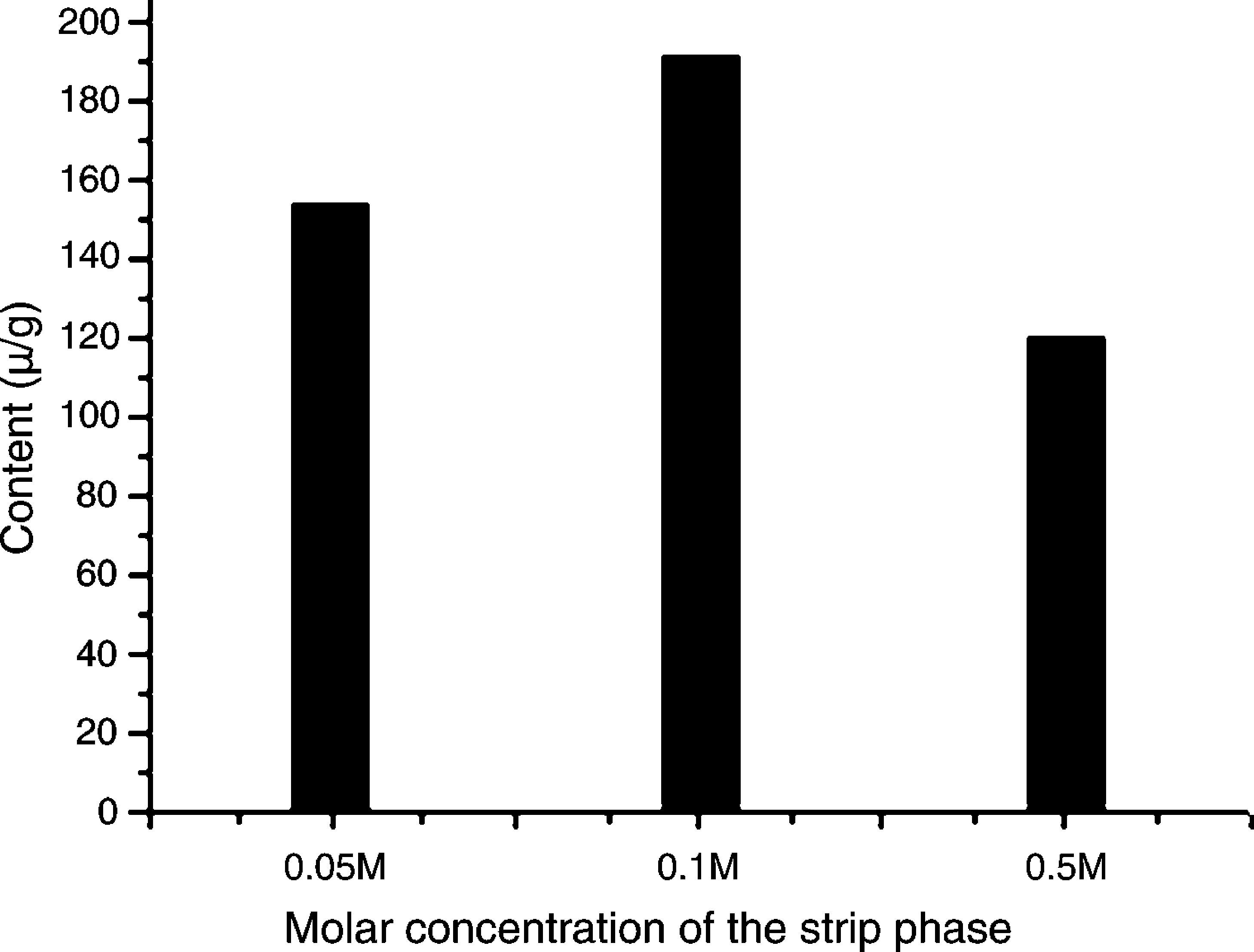
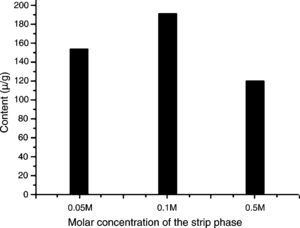
Effects of pH of the strip phase on mass transfer rateThe strip aqueous solution pH effect of mass transfer rate was studied because increasing acidity of the strip phase should not always contribute to analyte mass transfer rate according to the present literatures.14,41,42 For this purpose 0.05, 0.1 and 0.5M sulfuric acid aqueous solution were prepared as the strip phase and C. majus extracts as the feed phase. The CPC at three different sulfuric acid concentrations was shown in Fig. 2, which showed that the order of transfer protopine amount at different sulfuric acid concentrations was 0.05<0.5<0.1M, indicating that the solution of increasing acidity of strip phase for enhancing the mass transfer rate would be not always available. The reason should refer stability of the emulsion, because the stronger acid solution as the strip phase would lead to the W/O/W emulsion instability.
