The 5-hydroxytryptamine-1A receptors are known to be involved in the inhibition of seizures in epilepsy.
ObjectiveThe main aim of the present study is to determine the functional coupling to G-protein of the 5-HT1A receptor through 8-OH-DPAT-stimulated [35S]GTPγS binding assay in lateral temporal neocortex tissue of patients with mesial temporal lobe epilepsy (mTLE) and correlate it with clinical data.
Material and methodsThe activation of the G protein complex was determined by [35S]GTPγS binding assay. The temporal neocortex tissue was obtained from 5 patients with mTLE during epilepsy surgery and from 5 subjects (autopsies) who died due to an accident and without history of neurological disease.
ResultsWe found that values of maximal stimulation (Emax) of [35S]GTPγS binding significant decreased in the mTLE group (30.8%, p<.05) when compared to the autopsy samples (65.9%). The values of potency (pEC50) were similar in both groups. However, we found no significant differences between the Emax and age of patient, age of seizure onset, duration of epilepsy and frequency of seizures. Also, the pEC50 values revealed no significant correlations with the clinical data.
ConclusionsOur data provide evidence that the lateral temporal neocortex of patients with pharmacoresistant mTLE presents alterations in the functional coupling to G-protein of the 5-HT1A receptor. Such a change could be involved in hyperexcitability in the neocortex of patients with mTLE.
Los receptores 5-hidroxitriptamina-1A (5-HT1A) están implicados en la inhibición de las crisis en epilepsia.
ObjetivoEl presente estudio es determinar el acoplamiento funcional a la proteína-G del receptor 5-HT1A a través del ensayo de unión [35S]GTPγS estimulando al receptor por 8-OH-DPAT (agonista del receptor 5-HT1A) en tejido de neocorteza temporal de pacientes con epilepsia del lóbulo temporal mesial (ELTm) y correlacionar con datos clínicos.
Material y métodosLa activación del complejo de la proteína G del receptor de 5-HT1A se determinó por [35S]GTPγS por ensayos de unión. Tejido de neocorteza temporal de 5 pacientes con ELTm obtenido de la cirugía de epilepsia y 5 tejidos de autopsias de sujetos que fallecieron por accidente o por causas diferentes a una enfermedad neurológica.
ResultadosLos resultados demostraron estimulación máxima (Emax) significativamente disminuida en el grupo ELTm (30.8%, p<0,05), en comparación con tejido de muestras de autopsia (65.9%). Los valores de potencia (pEC50) fueron similares en ambos grupos. Sin embargo, no se encontraron diferencias significativas entre la Emax con la edad del paciente, la edad de inicio de las crisis, duración con epilepsia y la frecuencia de crisis. Además, los valores de pEC50 no revelaron correlación con los datos clínicos.
ConclusiónLos presente estudio proporcionan evidencia que la neocorteza temporal de pacientes con ELTm fármaco resistente presenta alteraciones en el acoplamiento funcional a la proteína-G del receptor 5-HT1A. Tal cambio podría estar implicado en la hiperexcitabilidad que se observa en la neocorteza de pacientes con ELTm.
