To evaluate the short-term effects of intravitreal ranibizumab (IVR) injection on visual and structural changes in diabetic macular edema (DME).
Patients and methodsA retrospective study including 108 eyes of 74 patients with DME in which IVR injection was conducted three times at one-month intervals. Retinal and choroidal layers, as well as the subretinal fluid area, were measured at baseline and during 3 months of treatment. The correlation between structural changes and visual acuity was investigated.
ResultsIn general, most of the retinal layers tended to decrease after treatment. Resolution of subretinal fluid remained relatively superior in predicting the outcome of ranibizumab injection. In addition, we found that reduction of photoreceptor layer (PRL) thickness provided the best estimation of visual acuity gain.
ConclusionNeural recovery in PRL is associated with better visual improvement. Individual retinal segmentation would be beneficial for monitoring and evaluating ranibizumab treatment in DME.
Evaluar los efectos a corto plazo de la inyección intravítrea de ranibizumab (IVR) sobre los cambios visuales y estructurales en el edema macular diabético.
Pacientes y métodos: Estudio retrospectivo que incluyó 108 ojos de 74 pacientes con edema macular diabético en los que se inyectó tres veces IVR 3 veces con intervalos de un mes. Las capas retinal y coroidea, así como el área de líquido subretiniano, se midieron al inicio del estudio y durante 3 meses de tratamiento. Se investigó la correlación entre los cambios estructurales y la agudeza visual.
ResultadosEn general, la mayoría de las capas de la retina tendieron a disminuir después del tratamiento. La resolución del líquido subretiniano siguió siendo relativamente superior para predecir el resultado de la inyección de ranibizumab. Además, encontramos que la reducción del grosor de la capa de fotorreceptores tuvo la mejor estimación de la ganancia de agudeza visual.
ConclusiónLa recuperación neural de de la capa de fotorreceptores se asocia con una mejor mejora visual. La segmentación individual de la retina sería beneficiosa para monitorear y evaluar el tratamiento con ranibizumab en el edema macular diabético.
Diabetes macular edema (DME) remains a major complication of diabetic retinopathy (DR) and is one of the most frequent causes of visual impairment among productive-age individuals1. Considering the large number of diabetic patients in the population, early identification and proper treatment for patients with DME are crucial due to the potential impact on our public health system and patient quality of life. The pathomechanism of DME is multifactorial. Nevertheless, mounting evidence indicates that it develops as a result of an increase in retinal vascular hyperpermeability due to the upregulation of vascular endothelial growth factor (VEGF)2,3. Although vascular dysfunction in the retina and choroid is the main contributor to the pathogenesis of DR and DME, retinal neurodegeneration seems independently associated with the severity of DR4–6. Hence, evaluating both vascular and neuronal changes are important in order to assess disease progression and therapeutic responses.
Laser photocoagulation has been the gold standard for the treatment of DME for several decades1,7. Today, however, intravitreal anti-VEGF injection is considered the first-line therapy for DME due to its significant effect on visual outcomes and tolerability risks. Additionally, it has been previously described that different anatomical responses to anti-VEGF therapy are observed in each morphologic subtype of DME1. Thus, identifying distinctive structural changes could be useful for the ophthalmologist in managing and monitoring DME evolution.
With the recent advancement of imaging techniques and technologies, the evaluation of morphological changes in the retina can be easily performed over time. In particular, spectral-domain optical coherence tomography (SD-OCT) can quantitatively examine intraretinal damage in DME8,9. Moreover, retinal layer changes are often used to monitor the effectiveness of anti-VEGF therapy for DME10,11. This study, therefore, aims to measure retinal and choroidal layer thickness, as well as subretinal fluid areas during a three-month follow-up to determine a possible association between visual acuity and structural changes in DME patients treated with intravitreal ranibizumab (IVR) injection, in addition to the identification of a promising biomarker for treatment response.
Patients and methodsStudy design and ethical considerationThis is a retrospective single-center observational study. This study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Karsa Husada General Hospital (Ref. No. 072/251/102.13/2022). Data were obtained from the ophthalmology clinic database in Karsa Husada General Hospital, Batu, Indonesia.