Effects of concentration of sulfuric acid aqueous solution in the strip phase on the ELME protopine efficiency. Experimental conditions – emulsions: volume ratios of rubber emulsifier to kerosene to internal strip phase are 1:12:7; emulsification speed is 10,000rpm; C. majus alcohol extracts aqueous solution (pH 12) is feed phase; 10mL of the emulsion is dispersed 40mL the feed phase for 4min at 150rmp of magnet stirring speed.
According to the mechanism of emulsion extraction,41 with addition of organic acid as carrier in the membrane phase should be the benefit of alkaloid mass transfer rate. The reactions of dissociated alkaloid anion A+ and the organic acid carrier CH in two interfaces of oil and water are
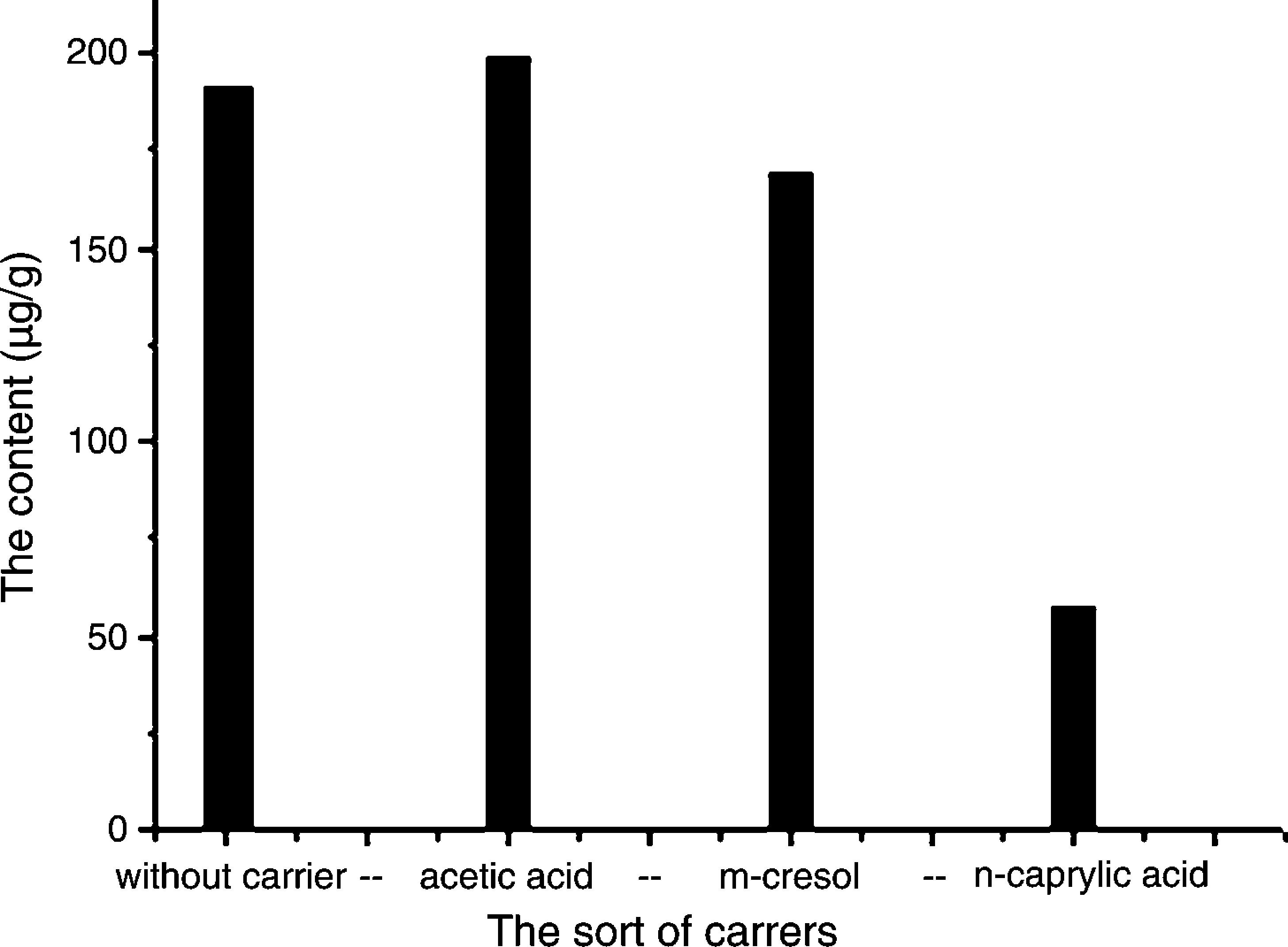
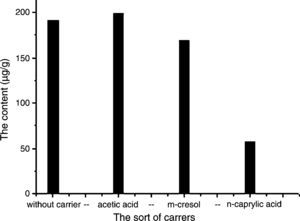
The effect of carrier was investigated using three of polar organic compounds as carrier according to their polarity order: acetic acid>metacresol>octanoic acid, C. majus extracts as the feed phase and the CPC also as the assessment.Experiments comparing the CPC extracted without added carrier in the membrane phase, as shown in Fig. 3, the CPC was increased as acetic acid added and