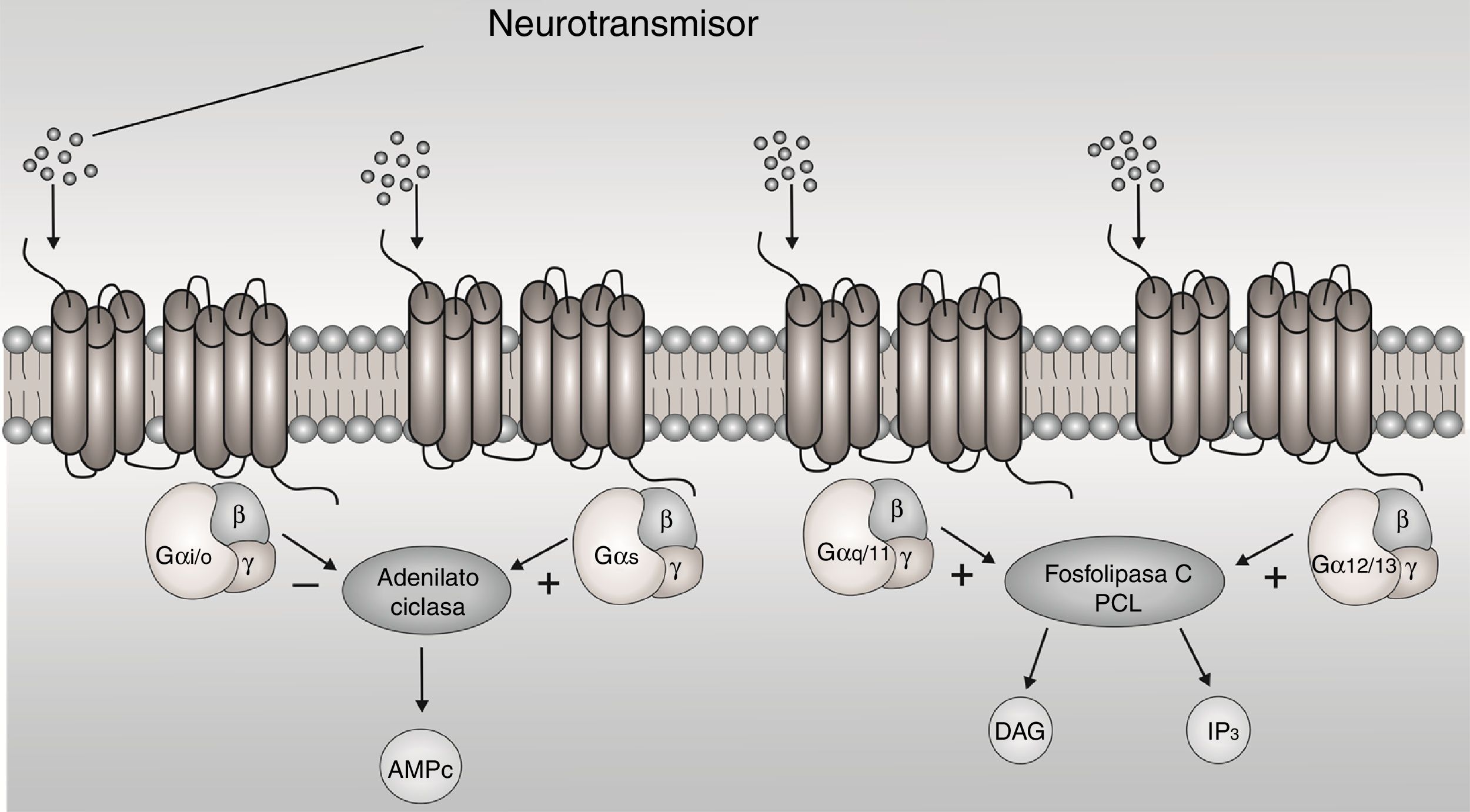
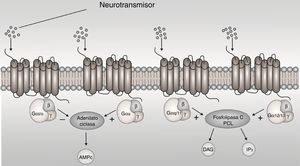
The G-protein-coupled receptors (GPCRs) are membrane proteins that sense signaling molecules such as hormones, light, odors, taste and neurotransmitters.1 GPCRs undergo conformational changes after ligand binding, causing the activation of signaling networks, resulting in a cellular response. G proteins are heterotrimers made of α, β and y subunit. When the receptor stimulates the G protein, the α subunit releases guanosine diphosphate (GDP) and binds to GTP (guanosine triphosphate). The Gα subunits are divided in Gαs, Gαi/o, Gαq/11, and Gα12/13 based on sequence homology and functional similarities, each one active a cascade of second messengers1 (Fig. 1). The Gαi/o subunit is interest, because it inhibits adenylyl cyclases (AC) and reduces the concentration of cyclic adenosine monophosphate (cAMP), this leads to mediate receptor-dependent inhibition of various types of adenylyl cyclases, in addition to being expressed more in brain.2
The metabotropic receptors Gαi/o-protein-coupled have been considered as promising drug targets in the treatment of several disease, related to excitation disorders such as epilepsy.