Patients and intravitreal ranibizumab injectionOne hundred eight eyes belonging to 74 patients were treated with three doses of IVR injection, at one month intervals, for DME treatment at the Karsa Husada General Hospital ophthalmology clinic between January 2021 and December 2022. The inclusion criteria were the presence of DME before therapy, no history of ocular surgery/treatment within the previous 10 weeks, and no macular involvements (such as retinal vein occlusion and age-related macular degeneration).
Before injection and at monthly follow-ups, patients underwent comprehensive ophthalmologic examinations, including measurement of best-corrected visual acuity (BCVA), intraocular pressure (IOP) evaluation with Goldmann applanation tonometry, as well as biomicroscopic examination of the anterior segment and funduscopy. Intravitreal injections were conducted monthly with 0.5mg/0.05mL ranibizumab (Patizra, Novartis). A topical antibiotic was applied after the IVR injection for six days.
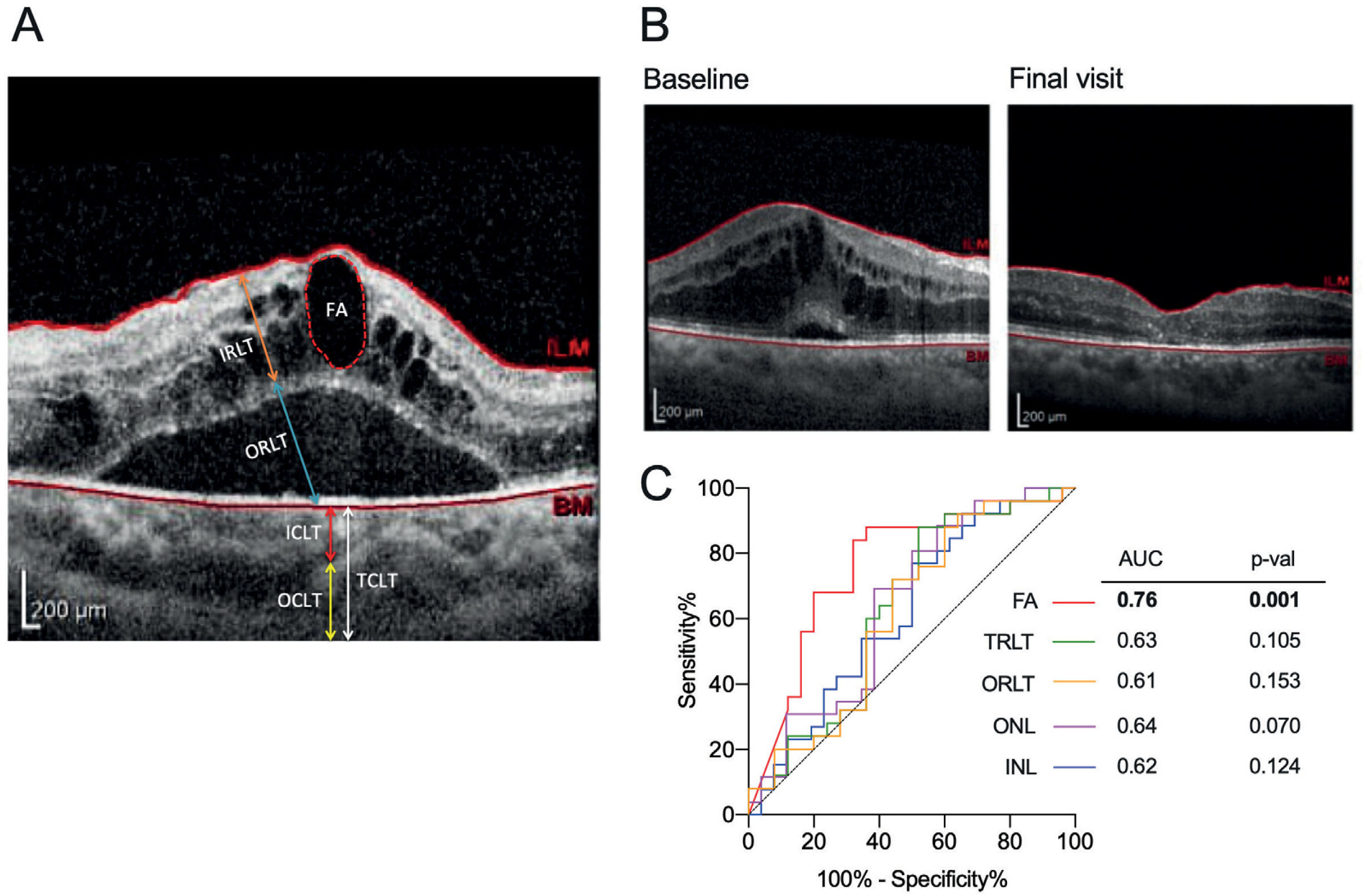
Structural outcomes evaluated by optical coherence tomography (OCT)Both horizontal A and vertical B scan images of the macular region concentrated at the fovea were examined using spectral domain optical coherence tomography (SD-OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany). Structural outcomes were examined by evaluating retinal layer thickness, choroidal layer thickness and subretinal fluid area. Individual retinal layer and choroidal layer thickness were measured manually according to the literature4,12. Briefly, the OCT image was loaded into ImageJ. The scale bar provided in the OCT image was used as a standard reference. The retinal layer thickness was then manually measured by outlining each retinal layer in the parafoveal ring (the average retinal thickness in the superior, inferior, nasal, and temporal regions was used), while choroidal layer thickness was quantified in the central fovea (Figure 1A). Basically, the scale bar was converted into the distance in pixels. Then unknown distance in pixels outlined in each layer will be automatically quantified to the corresponding scale by ImageJ.
Retina and choroid segmentation
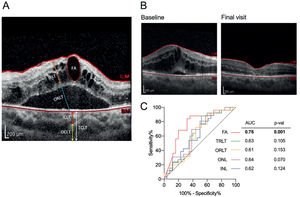