decreased, however, when metacresol and octanoic acid as carrier added in the membrane phase. The decrease of CPC was agreed with the decrease of the used carrier polarity. Increase in the CPC corresponds to increased diffusion of solute carrier complex across the membrane. Among all three carriers, acetic acid has the strongest polarity. Therefore, it can increase the protopine's concentration at the interface, providing a CPC increase. However, the carriers like metacresol and octanoic acid are the weaker polar compounds than acetic acid and hence an inhibition of ion exchange of solute carrier complex across the interfaces of oil and water providing the CPC decrease. Therefore, it appears that there is a limit to the carrier polarity that will give significant increase in the extraction rate, probably as a result of the equilibrium of the ion exchange reaction.
Effects of different carriers on the ELME protopine efficiency. Experimental conditions – 0.4mL of carrier was added to 13mL of the organic phase as membrane phase; volume ratios of the membrane phase to strip phase (0.1M sulfuric acid aqueous solution) is 13.4:7; emulsification speed is 10,000rpm; C. majus alcohol extracts aqueous solution (pH 12) is feed phase; 10mL of the emulsion is dispersed 40mL the feed phase for 4min at 150rmp of magnet stirring speed.
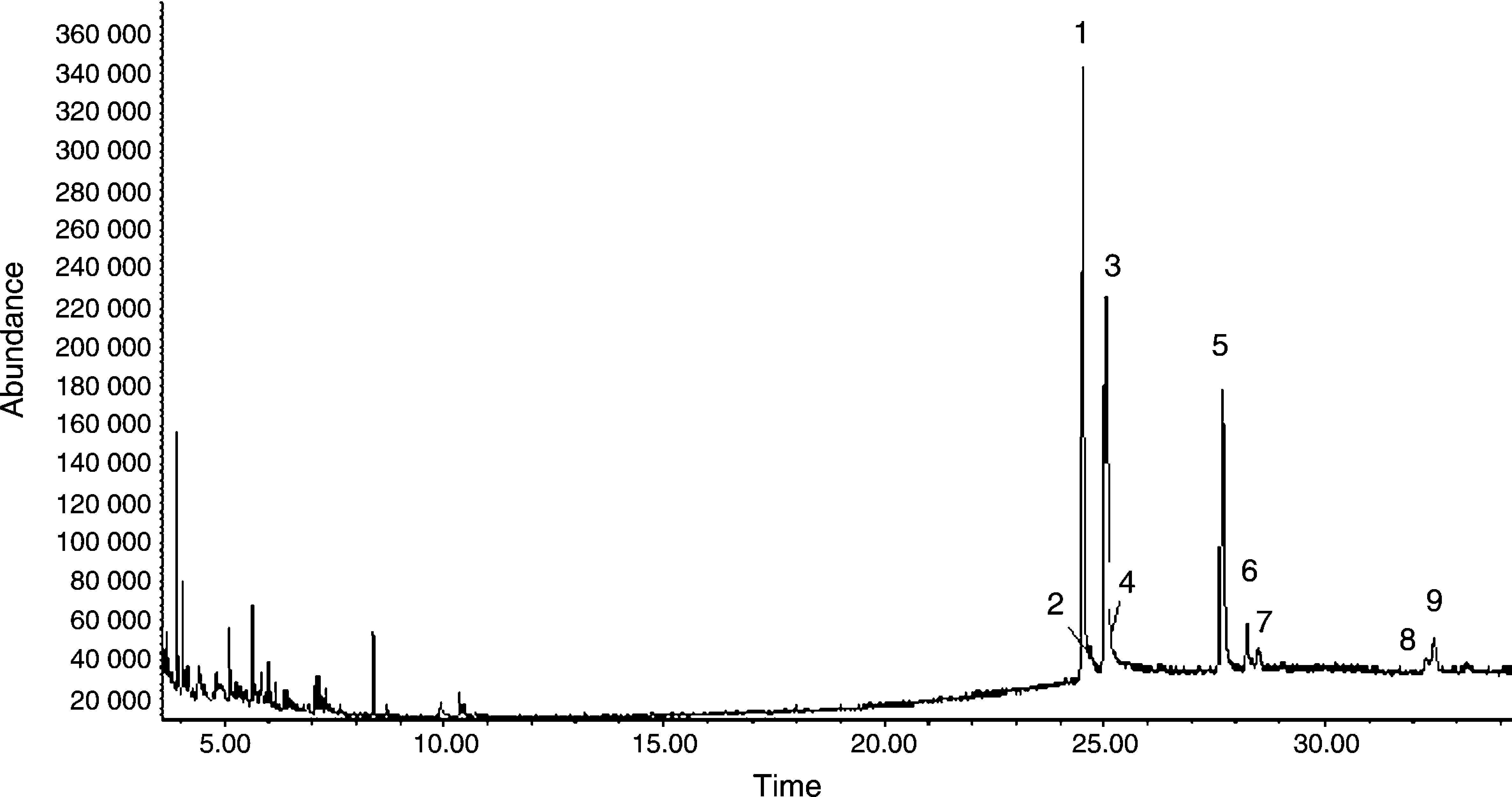
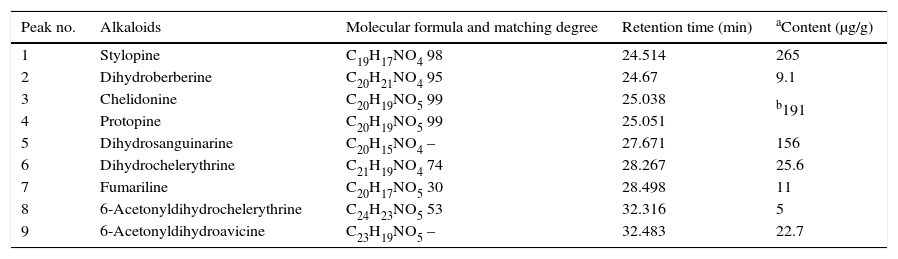
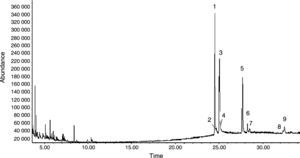
The above ELM individual optimization was employed for the extraction of alkaloids from C. majus extracts. A total ion chromatogram (TIC) of C. majus alkaloids extracted by emulsion, acquired in EI mode, is presented in Fig. 4. The peak numbers represent the elution order of identified peaks. Analysis using the methods employed here resulted in chromatograms that contained as many as 36 discernible peaks. Of these, 9 alkaloids identifications were made by matching sample mass spectra with those of the NBS library in varying degrees of certainty. They are labeled by number on the chromatogram. As seen in Fig. 4, the alkaloids of peaks were not wholly eluted prior to 20min and 3# peak should be correspond to both alkaloids chelidonine and protopine, which were not distinguished through their chromatogram behavior but they can be distinguished from their mass spectra. The peak numbers in Fig. 4 correspond to those listed in Table 1.