Epilepsy is a chronic neurological disorder characterized by increased excessive or synchronous abnormal neuronal activity in the brain.3 Numerous studies imply an imbalance between excitatory and inhibitory neurotransmission.4 The neurobiological basis of epilepsy involves alterations of neuronal functions at multiple levels. It is known that changes in neurotransmission systems as receptors coupled to G-proteins are involved in epileptic seizures.5–8 The activation of GPCR receptors specifically those coupled to Gαi/o proteins have demonstrated anticonvulsive, antiepileptic and neuroprotective effects in different experimental models of epilepsy.9–12 In contrast, brain tissue of patients with epilepsy showed alterations in their density and functional activity.13–15,30 These evidences in preclinical models and brain tissue of patients with epilepsy suggest the participation of receptors to Gαi/o proteins in epilepsy.
On the other hand, the 5-hydroxytryptamine-1A (5-HT1A) receptors are coupled to Gαi/o-proteins, It is known that induce inhibitory effect on seizure activity in models animals of epilepsy.16–18
Studies in patients with mesial temporal lobe epilepsy (mTLE) have demonstrated a decrease in 5-HT1A receptor binding in the insula, anterior cingulate, and medial and lateral temporal regions, ipsilateral to the epileptic focus in positron emission tomography (PET).19–21 Studies in vitro autoradiography on resected of neocortex tissues of surrounding the epileptic focus of patients with mTLE showed a decrease in 5-HT1A receptor binding in layers I-II,22 this could be associated to high excitability and facilitation of seizure activity.
The neocortical temporal tissues have important role in seizure generation and/or propagation.23,24 Previous studies describe that the neocortical temporal tissues of patients with mTLE presents a disorganization in synaptic circuits which consists of an increase and decrease of excitatory and inhibitory synapses, respectively.5,25
These studies (PET or autoradiography) provide information on the expression of receptor and their distribution in the brain. However, it does not provide information on the functional coupling of 5-HT1A receptor to the Gαi/o protein which could be implicated in the pathophysiology of the epilepsy, recalling that the activation of this Gαi/o protein induces inhibition.
The use of the [35S]GTPγS radioligand binding assay is a technique that allows the evaluation of the functional activity of the receptor occupation of G proteins. The [35S]GTPγS technique consists of the activation of receptors by selective agonists, promoting the exchange of GDP to GTP which facilitates the binding of sulfur-labeled GTP to the Gαi/o subunit, allowing the functional measurement of the receptors.26
Therefore, the aim of the present study was to examine 5-HT1A receptor-induced G-protein activation in lateral temporal neocortex tissue of patients with mTLE with analyses the [35S]GTPγS binding. Also, it is correlating of 5-HT1A receptor-induced G-protein functional activation with the subject's age, age of seizure onset, duration of epilepsy and frequency of seizures.
MethodsPatients groupThe protocol was approved for scientific committees of the institutions involved in the present research (protocol number DI/15/403/03/32) and each patient signed an informed consent.
The inclusion criteria of protocol were established by the Epilepsy Clinic Program of the General Hospital of Mexico included patients with pharmacoresistant mTLE who had a detailed seizure calendar of at least 3 months prior to surgery in order to confirm the presence of seizures, blood levels of antiepileptic drugs to verify that they were within therapeutic range, magnetic resonance imaging to detect the presence of hippocampal sclerosis or atrophy, four serial electroencephalograms to determine the presence of temporal epileptiform activity and neuropsychological assessment to determine basal cognitive condition and identify behavioral or memory disorders related to seizures.