(A) Retina and choroid segmentation using SD-OCT; (B) Structural changes before and after treatment in patients who received 3 doses of IVR injections due to DME; (C) Receiver operating characteristics (ROC) curve analysis. ROC curve of INL, ONL, TRLT, ORLT, and FA for the assessment of ranibizumab effectiveness. FA, fluid area; IRLT, inner retinal layer thickness; ORLT, outer retinal layer thickness; TRLT, total retinal layer thickness; ICLT, inner choroidal layer thickness; OCLT, outer choroidal layer thickness; TCLT, total choroidal layer thickness; INL, inner retina layer; ONL, outer retina layer.
The subretinal fluid area was evaluated by manually outlining the areas using ImageJ software (Figure 1A). The outer choroid layer thickness (OCLT, yellow line) was measured from the inner boundary of the choroid scleral junction to the innermost point of the choroidal large blood vessel observed closest to the fovea centralis. The inner choroidal layer thickness (ICLT, red line) was obtained by subtracting the OCLT from the total choroid layer thickness (TCLT, white line).
Statistical analysisAll continuous variables were expressed as the mean±standard deviation, while categorical variables were expressed as percentages. The variables between the two groups (baseline vs. final visit) were compared using either an independent or dependent t-test, depending on the nature of the data. Correlations between visual and structural outcomes were evaluated using Pearson correlation analysis. r>0.5 was considered positively correlated and r=0.3 to 0.5 as moderate positively correlated. The values of retinal changes were used to generate ROC (receiver operating characteristic) curves using the GraphPad Prism version 9 in order to determine the area under the curve (AUC). The optimal cut-off for each parameter was determined using Youden's index, and the corresponding sensitivity and specificity for the cut-off were also quantified. Individual clinical factors were subjected to univariate linear analysis and were subsequently entered into the multivariate analysis in a backward stepwise manner. The criterion for retention in the multivariate model was p<0.05. All statistical analyses were 2-tailed with a significance threshold of p<0.05 calculated with SPSS version 25.
ResultsDemographic and clinical characteristicsSeventy-four patients with DME, including 32 males and 42 females, were examined and followed up for 3 months. The baseline characteristics are depicted in Table 1. The mean age of patients with DME was 53.17±8.42 years old, with a duration of diabetes of 9.95±7.21 years. All patients were treated with ranibizumab (Patizra, Novartis) monotherapy. The mean baseline visual acuity (LogMAR BCVA) of the treated eye was 0.93±0.61 and decreased by an average of 0.65±0.51 at month three (p=0.0007) (Table 2).