Chromatogram of C. majus alcohol extracts. Experimental conditions is the same as the described in caption of Fig. 3 peak 1: stylopine, peak 2: dihydroberberine, peak 3: chelidonine and protopine, peak 4: dihydrosanguinarine, peak 5: dihydrochelerythrine, peak 6: fumariline, peak 7: 6-acetonyldihydrochelerythrine and peak 8: 6-acetonyldihydroavicine.
Alkaloids present or suspected present from extraction of C. majus using emulsion liquid membrane.
| Peak no. | Alkaloids | Molecular formula and matching degree | Retention time (min) | aContent (μg/g) |
|---|---|---|---|---|
| 1 | Stylopine | C19H17NO4 98 | 24.514 | 265 |
| 2 | Dihydroberberine | C20H21NO4 95 | 24.67 | 9.1 |
| 3 | Chelidonine | C20H19NO5 99 | 25.038 | b191 |
| 4 | Protopine | C20H19NO5 99 | 25.051 | |
| 5 | Dihydrosanguinarine | C20H15NO4 – | 27.671 | 156 |
| 6 | Dihydrochelerythrine | C21H19NO4 74 | 28.267 | 25.6 |
| 7 | Fumariline | C20H17NO5 30 | 28.498 | 11 |
| 8 | 6-Acetonyldihydrochelerythrine | C24H23NO5 53 | 32.316 | 5 |
| 9 | 6-Acetonyldihydroavicine | C23H19NO5 – | 32.483 | 22.7 |
–, Manual interpretation was used according to a specific EI fragmentation pattern and its molecular mass.
It reported that over 20 different C. majus alkaloids were identified and main alkaloids are benzylisoquinolines.14 But here only 9 sort of the alkaloids were separated and identified. The reason should be from the dried and aged C. majus sample.
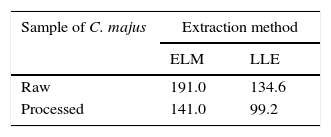
In order to illustrate the efficiency of the method employed here, same the sample was extracted by liquid–liquid extraction (LLE) method for comparing their extraction efficiency. The C. majus sample was divided into two groups, one group was the crude and the other was immersed in 2M of hydrochloric acid solution for 1h prior to extraction, calling the former raw sample and the later processed sample.
Protopine was also selected as the reference analyte for assessing the extraction efficiency because it was second main alkaloid content eluted in chromatogram (Fig. 4). Table 2 shows the separation efficiency achieved by ELME and LLE. Horizontal comparison from Table 2, it is observed that the CPC extracted by ELME is about 1.4 time to that by LLE, indicating the method described is more efficiency than LLE in extraction of alkaloids. Column comparison, the CPC extracted from the raw sample is also about 1.4 times to that from the processed sample, indicating the some alkaloid content lost as C. majus undergo a process of acid bath. The alkaloid content lost can be evaluated as an alkaloid dissolved in acid bath process. In other ward, a part of alkaloids content in C. majus can be removed through acid bath sample.
The content of protopine per gram C. majus extracted by ELM or LLE.
| Sample of C. majus | Extraction method | |
|---|---|---|
| ELM | LLE | |
| Raw | 191.0 | 134.6 |
| Processed | 141.0 | 99.2 |
Experimental conditions are the same as described in caption of Fig. 3.
An available and reliable method based on ELM combined with GC/MS was developed for the extraction of alkaloids from C. majus. The operational conditions here for an excellent stability of the W/O emulsion prepared with rubber emulsifier was emulsification time 3min at 10,000rpm stirring speed. The organic phase was kerosene with rubber emulsifier (1:15.25, v:v), acetic acid (36%) as carrier. The strip phase was 0.1M of sulfuric acid aqueous solution and the volume ratio of strip phase to organic phase was 3/7.
At the optimum experimental conditions, the emulsion extraction time should be limited within 4min. Total of 9 alkaloids were separated and identified from the dried C. majus using the present method. Of them, the most content of alkaloid was stylopine and second content was chelidonine and protopine. The content of alkaloids would be partly consumed as C. majus sample was underwent an acid bath process. In comparison with currently LLE method, the content of protopine per gram C. majus extracted by the ELME is 1.4 time to that by LLE and hence the present method offers an effective way for analysis of alkaloids in C. majus.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.