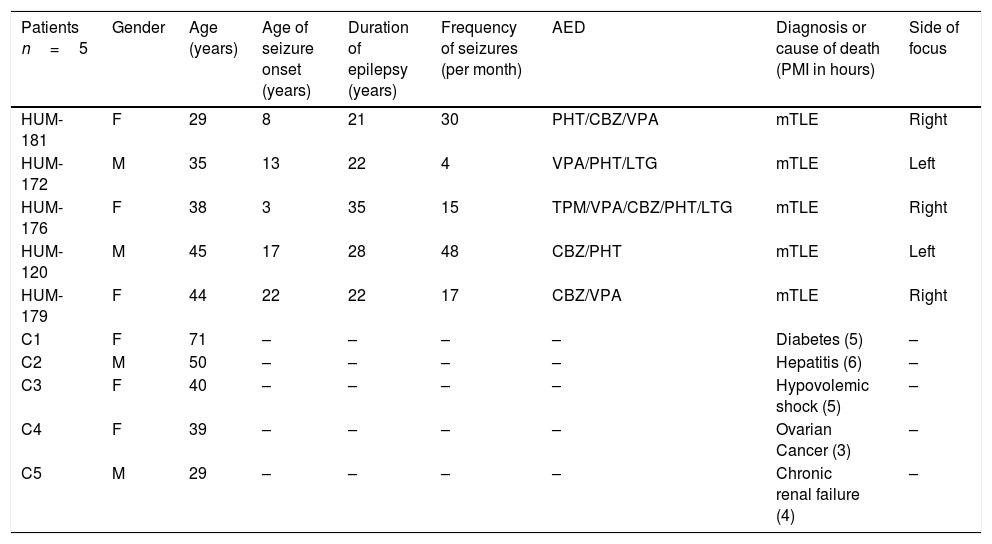
A standard anterior temporal lobectomy, ipsilateral to the epileptogenic zone, was performed in all patients after 48h from the last seizure occurrence. Epileptic lateral neocortex temporal tissue was obtained from five patients with pharmacoresistant mTLE history (Table 1). During the surgical procedure, lateral temporal neocortex tissue was collected immediately after resection then quickly frozen in pulverized dry ice and stored at −70°C.
Clinical data of patients with pharmacoresistant temporal lobe epilepsy and autopsies.
| Patients n=5 | Gender | Age (years) | Age of seizure onset (years) | Duration of epilepsy (years) | Frequency of seizures (per month) | AED | Diagnosis or cause of death (PMI in hours) | Side of focus |
|---|---|---|---|---|---|---|---|---|
| HUM-181 | F | 29 | 8 | 21 | 30 | PHT/CBZ/VPA | mTLE | Right |
| HUM-172 | M | 35 | 13 | 22 | 4 | VPA/PHT/LTG | mTLE | Left |
| HUM-176 | F | 38 | 3 | 35 | 15 | TPM/VPA/CBZ/PHT/LTG | mTLE | Right |
| HUM-120 | M | 45 | 17 | 28 | 48 | CBZ/PHT | mTLE | Left |
| HUM-179 | F | 44 | 22 | 22 | 17 | CBZ/VPA | mTLE | Right |
| C1 | F | 71 | – | – | – | – | Diabetes (5) | – |
| C2 | M | 50 | – | – | – | – | Hepatitis (6) | – |
| C3 | F | 40 | – | – | – | – | Hypovolemic shock (5) | – |
| C4 | F | 39 | – | – | – | – | Ovarian Cancer (3) | – |
| C5 | M | 29 | – | – | – | – | Chronic renal failure (4) | – |
M, male; F, female; AED, antiepileptic drugs; PHT, phenytoin; CBZ, carbamacepine; VPA, valproic acid; LTG, lamotrigine; TPM, topiramate; OXC, oxcarbazepine; PB, phenobarbital; CZP, clonazepam; RISP, Risperidone.