Demographic and clinical characteristics.
| Characteristics | Value |
|---|---|
| Sex (male/female) | 32/42 |
| Age, y, mean±SD | 53.17±8.42 |
| Duration of diabetes mellitus, y, mean±SD | 9.95±7.21 |
| LogMAR BCVA at baseline, mean±SD | 0.93±0.61 |
| IOP at baseline, mean±SD | 14.32±4.17 |
| Laterality, R/L | 64/44 |
| RBG, mg/dL, mean±SD | 152.17±75.07 |
| HbA1c, %, mean±SD | 8.96±1.01 |
BCVA, best-corrected visual acuity; IOP, intraocular pressure; logMAR, logarithm of the minimum angle of resolution; L, left; R, right; SD, standard deviation; RBG, random blood glucose, HbA1c, hemoglobin A1c.
Visual acuity and structural changes evaluated by SD-OCT.
| Parameters | Baseline | Final visit | p-val |
|---|---|---|---|
| LogMAR BCVA | 0.93±0.61 | 0.65±0.51 | 0.0007 |
| Retinal Layer (μm) | |||
| Retinal Nerve Fiber Layer (RNFL) | 18.12±9.68 | 13.57±9.59 | 0.249 |
| Ganglion Cell Layer (GCL) | 52.14±13.59 | 51.74±14.08 | 0.884 |
| Inner Plexiform Layer (IPL) | 45.99±13.38 | 43.09±10.64 | 0.257 |
| Inner Nuclear Layer (INL) | 59.03±17.70 | 50.98±11.25 | 0.007 |
| Outer Plexiform Layer (OPL) | 20.13±7.79 | 17.34±6.29 | 0.076 |
| Outer Nuclear Layer (ONL) | 128.60±95.38 | 81.42±32.41 | 0.007 |
| Photoreceptor Layer (PRL) | 78.01±49.40 | 63.43±25.27 | 0.085 |
| Total Retinal Layer Thickness (TRLT) | 410.40±115.00 | 358.20±79.52 | 0.010 |
| -Inner Retinal Layer Thickness (IRLT) | 182.40±38.32 | 166.40±33.46 | 0.072 |
| -Outer Retinal Layer Thickness (ORLT) | 228.00±98.08 | 184.50±60.15 | 0.015 |
| Choroidal Layer (μm) | |||
| Total Choroidal Layer Thickness (TCLT) | 358.4±104.5 | 355.6±61.41 | 0.911 |
| -Inner Choroidal Layer Thickness (ICLT) | 47.76±19.72 | 45.36±16.59 | 0.536 |
| -Outer Choroidal Layer Thickness (OCLT) | 310.70±98.64 | 310.30±65.80 | 0.987 |
| Subretinal Fluid Area (SFA) | 145,069±135,059 | 47,805±92,182 | 0.0005 |
Abbrev. LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; OCT, optical coherence tomography. Data presented as mean±SD. Bold indicates statistically significant.
The total retinal layer thickness (TRLT) decreased from 410.40±115.00μm at baseline to 358.20±79.52μm after three months of ranibizumab treatment (p=0.010, Table 2, figure 1B). Further examination indicated that only the outer retinal layer thickness (ORLT) was significantly lower at the final visit (p=0.015, Table 2). Although each individual retinal layer thickness (RLT) tended to decrease after IVR injection within three months of treatment, the thickness changed more significantly within the inner nuclear layer (INL, p=0.007) and outer nuclear layer (ONL, p=0.007) (Table 2). In contrast to RLT, no changes were observed in the choroidal layer thickness (CLT) (Table 2). In addition, we observed that three months of ranibizumab treatment effectively decreased the fluid area in the subretinal region (p=0.0005, Table 2). ROC analysis was performed in parameters with significant changes after ranibizumab injection in order to identify a possible biomarker for treatment evaluation. The changes in the subretinal fluid area were relatively superior to the other parameters (AUC=0.76, cut-off=78,257 with sensitivity of 84% and specificity of 68%, p=0.001) (Figure 1C).
Correlation of structural changes with visual acuityThe correlations of structural changes with logMAR BCVA were explored (Table 3). We found a significant positive correlation between logMAR BCVA and retinal nerve fiber layer (RNFL), ONL, TRLT, ORLT, and subretinal fluid area (r=0.419, 0.477, 0.483, 0.540, and 0.683, respectively, Table 3) at baseline, while other layers did not show good correlation. At the final visit, no significant correlations were detected (Table 3). We then examined whether there was any correlation between changes in the logMAR BCVA (ΔVA) and structural changes (Δstructural changes) from the baseline to the final visit. Similarly, no correlations were observed (Table 3). A multivariate analysis was further conducted using data at the final visit. The decrease in the PRL was associated with better visual acuity in the univariate analysis. However, this result was not statistically significant in the multivariate analysis (Table 4).
Association between visual acuity and structural changes at each time point.
| Parameters | Baseline | Final visit | LogMAR BCVA/structural changes |
|---|---|---|---|
| Retinal Layer (μm) | |||
| Retinal Nerve Fiber Layer (RNFL) | r=0.419; p=0.032 | r=0.292; p=0.198 | r=−0.004; p=0.982 |
| Ganglion Cell Layer (GCL) | r=0.306; p=0.093 | r=0.042; p=0.837 | r=−0.137; p=0.503 |
| Inner Plexiform Layer (IPL) | r=0.310; p=0.089 | r=0.303; p=0.131 | r=−0.096; p=0.639 |
| Inner Nuclear Layer (INL) | r=0.279; p=0.127 | r=0.037; p=0.856 | r=−0.157; p=0.443 |
| Outer Plexiform Layer (OPL) | r=0.281; p=0.125 | r=−0.178; p=0.384 | r=0.054; p=0.793 |
| Outer Nuclear Layer (ONL) | r=0.477; p=0.006 | r=0.255; p=0.102 | r=0.298; p=0.138 |
| Photoreceptor Layer (PRL) | r=0.069; p=0.710 | r=−0.327; p=0.102 | r=0.302; p=0.133 |
| Total Retinal Layer Thickness (TRLT) | r=0.483; p=0.006 | r=0.019; p=0.925 | r=0.061; p=0.771 |
| -Inner Retinal Layer Thickness (IRLT) | r=0.077; p=0.684 | r=−0.084; p=0.686 | r=−0.367; p=0.071 |
| -Outer Retinal Layer Thickness (ORLT) | r=0.540; p=0.002 | r=−0.207; p=0.318 | r=0.188; p=0.366 |
| Choroidal Layer (μm) | |||
| Total Choroidal Layer Thickness (TCLT) | r=0.0004; p=0.998 | r=0.192; p=0.346 | r=−0.102; p=0.617 |
| -Inner Choroidal Layer Thickness (ICLT) | r=0.186; p=0.315 | r=−0.084; p=0.682 | r=0.026; p=0.896 |
| -Outer Choroidal Layer Thickness (OCLT) | r=−0.037; p=0.876 | r=0.200; p=0.325 | r=−0.241; p=0.367 |
| Subretinal Fluid Area (SFA) | r=0.683; p=0.0001 | r=−0.081; p=0.698 | r=0.094; p=0.651 |
Multivariate linear regression analysis of factors with influence on LogMAR BCVA after 3 months ranibizumab treatment.