Lateral neocortex temporal tissue was obtained from the autopsy of five subjects, who died because of various causes, without evidence of neurological disease. The control autopsy samples were provided of the Institute of Forensic Sciences, in agreement with the Faculty of Medicine of the National Autonomous University of Mexico. Our tissue was dissected at the time of autopsy, with a postmortem interval of 3 to 6h, immediately stored at −70°C and then manipulated as described below.
Binding assaysMembrane preparationThe lateral temporal neocortex tissues were prepared according to the method previously described27 slight modifications. Briefly, samples (200–500mg) were homogenized on ice in 50mM Tris–HCl buffer (pH 7.4) using a Teflon-glass homogenizer. The homogenate was centrifuged at 15,000rpm for 25min at 4°C and the resulting pellet was re-suspended in fresh buffer and incubated for 30min at 35°C. The centrifugation step was repeated, and the final pellet was re-suspended in 50mM Tris–HCl buffer (pH 7.4) and stored at −70°C until use. Protein levels were determined by the method of Lowry et al. (1951).28
[35S]GTPγS functional assayThe 5-HT1A receptor-mediated G-protein activation experiments were carried as previously described for Spetea et al. (1998)29 with slight modifications. The membrane fractions (≈10μg of protein/sample) of lateral neocortex temporal tissue were incubated at 30°C for 60min in Tris–EGTA buffer (1M Tris–HCl, 0.2M EGTA, 1M MgCl2, 1M NaCl, pH 7.4), containing [35S]GTPγS (0.05nM) and increasing concentrations (Log 10−11 to 10−5 M) of 8-OH-DPAT (5-HT1A receptor agonist) in presence of excess GDP (10mM) in a final volume of 1ml. Total binding was measured in absence of the tested compound. Non-specific binding was determined in presence of 10mM unlabeled GTPγS and subtracted from total binding to calculate the specific binding. The reaction was initiated by adding [35S]GTPγS and completed with filtration of the samples through Whatman GF/B glass fiber filters; it was washed three times with ice-cold 50mM Tris–HCl buffer (pH 7.4) using Brandel M-48 Cell Harvester. The dried filters were immersed in a Sigma-FluorTM scintillation cocktail (Sigma) and then used to determine bound radioactivity as described above. The binding experiments were performed in triplicates.
Data analysisThe data were expressed as mean±S.E.M for the subject's age, age of seizure onset, duration of epilepsy and frequency of seizures. G-protein activation was given as percent of the specific [35S]GTPγS binding observed in absence of receptor ligands (basal activity). The binding data were subjected to non-linear regression analysis of concentration-effect curves performed by Prism (GraphPad Software, Inc.) for determined agonist potency (pEC50) and the maximum stimulation (Emax) values. The data were expressed as mean±S.E.M. The results from the specific binding were examined statistically by Student's t-test to find differences of Emax and pEC50 between autopsy samples and mTLE tissue. Pearson's correlation coefficients were calculated to establish a significant correlation (p<0.05) of Emax and pEC50 with the obtained values of patient's age, age of seizure onset, duration of epilepsy and frequency of seizures.