| Univariate analysis | Multivariate Analysis (Adjusted R2=0.235)* | ||||
|---|---|---|---|---|---|
| t | p-val | Unstandardized Regression Coefficient β | Unstandardized Regression Coefficient β | p-val | |
| Constant | 1.107 | 0.000 | |||
| Retinal Layer (μm) | |||||
| Retinal Nerve Fiber Layer (RNFL) | 1.889 | 0.076 | |||
| Ganglion Cell Layer (GCL) | −0.965 | 0.349 | |||
| Inner Plexiform Layer (IPL) | −0.731 | 0.475 | |||
| Inner Nuclear Layer (INL) | −0.866 | 0.399 | |||
| Outer Plexiform Layer (OPL) | 0.925 | 0.369 | |||
| Outer Nuclear Layer (ONL) | −1.805 | 0.090 | |||
| Photoreceptor Layer (PRL) | −2.556 | 0.020 | −0.007 | −0.327 | 0.103 |
| Total Retinal Layer Thickness (TRLT) | −0.375 | 0.712 | |||
| -Inner Retinal Layer Thickness (IRLT) | −0.503 | 0.622 | |||
| -Outer Retinal Layer Thickness (ORLT) | −0.109 | 0.914 | |||
| Choroidal Layer (μm) | |||||
| Total Choroidal Layer Thickness (TCLT) | −0.695 | 0.497 | |||
| -Inner Choroidal Layer Thickness (ICLT) | 0.302 | 0.767 | |||
| -Outer Choroidal Layer Thickness (OCLT) | −0.710 | 0.488 | |||
| Subretinal Fluid Area (SFA) | 0.348 | 0.733 |
Dependent variable: LogMAR best-corrected vision acuity (BCVA) at the final visit. Explanatory variable: retinal and choroidal layer thickness, subretinal fluid area.
This study showed that structural changes, marked by the reduction of TRLT (especially in the INL and ONL) and subretinal fluid accumulation, were observed in DME patients administered with three doses of IVR at one-month intervals. The efficacy of ranibizumab for DME treatment has been examined to some extent, and although the serum level of VEGF after 4 weeks of IVR did not statistically differ from baseline13, suppression of VEGF level is observed in aqueous humor samples14, reflecting that, locally, VEGF production within the eye is critical in progression and treatment of DME. The anti-VEGF drug ranibizumab also induced retinal reperfusion by inhibiting endothelial cell migration and neovascularization15,16. Additionally, ranibizumab treatments attenuated the vascular leakage in the DL-2-aminoadipic acid (DLAAA)-induced retinal neovascularization model17. Hence, a reduction of subretinal fluid accumulation as well as retinal layer thickness would be expected.
On the other hand, the effects of IVR injection on choroid layer thickness (CLT) in DME eyes are often inconsistent18–21. In this case, our results showed that CLT did not change after IVR treatment. Notably, a reduction of CLT is observed in DR treated with laser pan-retinal photocoagulation (PRP)22. Nonetheless, the exact mechanism underlying choroidal layer changes in DME eyes has yet to be elucidated. It has been demonstrated that vascular development in the choroidal layer is influenced by VEGF23. Therefore, it seems that CLT may be correlated with the upregulation of VEGF expression and subsequently increased CLT. Nonetheless, a recent study by Wang et al. suggests that choroidal response to ranibizumab treatment depends on DME types24. It is still unclear whether the onset of DME may also influence CLT. It is also possible to speculate that other treatment modalities may have influenced other factors rather than directly regulated VEGF expression. Hence, further related studies are required to explore the possible mechanism.
According to ROC analysis, reduction in the subretinal fluid area remained a crucial marker in determining the treatment outcome. Nevertheless, a decrease in the ONL can be considered a plausible alternative in several cases where fluid accumulations are minimal and hard to assess. Visual acuity gain was best associated with increased PRL, which concords with a previous study indicating photoreceptor thinning in DR/DME patients25. Indeed, the accumulation of fluid in the cystoid space has been proven to stimulate photoreceptor damage in patients with DME26,27. Although PRL degeneration has been previously demonstrated in the early onset of DR4,26, our current findings implied that ranibizumab treatment effectively reduces fluid accumulation and subsequently improves neural recovery in the PRL. Nonetheless, further studies with a larger sample size are needed to confirm this finding.
Despite the positive results, several limitations should be noted. Firstly, we found that six patients seemed non-responsive to ranibizumab treatment and required additional injections. Secondly, patients with better visual acuity at baseline had less room for visual improvement, which may influence the study outcome. Lastly, the retrospective nature and relatively small sample size of this study might have hindered the potential correlations between the variables measured.
In conclusion, individual segmentation in the retina, particularly in the PRL, may have a clinical value for monitoring a short-term response of ranibizumab therapy in DME patients. Further prospective and long-term studies with a large sample size are still required.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by funding from the Faculty of Medicine and Health Sciences, Maulana Malik State Islamic University with the Ref. No. DIPA-025.04.02.423812/2022.