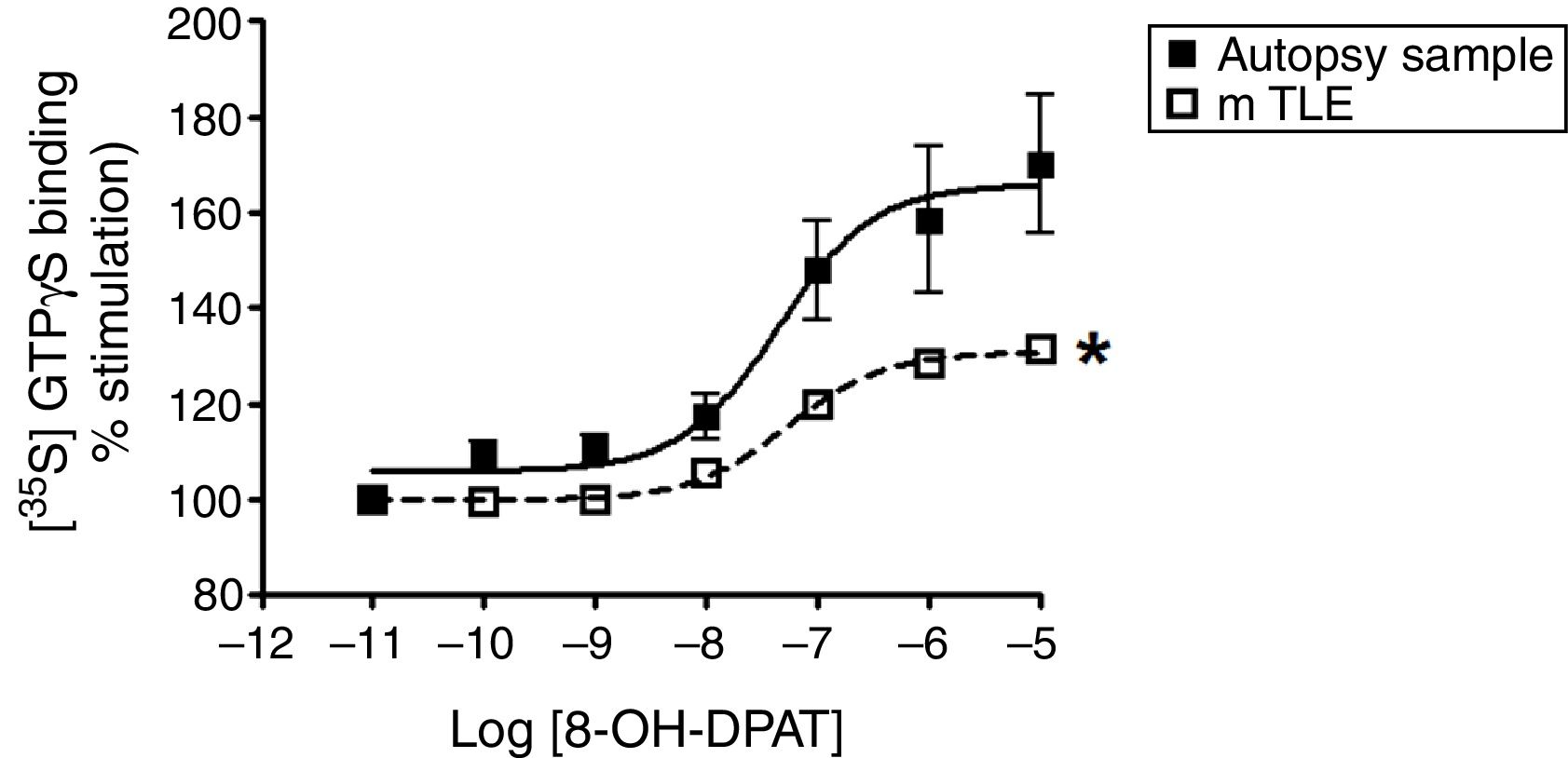
ResultsAutopsy samplesAutopsy samples were acquired from five subjects (two male and three female) ranging in age from 29 to 71 years (45.8±7.2 years), who died by different causes and without history of neurological disease. The postmortem interval was 3 to 6h (4.6±0.5h) (Table 1). 8-OH-DPAT-stimulated [35S]GTPγS binding was concentration-dependent and saturable. The binding assays revealed a Emax of 65.9% and a potency pEC50 value within the micromolar range (−7.3±0.3) (Fig. 2). We found no significant correlation in autopsy samples between the coefficients of the Emax of [35S]GTPγS binding and pEC50 values, with the age (r=0.7453, r=−0.0989, respectively) and postmortem interval (r=0.7092, r=−0.0305, respectively) (Table 2).
Specific [35S]GTPγS binding to membranes of samples from autopsies (--■--) and from patients with mTLE (-□-) as a function of increasing concentration of the 5-HT1A receptor agonist 8-OH-DPAT. Each point represents the mean±S.E.M of the percentage of stimulation over basal values. Note that in patients with mTLE, [35S]-GTPγS binding stimulation by 8-OH-DPAT was significantly different from that of autopsy samples.
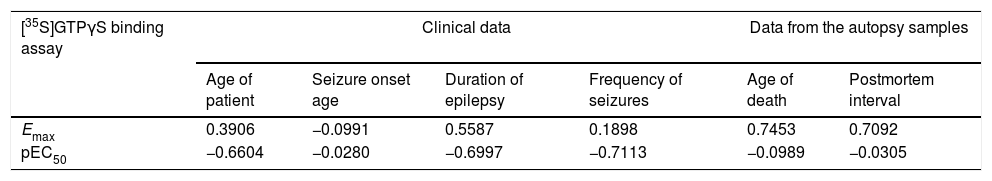
Correlations of [35S]GTPγS binding parameters between clinical data and data from the autopsy sample from neocortex temporal membranes in patients with temporal lobe epilepsy.
| [35S]GTPγS binding assay | Clinical data | Data from the autopsy samples | ||||
|---|---|---|---|---|---|---|
| Age of patient | Seizure onset age | Duration of epilepsy | Frequency of seizures | Age of death | Postmortem interval | |
| Emax | 0.3906 | −0.0991 | 0.5587 | 0.1898 | 0.7453 | 0.7092 |
| pEC50 | −0.6604 | −0.0280 | −0.6997 | −0.7113 | −0.0989 | −0.0305 |
Values represent Pearson Correlation Coefficients. Emax values (expressed as maximal stimulation of [35S]GTPγS binding) and pEC50 values (expressed as the negative logarithm of EC50).
The lateral temporal neocortex tissues were obtained from five patients (two male and three female) of age 38.2±2.9 years old (ranged from 29 to 45 years) with 25.6±2.6 years of epilepsy duration; the age of seizure onset was 12.6±3.3 years and they had 22.8±7.5 seizures per month (Table 1). We found no significant differences in age between mTLE patients and the autopsy samples (38.2±2.9 and 45.8±7.2 years, respectively, p=0.1766). Therefore, we found that Emax values significantly decreased in the mTLE group (30.8%, p<0.05) when compared to autopsy samples (65.9%) (Fig. 2). However, the pEC50 values in the micro molar range were similar in both groups (−7.3±0.3 and −7.2±0.1, autopsy samples and mTLE, respectively). Regarding the correlation analysis, we found no significant differences between the Emax with age of patient, age of seizure onset, duration of epilepsy and frequency of seizures. Also, the pEC50 values revealed no significant correlations with clinical data (Table 2).
DiscussionEven though the results showed a decrease of the 5-HT1A receptor-induced G-protein functional activation in mTLE, we found no correlation between the decrease in functional coupling to the 5-HT1A receptor with the patient's age, duration with epilepsy, age at onset with epilepsy, and frequency of seizures.
In the present study, we find limitations that describe below. Previous studies have indicated that 5-HT1A receptor density decreases with age in brain regions such as cortex, hippocampal regions, locus coeruleus and dorsal raphe nuclei.30,31 These results suggest that a decrease in receptor density could indirectly produce changes in functional 5-HT1A receptor activation. An important point to mention, we used tissue from mTLE surgical patients and autopsy samples from subjects of similar age, in order to reduce the possible influence of this variable. Also, our results show no significant differences in age between mTLE patients and the autopsy samples neither correlation with 5-HT1A receptor-induced G-protein functional activation. Our results suggest that decrease functional 5-HT1A receptor activation is directly related to changes associated with epilepsy rather than age.
Concerning the postmortem interval of the autopsy samples (4.6±0.5h) may produce changes that could alter the results, in this context, previous studies indicate that 5-HT1A receptors are preserved within a postmortem interval of 8–92h.32 Also, we results showed that postmortem interval did not correlate with the 5-HT1A receptor-induced G-protein functional activation. These previous studies and results obtained lead us to suggest that tissue from autopsy samples can be used as control.
Another limitation was the fabric storage time at −70°C. It has been reported that storage intervals of 1–82 months do not affect the functional activity of the 5-HT1A receptor.26 In our report the storage time was 4–12 months.
Regarding our results that showed a decrease in functional 5-HT1A receptor activation in patients with mTLE can be explained as a consequence of changes in density of receptor in neocortex. It is known that the cortical layers (I–II) of the neocortex present a dense pattern of these receptors.33 Rocha et al. (2007)22 showed decreased of 5-HT1A receptor binding in the external cortical layers (I–II) of patients with mTLE. Other studies with PET have shown decrease the 5-HT1A receptor binding in neocortex of patient with mTLE.19–21 These evidences may partly explain the decrease in the receptors functional activity in our results.
It is important to mention that 5-HT1A receptor-induced G-protein functional activation induces hyperpolarization via potassium channels and therefore inhibitory effects.34–36 Therefore, the decreased functional activity of 5-HT1A receptors associated with reduced density of these receptors in neocortex; it could explain the excitability and facilitation of seizure activity in neocortex of patients with mTLE. On the contrary, study in epileptic hippocampal tissue show an increase of the 5-HT1A receptor-induced G-protein functional activation37 suggest as a compensatory mechanism in response to the epileptic discharges that could contribute to the modulation of neuronal hyperexcitability.
The results of the present study indicate that patients with mTLE do not observe correlation with clinical aspects. These results suggest that clinical aspects do not play an important role in changes in the attachment of Gαi/o proteins to 5-TH1A receptors. However, we cannot rule out other factors such as antiepileptic drugs, cortical atrophy or other processes underlying epilepsy may be involved in the observed alterations in functional activity.
The development of drugs that target the activation of GPCR receptors specifically those coupled to Gαi/o proteins has recently gained new interest for its efficacy in preclinical models by their anticonvulsive, antiepileptic, and neuroprotective effects. However, it is important to consider that at present is insufficient evidence of the alterations in coupled to Gαi/o proteins have in pharmacoresistance in patients with epilepsy. These studies in surgically resected tissue of patients with pharmacoresistant epilepsy will allow us to investigate and provide the scientific basis for these alterations in epilepsy, thus leading to know whether they can be used as a therapeutic target to treat epilepsy in the future.
Conflict of interest statementNone of the authors has any conflict of interest to disclose.



![Specific [35S]GTPγS binding to membranes of samples from autopsies (--■--) and from patients with mTLE (-□-) as a function of increasing concentration of the 5-HT1A receptor agonist 8-OH-DPAT. Each point represents the mean±S.E.M of the percentage of stimulation over basal values. Note that in patients with mTLE, [35S]-GTPγS binding stimulation by 8-OH-DPAT was significantly different from that of autopsy samples. Specific [35S]GTPγS binding to membranes of samples from autopsies (--■--) and from patients with mTLE (-□-) as a function of increasing concentration of the 5-HT1A receptor agonist 8-OH-DPAT. Each point represents the mean±S.E.M of the percentage of stimulation over basal values. Note that in patients with mTLE, [35S]-GTPγS binding stimulation by 8-OH-DPAT was significantly different from that of autopsy samples.](https://static.elsevier.es/multimedia/01851063/0000008100000004/v1_201810010958/S0185106318300398/v1_201810010958/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
